Life Extension Magazine®
Elevated homocysteine is a proven risk factor for vascular disease.
Homocysteine can be lowered by supplementing with vitamins B12, B6, and folic acid.
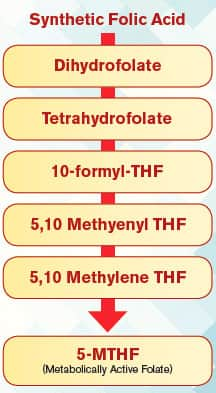
In order for folic acid to remove homocysteine, it first must be converted to its active form called L-methylfolate (5-MTHF). The diagram to the right shows the steps required in the body to convert folic acid to metabolically active L-methylfolate:
The conversion steps needed to form 5-MTHF outlined on the previous page require specific enzymes that in some people are impaired since birth. In other individuals, the activity of these enzymes slows with aging, which helps explain why homocysteine levels so often surge in the elderly.
Fortunately, we don’t have to rely on a perfect sequence of enzymes in our body to lower homocysteine. By taking L-methylfolate (also known as 5-methyltetrahydrofolate or 5-MTHF) directly, we can confidently lower homocysteine.
Published studies show that 5-MTHF achieves higher blood levels of active folate1,2—and more importantly, lowers homocysteine3 and extends survival in human study subjects.4
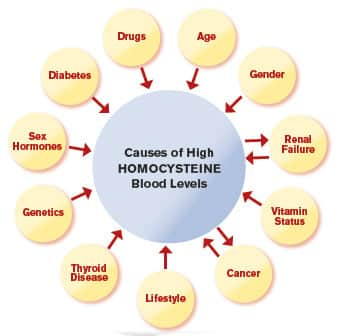
When was the last time you or your doctor checked your homocysteine levels? Can’t remember? Never? Either of these answers puts you at risk of a silent killer.5-7
Conventional doctors rarely test their patients’ homocysteine levels. Yet a low-cost blood test quickly reveals if you are in a danger zone.8,9
Most doctors don’t test for it and certainly don’t know how easy it is to treat high homocysteine with a unique form of folate known as 5-MTHF.
Devastating Impact Of High Homocysteine
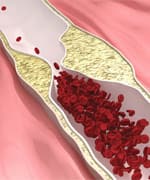
Elevated homocysteine levels (hyperhomocysteinemia) are an important, independent risk factor for cardiovascular diseases including atherosclerosis and its consequences, heart attack, stroke,10,11 and peripheral vascular disease.5,12,13
Homocysteine is an amino acid that’s very similar to the amino acids used by the body to make proteins.14 But, unlike these beneficial amino acids, homocysteine has toxic properties that contribute to poor health.15,16 High levels of homocysteine have been associated with numerous degenerative diseases.14
According to one study, 42% of patients with cerebrovascular disease (stroke or near stroke) had hyperhomocysteinemia, 28% of those with peripheral vascular disease (obstruction of blood vessels, especially in the legs) had elevated levels, and 30% of those with coronary artery disease (obstruction of the heart’s own blood supply, the coronary arteries) had elevated homocysteine levels.17
In a recent study of patients who had survived a heart attack, researchers divided over 800 subjects into two groups—those with high plasma homocysteine (defined as greater than 15 micromol/L in the study) and those with lower levels.5
After just 30 days, researchers found the rates of complications such as heart failure, heart rupture, death, and total adverse cardiovascular events were significantly higher in the elevated homocysteine group compared with those in the group with lower levels. Again, the higher the homocysteine level, the greater the incidence of adverse cardiovascular events.
Why Homocysteine Is So Dangerous
Scientists are still investigating the exact mechanism as to why homocysteine contributes to these grave health risks, but several convincing mechanisms are emerging. The most prominent ones involve homocysteine’s ability to elevate oxidant stress, alter lipid (fat) metabolism, and promote excessive blood clotting.18
Recently, links have been established between homocysteine and vital signaling information used by arteries to control blood pressure.19-21 There’s also evidence that homocysteine has damaging effects on the HDL-associated enzyme paraoxonase1, or PON1, which is required to protect LDL from oxidation.18,22-24
It is now clear that rising homocysteine levels are significantly associated with threats to the cardiovascular system, particularly to the aging adult. Mainstream medicine often ignores this information,25-27 and frequently offers little or no help to lower deadly homocysteine levels in patients.
