Life Extension Magazine®
Metformin and Glaucoma

Glaucoma is the second leading cause of blindness in the world.1
A medical group submitted a report to Life Extension Magazine® that provides persuasive data that the AMPK-activating drug metformin may be of significant benefit in protecting the eyes against the threat of blindness from open angle glaucoma.
This report is written with some technical language that may make it challenging for some of our readers to understand.
We choose to publish it with the caveat that a succinct practical suggestion on how to use metformin to potentially reduce glaucoma risk be made in the introduction.
So here is what the medical group that authored this report recommends:
“Those with elevated intraocular pressure (IOP) and/or glaucoma should ask their doctor about prescribing a modest 250 mg-500 mg dose of metformin twice a day after meals as it may have unique beneficial mechanisms in protecting against this blinding disorder.”
We welcome you to read the report beginning on the next page that describes underlying pathologies of open angle glaucoma and how metformin can help to counteract them.
Metformin is a decades-old antidiabetic drug used by millions of type II diabetics all over the world. It is inexpensive, quite commonly prescribed, and its effectiveness in reducing elevated blood glucose is well established.
In addition to its antidiabetic properties, metformin has also been shown to provide a number of other health benefits, including weight reduction, promoting longevity, and reducing cancer incidence, as well as reducing or eliminating chronic pain.2-7 It has calorie restriction mimetic cellular effects such as activating the energy enzyme adenosine monophosphate activated protein kinase (AMPK), and it favorably modulates certain genes thought to be involved in aging.8,9
Now, besides all these reported health effects, a new study reports metformin also reduces the development of open angle glaucoma (OAG).10
Aqueous Humor, Its Functions and Its Relation to Glaucoma Production
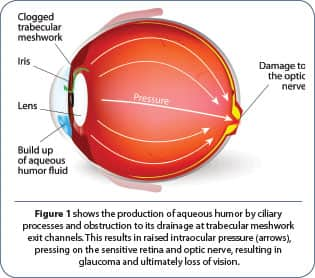
Glaucoma is a disease characterized by the increase of intraocular pressure due to various pathologies related to aqueous humor production, circulation, and drainage. In addition, the disease produces subsequent damage to the retina and atrophy of the optic nerve resulting in reduced visual acuity and ultimately leading to blindness.11
Aqueous humor is a transparent, watery fluid that provides nutrition to the front part of the eye. It also transports the metabolic debris produced there to the bloodstream, thus maintaining transparency of the lens and cornea so light rays can pass through cleanly and provide clear vision. Most importantly, it keeps the cornea inflated with hydrostatic pressure, like water in a balloon.
There are many varieties of glaucoma, the most common being open angle glaucoma, in which the angle where the cornea and the iris meet is as wide and open as it should be, but the aqueous humor drainage channels become blocked over time and aqueous humor builds up. This raises intraocular pressure.12,13
As pressure is exerted on the sensitive retina over time, it results in damage to nerve cells and their projection, the optic nerve.11 Once the optic nerve is damaged, it can’t be repaired, even if the raised intraocular pressure is corrected (figure 1).14,15 Abnormally high pressure inside the eye usually causes this retinal and optic nerve damage.
Because open angle glaucoma occurs due to the effects of aging, it may be that the disease is treatable with metformin, because of the drug’s general antiaging properties.
How Metformin Functions in the Body
Metformin works to reduce blood sugar in several ways. It decreases the amount of glucose made by the liver, decreases the amount of sugar absorbed into the body, and makes insulin receptors more sensitive. Metformin does not increase insulin levels as many antidiabetic medications do, which makes it unlikely to cause dangerously low drops in blood sugar.16,17 It’s therefore considered safe for nondiabetics to take.
Let’s examine how metformin works as an antiaging therapeutic agent and extrapolate the findings in terms of its ability to fight glaucoma.
AMPK activation helps to mimic the beneficial effects of calorie restriction.
