Life Extension Magazine®

I used to pay $340 for an FDA-approved drug called Cerefolin® that contained:
- 5-MTHF (L-methylfolate) 5,635 mcg
- Vitamin B12 (cyanocobalamin) 1,000 mcg
- Vitamin B6 (pyridoxine) 50 mg
- Vitamin B2 (riboflavin) 5 mg
I needed this prescription drug to reduce homocysteine blood levels. My insurance company refused to pay the outlandish price, so I had to cover it myself.
Move forward a few years and the superior 5-MTHF form of folate became available as a dietary supplement. You can now obtain better homocysteine-reducing formulas for less than $20 that provide:
- 5-MTHF (L-methylfolate) 5,000 mcg
- Vitamin B12 (methylcobalamin) 1,000 mcg
- Vitamin B6 (pyridoxal-5-phosphate) 100 mg
- Vitamin B2 (riboflavin) 25 mg
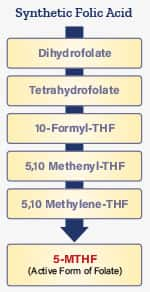
5-MTHF is the activated form of folate that supports methylation in your DNA. The chart on this page shows the processes required to convert folic acid into active 5-MTHF.
Pyridoxal-5-phosphate is the biologically active form of vitamin B6.1
Methylcobalamin is one of the most active forms of vitamin B12.
To enlighten politicians about slashing high drug costs, tell them to reduce the FDA’s authority and let the public access low-cost generic brands of all off-patent drugs—including nutrients that should not require a doctor’s prescription.
Enzymatic Conversions Required to Obtain Biologically Active 5-MTHF
Folic acid found in most supplements must undergo many enzymatic processes in your body before converting to its active 5-MTHF (5-methyltetrahydrofolate) form. Many people are unable to convert folic acid into enough 5-MTHF, which is why supplementing with 5-MTHF (instead of folic acid) makes sense.
In youth, all our body systems are expected to function well all the time.
This changes with aging.
An underlying culprit of degenerative aging is disrupted methylation.
Optimal methylation enables youthful reactions to occur throughout the body, including in our brain, heart, and critical liver detoxification systems.
Methylation controls genes that must precisely turn “on” and “off” in order to maintain cellular health.
Distorted DNA methylation is involved in a host of age-related disorders.
The good news is that nutrients most of you take, including activated B vitamins like 5-methyltetra-hydrofolate (5-MTHF), vitamin D, and magnesium facilitate healthy methylation.2,3
Measures of DNA methylation, called “epigenetic clocks,” are today’s premier predictors of human longevity.
Life Extension® has published many articles about methylation, but readers still find it challenging to understand.
According to research in mice, 20%-40% of age-related changes that are measured by epigenetic clocks can be favorably influenced by lifestyle changes such as calorie restriction.4
Evidence also suggests that, for many people, nutrients such as 5-MTHF and methylcobalamin can favorably influence healthy methyl-ation patterns.
Widespread Methylation Deficits

Methylation is an essential process for internal biochemical reactions. With aging, methylation patterns become disrupted.5
The MTHFR gene is responsible for the activity of the enzyme that enables folate to be converted into the bioactive form (called 5-methyltetrahydrofolate) that facilitates healthy methylation.
Approximately half the population carries a variant of the MTHFR gene that affects the activity of the enzyme, resulting in disrupted DNA methylation.6,7
To fulfill its role in maintaining proper methylation folate (or folic acid) needs to convert to bioactive 5-MTHF in the body.
Methylation Deficits Cause Excess Homocysteine

Homocysteine is an amino acid that, when elevated, has been associated with circulatory and neurological problems.8 Excess homocysteine creates inflammation of the endothelium (the inner wall of the arteries) which results in increased cardiovascular risks.9
Proper methylation is needed to detoxify homocysteine and reduce high levels of it.