What you need to know
 |
Lower Homocysteine With High-Potency Folate
- A high level of the amino acid homocysteine is a strong predictor of cardiovascular diseases such as heart attack and stroke, the leading killers of Americans.
- Folic acid supplementation is recommended as part of a B-vitamin package aimed at reducing homocysteine levels.
- But many people find their homocysteine levels stubbornly resist even high doses of folic acid.
- Such people may carry a gene variant that weakens their ability to generate the active form of folate, called 5-MTHF, resulting in elevated homocysteine levels.
- Supplementing directly with 5-MTHF evades the decrease in enzyme activity because it requires no further metabolism to achieve homocysteine-lowering effects.
- Get your homocysteine levels checked at least annually; if your levels resist therapy with standard folic acid, you should switch to the active, 5-MTHF form.
5-MTHF: The Best Form Of Folate Supplementation
In the body, the folate molecule goes through several enzymatic steps to become the active molecule 5-MTHF.28-30 It is the active 5-MTHF form of folate that participates in the homocysteine-lowering step that results in the production of the safe amino acid, methionine.28,30
It makes sense, then, that low levels of folate are predictive of elevated homocysteine levels.31
In theory, supplementing with folic acid—the most common form of folate in supplement form—should provide the body with the folate it needs to convert dangerous homocysteine into safe methionine. Unfortunately, this readily available homocysteine-lowering regimen doesn’t work for everyone.
That’s because in order to make the conversion from folate to the active 5-MTHF, an enzyme called methylenetetrahydrofolate reductase or MTHFR is necessary—and not everyone carries the same version of this important enzyme. It is estimated that between 5 and 10% of the population has a gene variant that reduces MTHFR activity by 70%,32 while nearly 50% of people of European descent have a genetic variation that decreases MTHFR activity by as much as 35%.28,29,33-36
If you fall into these categories, you are likely to be a victim of persistently high homocysteine levels— even if you are taking high doses of folic acid.37
The solution is simple: Instead of relying only on the folic acid in your multinutrient formula, supplement with 5-MTHF. By doing this, you’ll bypass the enzyme defect and provide your body with exactly the right molecule it needs to begin lowering those dangerous homocysteine levels.38
A wealth of laboratory and human studies demonstrate that 5-MTHF is the best form of folate required for lowering dangerous homocysteine levels.
5-MTHF: More Effective Than Folic Acid
Numerous animal and human studies have proven that 5-MTHF is more effective than folic acid at increasing serum active folate levels.
In a compelling animal study, mice were bred to have two copies of the defective gene for MTHFR, the enzyme involved in making 5-MTHF, which in turn, converts homocysteine into methionine.39 The animals had markedly elevated homocysteine levels as a result, and almost all died in infancy from homocysteine toxicity.
However, when their mothers (who survived because they carried one normal and one abnormal gene) were fed 5-MTHF during pregnancy, 64% of the offspring survived, compared with the 95% mortality rate seen in pups of mothers supplemented with folic acid. Encouragingly, the pups from mothers supplemented with 5-MTHF also showed improved appearance of structures in their brains that were previously affected by folate deficiency.
Human studies validate the superiority of 5-MTHF over folic acid.
One crossover study evaluated cardiovascular patients known to have two copies of the gene for the weakened MTHFR enzyme.38 Subjects received a single 5 mg oral dose of either folic acid or 5-MTHF, and then after a washout period, each patient received the opposite supplement from what they originally received. Researchers found that the subjects’ peak serum folate levels were nearly 7 times higher when taking the active 5-MTHF as they were when they took the folic acid supplement, demonstrating superior bioavailability of the 5-MTHF form.
Another crossover study involved a group of healthy women, one-third of whom had the typical gene variant for MTHFR, and two-thirds of whom had the weaker gene variant.35 Each woman received either 400 mcg of folic acid or 416 mcg of 5-MTHF. (The slightly higher dose in micrograms reflects chemical differences between folic acid and 5-MTHF; it represents the same dose of folate at the cellular level.) After the first phase of the study, the dosing regimen was reversed, and each woman received the opposite supplement from the first.
