Life Extension Magazine®

Countless numbers of men are alive today because a PSA blood test identified cancer early enough for curative therapies to be deployed. Some famous people diagnosed early with prostate cancer who continued to live productive lives include:
What we don’t know is how many treatment side effects, such as urinary incontinence, impotence, and chronic pain, these men may be dealing with.
Because as many as 1 in 7 American men will be diagnosed with prostate cancer, Life Extension® has sought to identify more effective and less side-effect-prone treatments. Our objective is to find innovative and compassionate doctors who are achieving impressive results without resorting to radical prostatectomy surgery and radiation.
This article describes a physician/scientist who has spent decades perfecting a minimally invasive diagnostic and treatment approach that may revolutionize conventional prostate cancer treatment.
The Dilemma…
Men have every reason to be confused about prostate cancer screening and treatment.1
On one hand, experts tell us that prostate cancer is the second most common malignancy in men, as well as the second leading cause of cancer-related death in modern industrialized countries.2,3
On the other hand, equally reliable sources tell us that prostate cancer screening is overused and can lead to a diagnosis of trivial cancers, resulting in excessive biopsies and surgical procedures.1,4-6
So what is a man to do?- skip blood tests and take a chance with a potential killer, or undergo screening with its possibility for a false alarm and the potential for treatment-associated side effects?
Modern medicine has arrived at this quandary with the best intentions. Advances in screening tests have enabled doctors to detect cancers at earlier stages. That’s generally considered a good thing in oncology circles.
What’s happened is that small-size/non-aggressive prostate cancers that pose only minimal risk can now be detected. But treatment technology has not kept up with diagnostics, leaving physicians with surprisingly crude techniques for biopsy and removal of suspect cancers. Virtually all conventional approaches to prostate cancer incur risk of incontinence and impotence, in addition to the possibility that a serious tumor mass could still be missed.
These concerns shift the benefit-risk calculation sharply and have caused some men and their physicians to take a dangerously conservative position, leaving open the possibility that they might be missing deadly cancers. These cancers could be amenable to treatment if we could only locate them with certainty and treat with sufficient precision to avert serious complications.
Delineating the PSA Quandary
 |
Widespread use of blood screening for PSA has permitted very early detection of prostate cancers. Too early, some experts believe, raising concerns that many cancers now being diagnosed are too trivial to justify a radical prostate operation, with its inherent risks to continence and potency.26 Yet at the same time, a large proportion of men are reluctant to accept the idea of “watchful waiting,” knowing that their bodies harbor a malignancy.26 Most men prefer a more active role in managing their own health.
This situation has led to a quandary in which neither patients nor physicians feel comfortable, yet official policy recommends against routine PSA screening.
This quandary may become moot if more patients and physicians recognize the value of optimized focal therapy for prostate cancer, using the tools and protocols developed by Dr. Onik and his team. By making prostate cancer detection more precise, and removing only the tiny amount of prostate tissue that has turned malignant, concerns on both sides of the PSA debate can be eliminated.
Equally important is evidence that this novel procedure may generate a localized reaction that destroys residual peripheral cancer cells, while generating a systemic immune response against malignant cells that have escaped the prostate gland.
A Better Technology
Gary Onik, MD, is a visionary prostate expert and inventor who thinks that this dilemma represents a false dichotomy. Using techniques Dr. Onik and his colleagues have developed over the past decade, it is now possible for a man to undergo the rough equivalent of a “lumpectomy” of the prostate.7 This procedure allows a urologist to precisely identify a tumor (or tumors) within a three-dimensional matrix of the prostate gland, then selectively destroy tissue only where malignant cells are documented.
Dr. Onik and his team recently reported the results of a 10-year average follow-up of 70 study participants that demonstrated superior results in medium- and high-risk patients compared with standard treatment of the whole prostate gland.7 Remarkably, Dr. Onik’s approach was equally successful at preventing recurrence for all risk categories of cancer, compared with standard treatments that are so side-effect prone.
What You Need to Know
 |
Breakthrough Prostate Cancer Testing and Treatment
- Prostate cancer is a common and sometimes deadly cancer.
- Early detection through screening and specific therapy is standard oncological practice for most malignancies.
- A 2008 report questioned routine blood testing for evidence of prostate cancer in men over 50. Physicians and patients have been at a loss for the best way forward in balancing the risk of unnecessary treatment against that of late diagnosis of a malignancy.
- Thanks to the work of Dr. Gary Onik and his team, that dilemma is largely out of date.