Age-related homocysteine increases are often caused by disrupted methylation.10 One reason for homocysteine buildup is that older people do not convert dietary folate or folic acid in supplements into biologically active methylated folate (5-MTHF).7
The solution is to use the 5-MTHF form of folate, which is a low-cost dietary supplement and no longer an outlandishly priced prescription drug.
Methylation Vital for Gene Expression
The 5-MTHF form of folate is necessary for a compound in the body called S-Adenosyl-Methionine (SAMe) to methylate genetic material, including DNA.
Methylation is a critical way the transcription (activation) of genes controlled. Without proper methyl-ation, harmful genes can be overly expressed.
Epigenetic age is an emerging scientific concept that involves analysis of methylation patterns to determine biological age.11
“Epigenetics” refers to external modifications that occur in gene expression as a result of what we do to our bodies, as opposed to the genetic code we are born with.
Cigarette smoke, for example, causes deleterious epigenetic changes to our cellular DNA.12
Vitamin D and omega-3 fatty acids, on the other hand, induce beneficial epigenetic effects.13-16
Published research shows that measuring epigenetic age can predict future disease and mortality.11
Scientists have invented tests they call epigenetic clocks that have been shown to be accurate predictors of lifespan, healthspan, and all-cause mortality.17
We expect to discuss more about epigenetic tests in future issues of Life Extension® magazine.
The B Vitamin That Became a Drug
Vitamin B6 comes in several forms that have shown varying degrees of beneficial effects.
Like folate and B12, vitamin B6 plays a role in reducing levels of homocysteine. It does this via a different detoxification pathway than by methylation.1,8
Pyridoxal-5-phosphate is the biologically active form of B6 that is obtainable today.1
Pyridoxamine may be a better form of vitamin B6 that is currently unobtainable.18
In 1999, a pharmaceutical company filed an application with the FDA to investigate pyridoxamine’s ability to treat diabetic nephropathy (kidney disease).19
In 2005, the same company asked the FDA to remove pyridoxamine as a supplement.
In 2009 the FDA complied with this drug company’s request.
Eleven years later pyridoxamine is not approved either as drug or supplement.
Fortunately, consumers have access to low-cost pyridoxal-5-phosphate, which many believe to have comparable benefits to the pyridoxamine form of vitamin B6 the FDA claims is a “drug.”
Consumers Won the Big War
For many decades, the FDA tried to convert low-cost dietary supplements into prescription drugs.19
This was done at the behest of pharmaceutical companies who stood to make a fortune charging consumers outlandish drug prices for what are low-cost nutrients.
Life Extension® initiated a political and legal campaign in the late 1980s that resulted in passage of the Dietary Supplement Health and Education Act in 1994. This law denies the ability of the FDA to classify most nutrients as drugs and allows truthful, non-misleading health claims.20
Consumers won a huge victory 26 years ago by inundating Congress with letters demanding that the FDA never be allowed to take away their supplements.20
This editorial reveals how much money consumers have saved by now paying less than $20 for a B vitamin formula that cost $340 when sold as a prescription drug.
For longer life,
William Faloon
What is Methylation?

Genes are stretches of DNA that determine our traits, from hair and eye color to susceptibility to certain diseases and even lifespan potential.
Genes can also be active or inactive. The science of epigenetics studies how and why genes are expressed, or not.21
One of the main “switches” that modulates the way genes are expressed is methylation, which occurs when methyl groups are added to our genetic material.22
SAMe (S-Adenosyl-Methionine) is a nutrient found naturally in the body that serves as the primary methyl donor. That means it’s involved in practically all methylation reactions.22-25
If we don’t have enough of it, the body cannot methylate properly. That can lead to excessive oxidative stress, chronic inflammation, tissue damage, and disease.26,27
B vitamins and SAMe offer a way to maintain healthy methylation.
References
- Ueland PM, McCann A, Midttun O, et al. Inflammation, vitamin B6 and related pathways. Mol Aspects Med. 2017 Feb;53:10-27.