Metformin enhances the activity of an enzyme found within all our cells called adenosine monophosphate-activated protein kinase, or AMPK for short.8AMPK activation helps to mimic the beneficial effects of calorie restriction and exercise, the best documented method of slowing and reversing degenerative aging processes and biomarkers of human aging.18
The biological effects of increased AMPK activity include inhibition of fat storage, reduced triglyceride synthesis, and increased glucose uptake into muscle for metabolism.19-27 AMPK activation also enhances destruction of diseased or dying cells as well as removal of intracellular metabolic debris – a method to slow and reverse degenerative aging processes of various organs.9
Further, experiments have shown that metformin, through AMPK activation, promotes the functional activity of the sirtuin family of genes, which is associated with longevity. Scientists have identified several signaling pathways involved in the regulation of aging processes that promote longevity. One of these signals, named p53, controls cell proliferation and is known as a tumor-suppressor gene. Loss of p53 predisposes normal cells to cancer. Metformin helps protect functional p53 so cells are less likely to become cancerous.9,28-31
What You Need to Know
 |
The Benefits of Metformin
- Metformin has been prescribed for decades as an effective treatment against type II diabetes. But studies have shown metformin to have a number of other beneficial effects as well. These include promoting longevity, weight loss, and reduced cancer risk, as well as reducing chronic pain.2-7 The drug also has antiaging effects that mimic calorie restriction, and it favorably modulates genes thought to be involved in aging.8,9
- Now, new research reveals metformin also reduces the development of open angle glaucoma, a progressive optic neuropathy and a leading cause of blindness.
- A University of Michigan study has found metformin to be linked with a 25% reduction in the risk of developing open angle glaucoma. Other medications used to treat type II diabetes did not have a similar benefit. Metformin is the only drug that has an intraocular pressure-reduction therapeutic effect.
- Everyone over age 50 would be well-advised to get tested for glaucoma and to ask their physician about possibly taking metformin, which could be preferable to typical antiglaucoma drugs, considering their common side-effects and lack of antiaging properties.
Metformin’s Inflammation-Reducing Property
Nuclear factor-kappa B (NF-kB) is an internal cell signal that induces chronic inflammation responsible for many diseases, from cancer to heart attack, neurodegenerative diseases and even glaucoma.32-35
NF-kB activation is blamed for many chronic diseases that ravage us as we age. Metformin produces higher AMPK activity which decreases expression of NF-kB.36
By blocking NF-kB, metformin is thought to promote longevity by inhibiting systemic inflammatory processes in the body, which play havoc in all our vital organs including the brain and heart, as well as the eyes.
A recent study has found that metformin relieves neuropathic and other pain by decreasing the activation of microglial cells in the spinal cord that are an integral part of the central nervous system and its proper functioning as discussed below.6
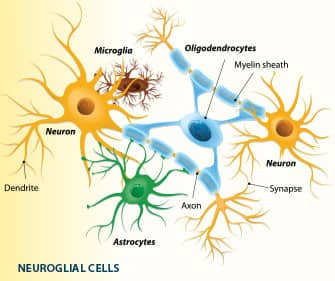
Metformin’s Effects on Nerve Cells (Neurons) and Their Physical Supporter-Glial Cells
Common characteristics for many neurodegenerative diseases include changes in glial cells, progressive neuronal loss, increased inflammation and oxidative stress.37 Thus decreasing the activation of glial cells in the brain is one promising approach to reducing the inflammation in the brain responsible for various neurodegenerative diseases including Parkinson’s and Alzheimer’s disease. This is exactly what a group of researchers found in an animal model of neuropathic pain treated with metformin—glial cell activation was decreased and chronic pain was reduced.6 We believe these findings may have implications to decrease neuropathic pain in thousands of patients treated with the expensive drug gabapentin (Neurontin®).
Glial cells have multiple functions:38,39
- They surround neurons and hold them in place.
- They supply nutrients and oxygen to neurons.
- They insulate one neuron from another.