- Dr. Onik combines precise, three-dimensional prostate mapping biopsy with equally precise three-dimensional destruction of tumor by freezing, to produce results equivalent to or better than standard approaches but with sharply reduced post-surgical side effects.
- Men should discuss optimized focal therapy for prostate cancer with their physicians, to allow for routine PSA testing and proper, lower side-effect risk treatment.
Introducing “Lumpectomy” for Men
Dr. Onik has written that “prostate cancer in men raises many of the same issues that breast cancer does in women. Complications of prostate cancer treatment, including impotence and incontinence, affect the self-image and psyche of a man no less than does the loss of a breast in a woman.”8
It was this insight that led Dr. Onik and his team to explore the possibility of a male version of lumpectomy. In other words, selective identification of a prostate tumor’s location and precise removal of the diseased tissue while sparing surrounding structures and their important functions.8-11
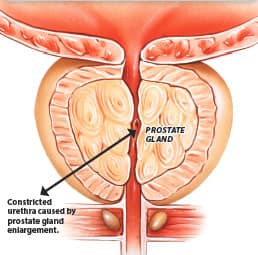
To understand the promise of Dr. Onik’s “lumpectomy” approach, one has to grasp the anatomical challenges posed by prostate cancer.
The prostate gland, unlike the breast, is in an extremely hard-to-reach location. Bounded by bone, bladder, and rectum, and containing the urine- and semen-carrying urethra, the gland cannot readily be exposed and directly examined.
This is further complicated by the fact that most men with early prostate cancer have no symptoms that might help guide or localize therapy. There is no palpable “lump” detected by patient. Instead, the concern about cancer is raised either on a rectal examination by a skilled clinician or by an elevated level of PSA in the blood.
Neither technique, of course, provides information about the potential severity or extent of any tumor found. To get that evidence, doctors turn to an ultrasound procedure, typically followed by a needle biopsy (tissue sampling) under ultrasound guidance.
The most common approach is called transrectal ultrasound biopsy. The main problem with this technique is that it’s inadequate for assuring that all of the tumor will be detected, and misses up to 46% of significant (high-grade) cancers.7,12 Studies have shown that transrectal ultrasound biopsies bear little resemblance to the actual pathological findings when the entire gland is removed, which clearly indicates room for improvement.12,13
Numerous sources suggest that the use of multiparametric MRI is useful for identifying and locating significant cancer in the prostate. While indications suggest that the use of the multiparametric MRI technology is more accurate than a transrectal ultrasound biopsy in the identification of cancer, findings have shown that its sensitivity for picking up clinically significant tumors in the peripheral zone (or outer area) of the prostate was 85% and just 62% in the transition zone (or innermost section) of the prostate.14
Not surprisingly, treatments based on an inadequate diagnostic technique have a high likelihood of being inadequate themselves, either overtreating a cancer that is in fact trivial or missing some parts of a viable tumor that will lead to a recurrence. In part to avoid those outcomes, current treatments target most or all of the prostate gland, to ensure that as much tumor as possible will be removed (such as radical prostatectomy).
Thus, inaccurate diagnoses coupled with heavy-handed, one-size-fits-all surgical approaches incur substantial risk of damaging important nerves and other structures that can potentially leave a man with urinary or erectile dysfunction, without necessarily providing a cure of the cancer itself.8
More Accurate Detection and Diagnosis Changes Tumor Management

In the early 2000s, Dr. Onik and his team began exploring better techniques for prostate cancer detection, using an array of emerging high-technology devices in the hope that they could improve on the performance of standard transrectal ultrasound guided prostate biopsies.
First, they began using a highly accurate means of examining the prostate gland in three dimensions and obtaining biopsy specimens from the entire bulk of the gland. A technique called three-dimensional prostate mapping biopsy, 3D-PMB, provides more accurate information about a tumor’s extent and location compared with a standard transrectal ultrasound biopsy.12,15
This three-dimensional prostate mapping biopsy is extraordinarily accurate and painless, and it does not involve puncturing the rectal wall. This sterile procedure greatly lowers the chance for life-threatening sepsis and debilitating prostatitis, which are sometimes the result of standard transrectal ultrasound biopsies.
By 2009, with five years of experience using three-dimensional prostate mapping biopsy, Dr. Onik’s team published a study of men who had previously undergone a standard biopsy using transrectal ultrasound guidance and whose results showed tumor on only one side of the gland.12 These men were considering “conservative” management, meaning that they were likely to receive no further treatment while being carefully observed.