- Wessels I. Epigenetics and Minerals: An Overview. Handbook of Nutrition, Diet, and Epigenetics 2017:1-19.
- Beckett EL, Duesing K, Martin C, et al. Relationship between methylation status of vitamin D-related genes, vitamin D levels, and methyl-donor biochemistry. Journal of Nutrition & Intermediary Metabolism. 2016 2016/12/01/;6:8-15.
- Unnikrishnan A, Freeman WM, Jackson J, et al. The role of DNA methylation in epigenetics of aging. Pharmacol Ther. 2019 Mar;195:172-85.
- Xiao FH, Wang HT, Kong QP. Dynamic DNA Methylation During Aging: A “Prophet” of Age-Related Outcomes. Front Genet. 2019 2019-February-18;10(107):107.
- Moll S, Varga EA. Homocysteine and MTHFR Mutations. Circulation. 2015 Jul 7;132(1):e6-9.
- Hankey GJ, Eikelboom JW. Homocysteine and vascular disease. Lancet. 1999 Jul 31;354(9176):407-13.
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217-46.
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015 Jan 10;14:6.
- Araujo JR, Martel F, Borges N, et al. Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res Rev. 2015 Jul;22:9-19.
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018 Apr 18;10(4):573-91.
- Kaur G, Bagam P, Pinkston R, et al. Cigarette smoke-induced inflammation NLRP10-mediated mechanisms. Toxicology. 2018 Apr 1;398-399:52-67.
- Carlberg C. Nutrigenomics of Vitamin D. Nutrients. 2019 Mar 21;11(3).
- Huang Q, Wen J, Chen G, et al. Omega-3 Polyunsaturated Fatty Acids Inhibited Tumor Growth via Preventing the Decrease of Genomic DNA Methylation in Colorectal Cancer Rats. Nutr Cancer. 2016;68(1):113-9.
- Tremblay BL, Guenard F, Rudkowska I, et al. Epigenetic changes in blood leukocytes following an omega-3 fatty acid supplementation. Clin Epigenetics. 2017;9:43.
- Chen L, Dong Y, Bhagatwala J, et al. Effects of Vitamin D3 Supplementation on Epigenetic Aging in Overweight and Obese African Americans With Suboptimal Vitamin D Status: A Randomized Clinical Trial. J Gerontol A Biol Sci Med Sci. 2019 Jan 1;74(1):91-8.
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019 Jan 21;11(2):303-27.
- Voziyan PA, Hudson BG. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci. 2005 Aug;62(15):1671-81.
- Available at: https://www.lifeextension.com/magazine/2009/7/fda-seeks-to-ban-pyridoxamine. Accessed,
- Dickinson A. History and overview of DSHEA. Fitoterapia. 2011 Jan;82(1):5-10.
- Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009 Sep;27(5):351-7.
- Glier MB, Green TJ, Devlin AM. Methyl nutrients, DNA methylation, and cardiovascular disease. Mol Nutr Food Res. 2014 Jan;58(1):172-82.
- Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem. 2009 Aug 21;284(34):22507-11.
- Lin H. S-Adenosylmethionine-dependent alkylation reactions: when are radical reactions used? Bioorg Chem. 2011 Dec;39(5-6):161-70.
- Laurino P, Tawfik DS. Spontaneous Emergence of S-Adenosylmethionine and the Evolution of Methylation. Angew Chem Int Ed Engl. 2017 Jan 2;56(1):343-5.
- Rotondo JC, Bosi S, Bazzan E, et al. Methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples of infertile couples correlates with recurrent spontaneous abortion. Hum Reprod. 2012 Dec;27(12):3632-8.
- Rotondo JC, Selvatici R, Di Domenico M, et al. Methylation loss at H19 imprinted gene correlates with methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples from infertile males. Epigenetics. 2013 Sep;8(9):990-7.