- They destroy pathogens and remove dead neurons with various processes, including production of inflammatory cytokines that, besides attacking the invading microorganism, can promote neurodegenerative diseases.
- They transport the brain’s interstitial fluids between neurons to the cerebrospinal fluid, through drainage channels they create called glymphatics.
- They play a role in synapse formation and the transmission of electrical signals from one nerve cell to another, especially in memory centers of the brain. Basically, glial cells are caregivers to nerve cells and facilitate nerve cell activity in the central nervous system and maintain a homeostatic milieu for nerve cells to function properly.
Now let us examine how metformin can help fight glaucoma and age-related neurodegenerative diseases related to glial cell pathology.
Originally marketed as an agent for type II diabetes, metformin has been found to have a number of other uses in clinical practice, including, in one study, the ability to decrease the activation of glial cells in the spinal cord.6 Researchers reported complete resolution of suffering in some rats with induced neuropathic pain. This study reveals the impact of metformin on the nervous system glial cells, which are believed to be associated with chronic pain. If that is the case, is it possible that metformin can protect other parts of the nervous system, such as the retinal ganglion cells, by inhibiting the activity of glial cells that produce inflammatory cytokines that are toxic to neurons? This would also explain our finding and the findings of other scientists that those on metformin have better cognition with reduction in dementia.40,41 In our practice we routinely prescribe metformin for people over the age of 50 to be taken twice daily after meals to prevent future neurodegenerative diseases and aging.
Autophagy: Cellular House Cleaning
 |
Christian de Duve, 1974 Nobel Laureate in physiology or medicine, coined the term autophagy (meaning “self-eating”) in 1963. This year, biologist Yoshinori Ohsumi, of the Tokyo Institute of Technology, has been awarded the Nobel Prize in physiology or medicine for his discoveries in autophagy, the process whereby a cell recycles part of its own cellular debris (cellular house cleaning).
Scientists had been aware of autophagy for decades, but knew little about how it worked—until Ohsumi’s pioneering experiments in the 1990s. It’s important because autophagy can eliminate invading intracellular bacteria. Disrupted autophagy has been linked to Parkinson’s and Alzheimer’s disease, type II diabetes and other disorders that particularly affect the elderly.
We know that metformin enhances autophagy, which is how it reduces diseases of aging such as Parkinson’s and Alzheimer’s, and may reduce the incidence of glaucoma. This is one more reason to prescribe metformin.
How Does Metformin Reduce Glaucoma?
A recent study found that metformin reduces the intraocular pressure of primary open angle glaucoma.10 Open angle glaucoma is a progressive optic neuropathy characterized by loss of retinal ganglion cells and optic nerve atrophy.42 It’s the most common form of glaucoma and is often asymptomatic and may even go undetected for a while.43 By the time vision is noticeably impaired, the loss is irreversible, because once the nerve cells are dead in the retina with degeneration of the connected nerve fibers, nothing can restore them.
Open angle glaucoma is a manifestation of aging along with other neurodegenerative diseases. Normally, through autophagy, our cells purge themselves of accumulated debris, often called “cellular metabolic junk.” Autophagy is a natural mechanism that disassembles cells’ unnecessary or dysfunctional components as they age and lose their function. But over time, our cells lose this housekeeping ability.44,45 Metformin has been shown to promote this process.46
According to a study at the University of Michigan, metformin was associated with a 25% reduction in the risk of developing open angle glaucoma. They also found that other oral antidiabetic medications used to treat type II diabetes did not confer a similar risk reduction. Metformin is the only drug endowed with this intraocular pressure-reduction therapeutic effect.
This retrospective cohort study was based on longitudinal data from more than 150,000 patients with type II diabetes and no preexisting record of open angle glaucoma. Forty percent filled at least one metformin prescription. During the 10-year study period, 5,893 (3.9%) of the patients of a large health care network developed the disease. The researchers compared users of metformin with nonusers, analyzing the data by means of regression modeling. Each model demonstrated substantial reductions in open angle glaucoma risk among those using metformin. In two years, a diabetic patient taking a daily 2,000 mg dose of metformin would have a 20.8% reduction in open angle glaucoma risk, compared with a diabetic patient who had no metformin exposure.10
Metformin was associated with a 25% reduction in the risk of developing open angle glaucoma.