As a result of performing the three-dimensional prostate mapping biopsy on the 180 men who were identified as having cancer on one side of the gland by the standard transrectal ultrasound biopsy, Dr. Onik identified:
- 110 patients (61.1%) who had tumor on both sides
- 41 patients (22.7%) whose tumor grade (severity) increased from a low-grade score to an intermediate-grade score
- 35 patients (19.4%) who were found to have cancer dangerously close to nerve and blood vessel bundles
Overall, 69.4% of the men originally diagnosed by transrectal ultrasound to have low-grade, one-sided tumors meriting just “watchful waiting” (active surveillance) turned out to have at least one finding that might have changed their cancer management plan to a more aggressive approach.12
Dr. Onik’s findings are being corroborated by other inventive physicians using enhanced imaging techniques.14
Optimized Focal Therapy for Prostate Cancer
Having clearly identified the more accurate higher-resolution diagnostic properties of three-dimensional prostate mapping biopsy, Dr. Onik and his team went on to study the results of treating men according to its results. To do so, they used an approach called optimized focal therapy.7
“Focal” is medically defined as “localized.” So “focal ablation” in this context refers to precise localized removal of malignant prostate tissues.
In this technique, patients first undergo biopsies by three-dimensional prostate mapping to accurately locate the tumor or tumors in three-dimensional imaging of the prostate gland. The location of each specimen is carefully noted and correlated with the pathology report for each site. This allows physicians to see not only the three-dimensional extent of the tumor but also its most concerning areas.7
Next, using their detailed three-dimensional map of each man’s prostate, the team returns to the diagnostic suite armed with a cryoablation device. Cryoablation means “destruction by cold.” It is a technique widely used for removal of focal areas of diseased tissue while assuring minimal collateral damage to adjacent healthy structures (anyone who has had a wart “burned off” with liquid nitrogen has undergone a simplified version of this technique).
Dr. Onik’s team used their three-dimensional map of tumor extent and severity to guide their ablation tool, aiming to destroy all known areas of tumor while sparing vital structures such as nerve/blood vessel bundles. They carefully control the freezing temperatures to assure that they deliver the precise amount of tissue-killing cold to each area of the tumor.7
Findings from 10-Year Study Using Focal Ablation Therapy

A long-term clinical trial was initiated to ascertain the accuracy, safety, and efficacy of focal cryoablation therapy using precise three-dimensional prostate mapping biopsy.
Dr. Onik’s team treated 70 men in this fashion, giving them a blood test for the PSA tumor marker at quarterly intervals for two years, then every six months. Men were determined to be “biochemically disease free” when their PSA level stabilized, indicating no actively growing tumor.7
Additional follow-up determined the degree to which each man’s potency and urinary continence had been affected, to identify common side effects of prostate surgery.
By the end of the study, Dr. Onik had data for an average of over 10 years on 70 subjects aged 45 to 77 years at the time of the procedure, an ample number for analysis.
Overall, 66 men survived to the end of the study, and none died of prostate cancer, yielding a “disease-specific” survival rate of 100%. Biochemical disease-free survival (proportion of men achieving PSA stability) was 89% overall.
Men with prostate cancer are categorized according to risk level based on the characteristics of the cancer, the Gleason score (a measure of the aggressiveness of the cancer), the stage (extent of the cancer), and the PSA level. With conventional treatments, patients with low-risk cancer have an approximate 85% long-term disease-free survival rate while men at high risk have a success rate that falls to 45%.16 Surprisingly, with the focal cryoablation treatment, there seems to be no difference in patient results based on risk level.7
Biochemical disease-free survival was 90% in men categorized as low-risk, 88% in medium-risk, and 89% in high-risk men, showing no statistically significant difference among the risk levels.7 In other words, unlike existing therapies, all men had superb results regardless of their original risk category.