Regardless of the women’s genetic makeup, the total amount of folate in blood, as well as the maximum concentration reached, were significantly higher when the women were taking 5-MTHF compared to when they were taking folic acid.35
5-MTHF Lowers Homocysteine Levels
The superiority of 5-MTHF for increasing blood levels of folate directly translates to lower homocysteine levels.
This was first shown in a study of healthy people.40 For the study, a low dose of 5-MTHF (113 mcg per day) was compared with a low dose of folic acid (100 mcg per day). After six months, the mean total homocysteine was reduced by 14.6% in the 5-MTHF group, compared with only 9.3% in the folic acid group.
Further support for the superiority of 5-MTHF as a homocysteine-lowering supplement comes from a study of patients with kidney disease on dialysis.4 This group of people is at very high risk for elevated homocysteine levels,41,42 as well as high rates of complications and cardiovascular disease that occur as a result. In fact, 93% of this group of people had homocysteine levels above the upper limit of normal, which is considered in this study to be 12 micromol/L.
Subjects were divided into two treatment groups: The first one received 50 mg of intravenous 5-MTHF at the end of each dialysis section and the second received 5 mg per day of folic acid, orally. Both groups received the same intravenous dose of vitamins B6 and B12. After six months, homocysteine levels were reduced to an average of 20.7 micromol/L in the 5-MTHF group, compared with stubbornly high levels of 35.0 micromol/L in the folic acid group.4 Along with the greater homocysteine reduction, after 24 months, those in the 5-MTHF group also had significantly lower levels of the inflammatory marker C-reactive protein, or CRP.
Most importantly, however, was the impact 5-MTHF had on survival. Subjects in the 5-MTHF group survived on average 36.2 months after beginning treatment, while those in the folic acid group survived an average of only 26.1 months—that’s a 39% increase in survival in the 5-MTHF group!4 Interestingly, the doctors who conducted this study attributed the improved survival on the reduction in CRP observed in the 5-MTHF group.
5-MTHF And Depression

5-MTHF is clearly a superior way to achieve optimal blood levels of active folate, as well as the best means known for lowering dangerous homocysteine levels. But it has other benefits as well. Because folate is required in processes that produce the brain’s neurotransmitters,43 5-MTHF has also attracted the interest of researchers that investigate major depression, a condition that reflects imbalances in neurotransmitter quantity or effect.44
Major depression is a debilitating illness that is very difficult to treat: Only about 30% of patients treated with a single antidepressant drug achieve remission of their symptoms, a figure that only rises to 50 to 55% when a second drug is added.45,46 As a result, there has been a major push to find non-pharmacological therapies that could improve response rates. Once researchers discovered that people with low serum and red blood cell levels of folate have poorer responses to antidepressant therapy, they decided to see if adding 5-MTHF to antidepressant drugs could improve response rates.45 The results have been dramatic.
In one study, 19% of patients taking 5-MTHF plus a regular antidepressant drug experienced major improvement on a standard depression score, compared with just 7% of those who only took an antidepressant drug.47 That response rate was even higher among patients with the worst degree of impairment from depression, with 40% of those taking 5-MTHF in addition to their regular antidepressants experiencing major improvement versus just 16% of those taking antidepressants only.
In addition, the 5-MTHF group experienced these improvements significantly faster than the control group—in 177 days versus 231 days. Once again, those with the most severe depression experienced the most dramatic results, with the median time to improvement in the 5-MTHF group being 85 days, compared with 150 days in control subjects. Impressively, nearly twice as many people in the antidepressant-only group stopped therapy because of adverse events (34%) versus the 5-MTHF group (17.9%), a testament to the supplement’s safety.
Numerous other studies have achieved similar results when 5-MTHF is added to a standard antidepressant drug; doses used are typically 15 mg day.48-50 An abnormal gene variant for 5-MTHF synthesis and metabolism may predict resistance to antidepressant therapy and help identify patients who may be responsive to adjunctive therapy with 5-MTHF.51
5-MTHF And Diabetic Peripheral Neuropathy
Diabetic peripheral neuropathy is another condition that reflects imbalances in neurotransmitter quantity or effect,52 leading researchers to investigate 5-MTHF as a potential treatment. Diabetic peripheral neuropathy is a painful condition that causes slowing and abnormal transmission of nerve impulses,53 which results in both pain and loss of sensitivity to normal touch.54
The combination of 5-MTHF with vitamin B12 (methylcobalamin) and an active form of vitamin B6 (pyridoxal-5’-phosphate) has been used to treat endothelial dysfunction, and is now under active exploration for use in diabetic neuropathy.