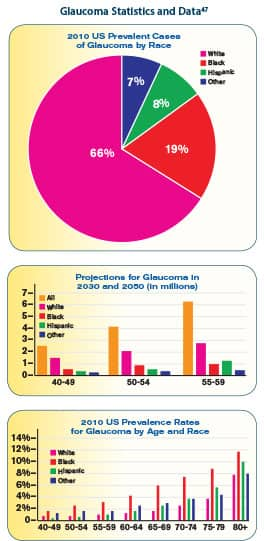
Glaucoma is the second leading causes of blindness in the world.1 It is estimated that in excess of 2.5 million people have glaucoma in the United States, and that more than 120,000 people are legally blind from the disease.42 Many people who have it aren’t aware of it. Blindness from glaucoma is six to eight times more common in African Americans than Caucasians, and, after cataracts, is the leading cause of blindness among them.43 We advise all those over the age of 50, especially African Americans and women, to get their eyes tested for glaucoma and ask their physician if it is appropriate to start taking metformin, not as an antidiabetic, but for its antiaging, antiglaucoma properties. Women have longer life expectancy and are more likely than men to develop age-related eye diseases like glaucoma.47
There are dozens of drugs available to treat glaucoma.48,49 Many of them have systemic complications and lack the antiaging effect of metformin on the rest of the body. Wouldn’t it make sense to prescribe metformin to prevent glaucoma and at the same time delay and/or reverse the ravages of aging?
Researchers at the University of Michigan Kellogg Eye Center have suggested a clinical trial protocol in which newly diagnosed glaucoma patients would be randomized to receive either an IOP (intraocular pressure)-lowering drug plus metformin or a glaucoma drug plus a placebo.50 From our point of view, a randomized clinical trial which might take decades may not be needed. Metformin is inexpensive, widely used to treat type II diabetics, and has hardly any adverse effects.
Summary of How Metformin Wards Off Glaucoma

Given how aqueous humor is formed, how it circulates and exits the eye, there are numerous possible explanations for how metformin works. It may act to reduce open angle glaucoma risk at multiple levels, which need to be further examined. The possible mechanisms are:
- Metformin, by inhibiting an inflammatory reaction and its related cytokines, may reduce aqueous humor production by ciliary processes, and bring it to stability.
- By promoting autophagy, it may prevent exfoliated cells from blocking the aqueous humor drainage channels of the meshwork and the Schlemm’s canal.
- By AMPK activation, it may reverse the biomarkers of human aging in the uveal aqueous humor production structures and transportation channels of aqueous humor.
- Due to increased AMPK activation, as the aqueous humor circulates, it comes in contact with trabecular meshwork and may cleanse the glycation around the endothelial cells of the trabecular meshwork, thus allowing the aqueous humor to pass to exits without resistance.
- Metformin in the aqueous humor may cleanse and open the pores in the Schlemm’s canal and uvea-scleral pathways by activation of AMPK, resulting in autophagy within the disease-afflicted lining cells of trabecular meshwork.
- By autophagy, it may effectively cleanse the platelet clumps and lipid deposits in the trabecular meshwork and the Schlemm’s canal that facilitates the easy drainage of the aqueous humor without increasing intraocular pressure.
- Metformin protects the functional p53 gene while repressing and/or blocking the pro-inflammatory NF-kB by reversing or inhibiting inflammatory process in the body, including the eyes.29,30,36 By reduction of inflammatory cytokines, it may protect the retina and prevent the degeneration of ganglion cells and optic nerve fibers, thus reducing the chances of blindness.