Dr. Onik’s approach of focal cryoablation represents the first time that statistically identical survival rates were obtained across all cancer risk levels through use of a localized, minimally invasive prostate cancer treatment. Importantly, the use of the three-dimensional prostate mapping biopsy also lowered the local recurrence (patients who needed to be retreated due to more cancer found within the gland) to just 4%.7
Complications occurred with extremely low frequency after optimized focal therapy: A full 100% of men retained urinary continence, requiring no absorbent pad use, while 94% of men remained normally potent after the first treatment.7
There are a number of potential reasons for these results:
“A cryoimmunological response must also be considered for these results in medium- and high-risk patients,” wrote Dr. Onik and colleagues in a paper published in the Journal of Men’s Health. “Based on the human and animal data, it is likely that in some patients there is exposure of tumor antigens at the time of the procedure that acts as an in vivo cancer vaccine, preventing later metastasis from occurring.”7
Said differently, as the prostate tumor mass is being damaged/destroyed by the cryoablation (freezing) technique, barriers to recognition by the immune system are broken down. This can enable immune cells to take notice of the tumor’s genetic makeup and initiate attack against malignant lesions in other parts of the body that have spread or metastasized beyond the prostate capsule. This immune-boosting phenomenon has been observed in response to laser and certain localized radiation procedures.17-20 Interestingly, another medical team utilizing cryoablation reported the spontaneous remission of metastatic prostate cancer after freezing of the primary tumor for palliation.21
Dr. Onik’s team identified another mechanism by which focal cryoablation may be effective in treating malignant disease outside the prostate capsule. They wrote: “Focal cryoablation has an ability to treat extra-capsular disease. Patients at high risk for positive margins at prostatectomy have a better chance of local control with ablative therapy.”7
Comparative Studies
Optimized focal therapy appears to be superior to several other modern techniques at producing biological disease-free survival. In one study of prostate patients treated with specialized radiation therapy, 10-year biochemical disease-free survival was 81% for low-risk, 78% for medium-risk, and just 62% for high-risk men, using identical criteria for success.22
In a study of robotic radical prostate removal—considered by many to be the “gold standard” of prostate cancer treatment7—the biochemical disease-free survival rate in all patients was just 72%, even when measured at only five years, rather than 10 years, after the procedure.23
That study also found substantial amounts of cancer on the margins of the removed tissue—indicating some cancer remained—in 23% of low-risk, 29% of medium-risk, and 42% in high-risk patients.23 These patients would, under current guidelines, be offered additional radiation therapy, which adds both risk and cost to an already complicated procedure.24
Thus, Dr. Onik’s team appears to have realized their dream of performing the equivalent of a lumpectomy in men with prostate cancer. They identified the extent and severity of the disease in a large group of men using three-dimensional mapping and biopsies, then used that information to selectively destroy tissue in a fashion personalized for each individual patient. Their results are equivalent to more radical surgical approaches in low-risk patients, and superior to those in medium- and high-risk patients for achieving cancer-free status.7
An Answer to the PSA Screening Quandary
Because of these compelling results, Dr. Onik has suggested that the debate regarding PSA screening can easily be resolved.
That quandary was first produced by the United States Preventive Services Task Force, which in 2008 recommended discontinuation of routine PSA blood testing in most men, citing the emotional turmoil and potential for overtreatment raised by excessively early detection of small prostate tumors.25
But that United States Preventive Services Task Force recommendation made the assumption that prostate cancer diagnosis and treatment were static and unchanging, making the risk-benefit choice in some cases favor watchful waiting rather than aggressive treatment.
With the dawn of the era of optimized focal therapy for prostate cancer, the calculus has changed. Tumors can now be identified, localized, and staged with unprecedented accuracy, using three-dimensional mapping and biopsies. Focal cryoablation based on the three-dimensional map allows each man’s therapy to be personally customized, resulting in higher rates of disease elimination with few of the complications that spurred the task force’s fears.
Dr. Onik firmly believes that, with the availability of these enhanced techniques of diagnosis and treatment, all men should once again undergo routine PSA screening and digital rectal exam, with much less fear of unintended consequences if a suspicious lesion is discovered.
In his opinion, there never was anything wrong with the PSA test. It was simply being applied in a group of patients for whom tailored therapy was not available, resulting in a one-size-fits-all approach that did no favors for many men. But that argument can now be convincingly retired, Dr. Onik says, with the net result that more men will receive more appropriate therapy for their own specific prostate cancers.
Although the results of the Onik study are impressive, additional confirmatory studies will be required for widespread acceptance.
Summary
Ever since the 2008 publication of the United States Preventive Services Task Force report condemning routine use of PSA testing to detect early prostate cancers, physicians and their patients have been at in a quandary regarding the best approach for detection and treatment of prostate cancer.
The options seemed limited to one of two extremes: Test early and run the risk of unnecessarily invasive surgery and its performance-related side effects, or refrain from testing until obvious symptoms arise, risking the chance of more advanced and potentially fatal cancer.
A proper balance has been restored with an approach championed by Gary Onik, MD. Men can now return to regular PSA screening, and, if found to be at risk of harboring a prostate tumor, undergo precise three-dimensional prostate mapping biopsy followed by equally precise cryoablation of just the malignant tissue, largely sparing the vital structures needed to sustain potency and continence.
As discussed in the opening editorial in this month’s issue of Life Extension® magazine, there are also opportunities for men with a rising PSA to reverse it via modifications to their diet, lifestyle, supplement, hormone, and medication program.