An animal study showed that this combination, at a human equivalent dose, increased the density of nerve fibers in the skin and improved nerve sensory conduction and responses to temperature and mechanical touch.55 These improvements occurred in the absence of changes in blood sugar.
Studies have shown that when humans with diabetic peripheral neuropathy are treated with this supplement combination, they experience improvements of skin sensitivity to touch and reduction in painful symptoms.56,57 One such trial also showed improvements in the density of nerve fibers in the skin,57 and a different trial also noted a significant decrease in homocysteine levels compared with a small increase in placebo recipients.56
Testing For Homocysteine
 |
Your homocysteine levels can be determined by a simple blood test. Knowing your homocysteine status will allow you take therapeutic action if your levels are too high. Remember to fast for eight to 12 hours before the blood test. Do not eat any food or drink liquids other than water before the test and take any medications as prescribed. Do not take your supplements the morning of the test.
Summary
High levels of the amino acid homocysteine are a major threat to an aging person’s health, raising the risk for cardiovascular disorders such as heart attack and stroke. Folate is known to be effective in lowering homocysteine levels, but a large proportion of people find that their homocysteine levels remain stubbornly high, even on folic acid supplements.
This could be caused by the fact that 5 to 10% of the population and nearly half of people of European descent carry a gene variant that reduces the activity of the enzyme required to efficiently convert folic acid into the active, homocysteine-lowering form of the vitamin, 5-MTHF.
For people with persistently high homocysteine levels, high-dose 5-MTHF is now available as a supplement.
Published studies show that 5-MTHF not only lowers homocysteine and CRP, but also improves human survival.
If your homocysteine-lowering regimen seems stalled, don’t simply raise your dose of folic acid. Instead, switch to 5-MTHF, the active form of the supplement. Then test your homocysteine levels again in 30 days to ensure you are taking the proper dose of 5-MTHF. Some people with high homocysteine may only need 5 mg (5,000 mcg) of 5-MTHF once daily while others will need to take 5 mg of 5-MTHF twice a day.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
References
- Lamers Y, Prinz-Langenohl R, Bramswig S, Pietrzik K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am J Clin Nutr. 2006 Jul;84(1):156-61.
- Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2010 Aug;49(8):535-48.
- Caruso R, Campolo J, Sedda V, et al. Effect of homocysteine lowering by 5-methyltetrahydrofolate on redox status in hyperhomocysteinemia. J Cardiovasc Pharmacol. 2006 Apr;47(4):549-55.
- Cianciolo G, La Manna G, Coli L, et al. 5-methyltetrahydrofolate administration is associated with prolonged survival and reduced inflammation in ESRD patients. Am J Nephrol. 2008;28(6):941-8.
- Ma Y, Li L, Geng XB, et al. Correlation between hyperhomocysteinemia and outcomes of patients with acute myocardial infarction. Am J Ther. 2014 Nov 17.
- Martí-Carvajal AJ, Solà I, Lathyris D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2015 Jan 15;1:CD006612.
- Vollset SE, Refsum H, Tverdal A, et al. Plasma total homocysteine and cardiovascular and noncardiovascular mortality: the Hordaland Homocysteine Study. Am J Clin Nutr. 2001 Jul;74(1):130-6.
- Available at: http://www.medicinenet.com/homocysteine/page3.htm. Accessed February 11, 2015.
- Available at: http://elcaminogmi.dnadirect.com/grc/patient-site/mthfr-pregnancy-complications/testing-for-high-homocysteine.html. Accessed February 11, 2015.
- Tascilar N, Ekem S, Aciman E, et al. Hyperhomocysteinemia as an independent risk factor for cardioembolic stroke in the Turkish population. Tohoku J Exp Med. 2009 Aug;218(4):293-300.