- Metformin reduces resistance to insulin, thus helping uptake and metabolism of circulating sugar, and preventing the adverse effects of hyperglycemia such as glycation—the bonding of a protein or lipid molecule with a sugar molecule.16,17,51
- Loss of ganglion cells in the retina is a leading cause of blindness in open angle glaucoma.42 This could be prevented with metformin by decreasing activation of glial cells in the retina and optic nerve.
FDA Approves Human Trials on Metformin Antiaging Effects

Further studies will point out the multiple ways metformin reduces the incidence of open angle glaucoma in older people as it provides antiaging protection in other organs and tissues and possibly even prevents or reduces the incidence of age-related macular degeneration.
Interestingly, the FDA’s approval of the first human trials to see if metformin can protect against diseases of aging was headlined in news media reports. We hope this study includes the drug’s effect on the eyes of the aging population.
For decades, Life Extension has discussed the antiaging effects of metformin. Finally, the FDA has heard their call. This study may take decades to reveal its findings, hence our practice has started advocating for metformin use for people over the age of 50 to promote good health and reverse, inhibit, or stop the ravages of aging.
Although it can cause lactic acidosis if taken in doses that are much larger than required for treatment, metformin is essentially very safe. The public should demand that the FDA approve metformin for use without prescription as an over-the-counter medication, both in oral form and as ophthalmic drops.
This will reduce medical cost and improve the health of many, with reduction in age-related diseases (which cost billions to care for). It will also bestow longevity, with probable reductions of neurodegenerative diseases such as Parkinson’s and Alzheimer’s, and at the same time provide good eyesight. Until that happens, the best alternative is for patients to ask their physicians to prescribe metformin for them and put it in writing that they, the patients, will not hold their doctors responsible for any untoward effects. Those with elevated intraocular pressure and/or glaucoma should ask their doctor about prescribing a modest 250 mg-500 mg dose of metformin twice a day after meals.
When developed, metformin ophthalmic drops, besides preventing open angle glaucoma, may also prevent or delay the development of age-related macular degeneration and diabetic retinopathy, and restore good vision to the aging population inexpensively. We appeal to the pharmaceutical industry to develop metformin ophthalmic drops with other adjuvant therapeutic agents to treat various eye diseases such as open angle glaucoma, age-related macular degeneration, retinitis pigmentosa, diabetic retinopathy and uveitis.
Jessica G. Shantha, MD, Fellow in Uveal diseases; Proctor Foundation, University of San Francisco, San Francisco California. T. R. Shantha MD, PhD, FACA
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
References:
- Available at: http://www.who.int/bulletin/volumes/82/11/feature1104/en/. Accessed September 28, 2016.
- Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes. 2013;121(1):27-31.
- Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):323-9.
- Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192.
- Gong Z, Aragaki AK, Chlebowski RT, et al. Diabetes, metformin and incidence of and death from invasive cancer in postmenopausal women: Results from the women’s health initiative. Int J Cancer. 2016;138(8):1915-27.
- Inyang K, Szabo-Pardi T, Price T. (309) Treatment of Chronic pain: long term effects of Metformin on chronic neuropathic pain and microglial activation. The Journal of Pain.17(4):S53.
- Taylor A, Westveld AH, Szkudlinska M, et al. The use of metformin is associated with decreased lumbar radiculopathy pain. J Pain Res. 2013;6:755-63.
- Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167-74.
- Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11(2):230-41.
- Lin HC, Stein JD, Nan B, et al. Association of Geroprotective Effects of Metformin and Risk of Open-Angle Glaucoma in Persons With Diabetes Mellitus. JAMA Ophthalmol. 2015;133(8):915-23.
- Aung T, Lim MC, Chan YH, et al. Configuration of the drainage angle, intraocular pressure, and optic disc cupping in subjects with chronic angle-closure glaucoma. Ophthalmology. 2005;112(1):28-32.
- Available at: http://www.glaucoma.org/glaucoma/types-of-glaucoma.php. Accessed September 28, 2016.