Any man over 40 years old should include the PSA test with their annual screening for modifiable disease risk factors. If the result comes back very high, or if there is a consistent rise in PSA readings over time, the use of three-dimensional prostate mapping biopsy should be considered for diagnosis to assess if one has prostate cancer.
If the biopsy comes back positive, then focal cryoablation therapy can be performed during the same diagnostic procedure as a potential curative treatment.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
References
- Murphy DG, Ahlering T, Catalona WJ, et al. The Melbourne Consensus Statement on the early detection of prostate cancer. BJU Int. 2014;113(2):186-8.
- Colloca G, Venturino A, Governato I, et al. Incidence and correlates of fatigue in metastatic castration-resistant prostate cancer: a systematic review. Clin Genitourin Cancer. 2016;14(1):5-11.
- Pugliese D, Palermo G, Totaro A, et al. Clinical, pathological and molecular prognostic factors in prostate cancer decision-making process. Urologia. 2016;82(1):14-20.
- Hugosson J, Carlsson S. Overdetection in screening for prostate cancer. Curr Opin Urol. 2014;24(3):256-63.
- Pezaro C, Woo HH, Davis ID. Prostate cancer: measuring PSA. Intern Med J. 2014;44(5):433-40.
- Vickers AJ, Eastham JA, Scardino PT, et al. The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening. Urology. 2016.
- Onik G, Barrie K, Miessau M, et al. Long-term results of optimized focal therapy for prostate cancer: average 10-year follow-up in 70 patients. J Men’s Health. 2014;11(2):64-74.
- Onik G. Rationale for a “male lumpectomy,” a prostate cancer targeted approach using cryoablation: results in 21 patients with at least 2 years of follow-up. Cardiovasc Intervent Radiol. 2008;31(1):98-106.
- Onik G. The male lumpectomy: rationale for a cancer targeted approach for prostate cryoablation. A review. Technol Cancer Res Treat. 2004;3(4):365-70.
- Onik G, Vaughan D, Lotenfoe R, et al. “Male lumpectomy”: focal therapy for prostate cancer using cryoablation. Urology. 2007;70(6 Suppl):16-21.
- Onik G, Vaughan D, Lotenfoe R, et al. The “male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol. 2008;26(5):500-5.
- Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27(26):4321-6.
- Bulbul MA, El-Hout Y, Haddad M, et al. Pathological correlation between needle biopsy and radical prostatectomy specimen in patients with localized prostate cancer. Can Urol Assoc J. 2007;1(3):264-6.
- Delongchamps NB, Beuvon F, Eiss D, et al. Multiparametric MRI is helpful to predict tumor focality, stage, and size in patients diagnosed with unilateral low-risk prostate cancer. Prostate Cancer Prostatic Dis. 2011;14(3):232-7.
- Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate--a 4-year experience. Urology. 2007;70(6 Suppl):27-35.
- Sylvester JE, Blasko JC, Grimm PD, et al. Ten-year biochemical relapse-free survival after external beam radiation and brachytherapy for localized prostate cancer: the Seattle experience. Int J Radiat Oncol Biol Phys. 2003;57(4):944-52.
- Sabel SM. The interrelationship between cryoablation, the immune response and the tumor microenvironment: stimulatory and suppressive effects. In: Keisari Y, ed. Tumor Ablation: Effects on Systemic and Local Anti-Tumor Immunity and on Other Tumor-Microenvironment Interactions. Dordrecht: Springer Netherlands; 2013:77-107.
- Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535-45.
- Reginato E, Wolf P, Hamblin MR. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J Immunol. 2014;4(1):1-11.
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718-26.
- Ablin RJ, Fontana G. Cryoimmunotherapy: continuing studies toward determining a rational approach for assessing the candidacy of the prostatic cancer patient for cryoimmunotherapy and postoperative responsiveness. An interim report. Cryobiology. 1980;17(2):170-7.
- Alicikus ZA, Yamada Y, Zhang Z, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117(7):1429-37.
- Ginzburg S, Nevers T, Staff I, et al. Prostate cancer biochemical recurrence rates after robotic-assisted laparoscopic radical prostatectomy. JSLS. 2012;16(3):443-50.
- Valicenti RK, Thompson I, Jr., Albertsen P, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013;86(5):822-8.
- Available at: http://www.uspreventiveservicestaskforce.org/page/document/recommendationstatementfinal/prostate-cancer-screening. Accessed March 15, 2016.
- Eggener SE, Scardino PT, Carroll PR, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007;178(6):2260-7.