- Ashjazadeh N, Fathi M, Shariat A. Evaluation of homocysteine level as a risk factor among patients with ischemic stroke and its subtypes. Iran J Med Sci. 2013 Sep;38(3):233-9.
- Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049-57.
- Fowler B. Homocysteine—an independent risk factor for cardiovascular and thrombotic diseases. Ther Umsch. 2005 Sep;62(9):641-6.
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015 Jan 10;14(1):6.
- Jakubowski H. Molecular basis of homocysteine toxicity in humans. Cell Mol Life Sci. 2004 Feb;61(4):470-87.
- Boldyrev AA. Molecular mechanisms of homocysteine toxicity. Biochemistry (Mosc). 2009 Jun;74(6):589-98.
- Clarke R, Daly L, Robinson K, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991 Apr 25;324(17):1149-55.
- Eren E, Ellidag HY, Aydin O, Yilmaz N. Homocysteine, paraoxonase-1 and vascular endothelial dysfunction: omnibus viis romam pervenitur. J Clin Diagn Res. 2014 Sep;8(9):Ce01-4.
- Kim CS, Kim YR, Naqvi A, et al. Homocysteine promotes human endothelial cell dysfunction via site-specific epigenetic regulation of p66shc. Cardiovasc Res. 2011 Dec 1;92(3):466-75.
- Schiffrin EL; Canadian Institutes of Health Research Multidisciplinary Research Group on Hypertension. Beyond blood pressure: the endothelium and atherosclerosis progression. Am J Hypertens. 2002 Oct;15(10 Pt 2):115S-122S.
- Spieker LE, Flammer AJ, Lüscher TF. The vascular endothelium in hypertension. Handb Exp Pharmacol. 2006;(176 Pt 2):249-83.
- Rozenberg O, Rosenblat M, Coleman R, Shih DM, Aviram M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic Biol Med. 2003 Mar 15;34(6):774-84.
- Efrat M, Aviram M. Paraoxonase 1 interactions with HDL, antioxidants and macrophages regulate atherogenesis - a protective role for HDL phospholipids. Adv Exp Med Biol. 2010;660:153-66.
- Sampson MJ, Braschi S, Willis G, Astley SB. Paraoxonase-1 (PON-1) genotype and activity and in vivo oxidized plasma low-density lipoprotein in Type II diabetes. Clin Sci (Lond). 2005 Aug;109(2):189-97.
- Jacobsen DW, Gatautis VJ, Green R, et al. Total plasma homocysteine: the mediator/marker controversy continues. 1994. Clin Chem. 2009 Sep;55(9):1742-3.
- Schaffer A, Verdoia M, Cassetti E, Marino P, Suryapranata H, De Luca G. Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. 2014 Aug;134(2):288-93.
- Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. 2011 Feb;34(1):93-9.
- Obeid R, Holzgreve W, Pietrzik K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J Perinat Med. 2013 Sep 1;41(5):469-83.
- Leemans L. Does 5-methyltetrahydrofolate offer any advantage over folic acid? J Pharm Belg. 2012 Dec (4):16-22.
- Bailey LB, Gregory JF 3rd. Folate metabolism and requirements. J Nutr . 1999 Apr;129(4):779-82.
- Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clin Chem. 2000 Aug;46(8 Pt 2):1277-83.
- Bezold G, Lange M, Peter RU. Homozygous methylenetetrahydrofolate reductase C677T mutation and male infertility. N Engl J Med. 2001 Apr 12;344(15):1172-3.
- Fukuda N, Hamajima N, Wakai K, Suzuki K. A cross-sectional study to find out the relationship of methylenetetrahydrofolate reductase (MTHFR) C677T genotype with plasma levels of folate and total homocysteine by daily folate intake in Japanese. J Nutr Sci Vitaminol (Tokyo). 2014;60(4):231-8.
- Lovricevic I, Franjic BD, Tomicic M, et al. 5, 10-Methylenetetrahydrofolate reductase (MTHFR) 677 C --> T genetic polymorphism in 228 Croatian volunteers. Coll Antropol. 2004 Dec;28(2):647-54.
- Prinz-Langenohl R, Bramswig S, Tobolski O, et al. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C-->T polymorphism of methylenetetrahydrofolate reductase. Br J Pharmacol. 2009 Dec;158(8):2014-21.