- Available at: https://nei.nih.gov/health/glaucoma/glaucoma facts. Accessed September 28, 2016.
- Moore DL, Goldberg JL. Four steps to optic nerve regeneration. J Neuroophthalmol. 2010;30(4):347-60.
- Shum JW, Liu K, So KF. The progress in optic nerve regeneration, where are we? Neural Regen Res. 2016;11(1):32-6.
- Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012;122(6):253-70.
- Gong L, Goswami S, Giacomini KM, et al. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22(11):820-7.
- Available at: http://www.lifeextension.com/magazine/2015/2/major-advance-in-slowing-aging/page-01. Accessed September 29, 2016.
- Villena JA, Viollet B, Andreelli F, et al. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53(9):2242-9.
- Anthony NM, Gaidhu MP, Ceddia RB. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (Silver Spring). 2009;17(7):1312-7.
- Daval M, Diot-Dupuy F, Bazin R, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280(26):25250-7.
- Henin N, Vincent MF, Gruber HE, et al. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. Faseb j. 1995;9(7):541-6.
- Henriksen BS, Curtis ME, Fillmore N, et al. The effects of chronic AMPK activation on hepatic triglyceride accumulation and glycerol 3-phosphate acyltransferase activity with high fat feeding. Diabetol Metab Syndr. 2013;5:29.
- Friedrichsen M, Mortensen B, Pehmoller C, et al. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. 2013;366(2):204-14.
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328-41.
- Rutter GA, Da Silva Xavier G, Leclerc I. Roles of 5’-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J. 2003;375(Pt 1):1-16.
- Pirkmajer S, Kulkarni SS, Tom RZ, et al. Methotrexate promotes glucose uptake and lipid oxidation in skeletal muscle via AMPK activation. Diabetes. 2015;64(2):360-9.
- Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298(4):E751-60.
- Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Ann Transl Med. 2014;2(6):57.
- Del Barco S, Vazquez-Martin A, Cufi S, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2(12):896-917.
- Available at: https://www.ncbi.nlm.nih.gov/books/NBK26902/. Accessed September 30, 2016.
- Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1(5):a000141.
- Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108(9):1122-32.
- Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107(3):247-54.
- Erb C. [Importance of the nuclear factor kappaB for the primary open angle glaucoma--a hypothesis]. Klin Monbl Augenheilkd. 2010;227(2):120-7.
- Gu J, Ye S, Wang S, et al. Metformin inhibits nuclear factor-kappaB activation and inflammatory cytokines expression induced by high glucose via adenosine monophosphate-activated protein kinase activation in rat glomerular mesangial cells in vitro. Chin Med J (Engl). 2014;127(9):1755-60.
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354-65.
- Available at: http://www.newworldencyclopedia.org/entry/Glial cell. Accessed September 30, 2016.
- Jessen NA, Munk AS, Lundgaard I, et al. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015;40(12):2583-99.
- Ng TP, Feng L, Yap KB, et al. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41(1):61-8.
- Hsu CC, Wahlqvist ML, Lee MS, et al. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis. 2011;24(3):485-93.
- Distelhorst JS, Hughes GM. Open-angle glaucoma. Am Fam Physician. 2003;67(9):1937-44.
- Available at: http://www.glaucoma.org/glaucoma/glaucoma-facts-and-stats.php. Accessed September 30, 2016.
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3-12.
- Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24(12):604-12.
- Feng Y, Ke C, Tang Q, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088.
- Available at: https://nei.nih.gov/eyedata/glaucoma. Accessed October 4, 2016.
- Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging. 2005;22(1):1-21.
- Sambhara D, Aref AA. Glaucoma management: relative value and place in therapy of available drug treatments. Ther Adv Chronic Dis. 2014;5(1):30-43.
- Available at: http://www.aao.org/eyenet/article/metformin-associated-with-reduced-risk-of-oag. Accessed October 4, 2016.
- Ishibashi Y, Matsui T, Takeuchi M, et al. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm Metab Res. 2012;44(12):891-5.