- Yafei W, Lijun P, Jinfeng W, Xiaoying Z. Is the prevalence of MTHFR C677T polymorphism associated with ultraviolet radiation in Eurasia? J Hum Genet. 2012 Dec;57(12):780-6.
- Shojaei MH, Djalali M, Siassi F, Khatami MR, Boroumand MA, Eshragian MR. Serum levels of lipoprotein(a) and homocysteine in patients on hemodialysis who take hydroxymethylglutaryl-CoA reductase inhibitors, vitamin B6, and folic acid. Iran J Kidney Dis. 2009 Jul;3(3):141-4.
- Willems FF, Boers GH, Blom HJ, Aengevaeren WR, Verheugt FW. Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease. Br J Pharmacol. 2004 Mar;141(5):825-30.
- Li D, Karp N, Wu Q, et al. Mefolinate (5-methyltetrahydrofolate), but not folic acid, decreases mortality in an animal model of severe methylenetetrahydrofolate reductase deficiency. J Inherit Metab Dis. 2008 Jun;31(3):403-11.
- Venn BJ, Green TJ, Moser R, Mann JI. Comparison of the effect of low-dose supplementation with L-5-methyltetrahydrofolate or folic acid on plasma homocysteine: a randomized placebo-controlled study. Am J Clin Nutr. 2003 Mar;77(3):658-62.
- Bostom AG, Shemin D, Lapane KL, Hume AL, Yoburn D, Nadeau MR, Bendich A, Selhub J, Rosenberg IH. High dose-B-vitamin treatment of hyperhomocysteinemia in dialysis patients. Kidney Int. 1996 Jan;49(1):147-52.
- McDonald SP, Whiting MJ, Tallis GA, Barbara JA. Relationships between homocysteine and related amino acids in chronic hemodialysis patients. Clin Nephrol. 2001 Jun;55(6):465-70.
- Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008 Sep;13(3):216-26.
- Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69 Suppl E1:4-7.
- Farah A. The role of L-methylfolate in depressive disorders. CNS Spectr. 2009 Jan;14(1 Suppl 2):2-7.
- Wade RL, Kindermann SL, Hou Q, Thase ME. Comparative assessment of adherence measures and resource use in SSRI/SNRI-treated patients with depression using second-generation antipsychotics or L-methylfolate as adjunctive therapy. J Manag Care Pharm. 2014 Jan;20(1):76-85.
- Ginsberg LD, Oubre AY, Daoud YA. L-methylfolate Plus SSRI or SNRI from treatment initiation compared to SSRI or SNRI monotherapy in a major depressive episode. Innov Clin Neurosci. 2011 Jan;8(1):19-28.
- Papakostas GI, Cassiello CF, Iovieno N. Folates and S-adenosylmethionine for major depressive disorder. Can J Psychiatry. 2012 Jul;57(7):406-13.
- Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012 Dec;169(12):1267-74.
- Shelton RC, Sloan Manning J, Barrentine LW, Tipa EV. Assessing effects of l-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4).
- Papakostas GI, Shelton RC, Zajecka JM, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014 Aug;75(8):855-63.
- Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury, and schizophrenia. Trends Pharmacol Sci. 2005 Sep;26(9):477-84.
- Skundric DS, Lisak RP. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy: from glucose metabolism to neurodegeneration. Exp Diabesity Res. 2003 Oct-Dec;4(4):303-12.
- Said G. Diabetic neuropathy—a review. Nat Clin Pract Neurol. 2007 Jun;3(6):331-40.
- Shevalye H, Watcho P, Stavniichuk R, Dyukova E, Lupachyk S, Obrosova IG. Metanx alleviates multiple manifestations of peripheral neuropathy and increases intraepidermal nerve fiber density in Zucker diabetic fatty rats. Diabetes. 2012 Aug;61(8):2126-33.
- Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes with peripheral neuropathy: a randomized trial. Am J Med. 2013 Feb;126(2):141-9.
- Jacobs AM, Cheng D. Management of diabetic small-fiber neuropathy with combination L-methylfolate, methylcobalamin, and pyridoxal 5’-phosphate. Rev Neurol Dis. 2011;8(1-2):39-47.

