Life Extension Magazine®
Article reviewed & critiqued by Stephen B. Strum, MD, FAC P, LE Scientific Advisory Board Member
Health care corruption is driving this nation into economic insolvency. A particularly disgusting method used to inflate drug prices is the filing of frivolous lawsuits against generic drug makers. Each day a court delays approval of a lower-cost generic can enable a pharmaceutical company to earn millions of dollars in illicit revenue. It thus becomes cost-efficient for pharmaceutical companies to initiate limitless made-up claims against generic makers knowing that legal fees are far less than the profits earned each extra day that its expired-patent drug retains market exclusivity. The filing of lawsuits against generic manufacturers for the purpose of delaying FDA approval has gone on for years. Once the generic maker defeats the imaginary claims, it then has to charge more to the consumer because of the millions of dollars it was forced to squander on legal fees. This is just one reason why generic drugs cost far more than they should.1-11 As Americans are painfully aware, runaway medical costs are adversely impacting every segment of the US economy. Government health care programs are mathematically insolvent, medical insurance premiums are at unprecedented levels, and consumers are shouldering a far greater portion of their health care with out-of-pocket dollars.12,13 How the Corrupt System OperatesWhen considering whether to approve a generic drug, the FDA is caught in the middle as pharmaceutical companies who own name-brand drugs create myriad obstacles to deny others the right to sell generic versions. A pharmaceutical company with an expiring patent may pay enormous legal and lobbying fees to file petitions with the FDA arguing why it is not in the public interest to allow a low-cost generic to compete. Generic companies then have to respond by paying their own legal and lobbying costs to make their counterargument. The FDA plays referee in what often turns out to be a multi-year battle between a large pharmaceutical company and generic makers.14 None of this bureaucratic waste would occur in a free-market setting, but our cowardly Congress has failed to enact reforms—measures that Life Extension® long ago proposed to eliminate the corrupt behavior that routinely transpires between drug companies and the FDA.15,162 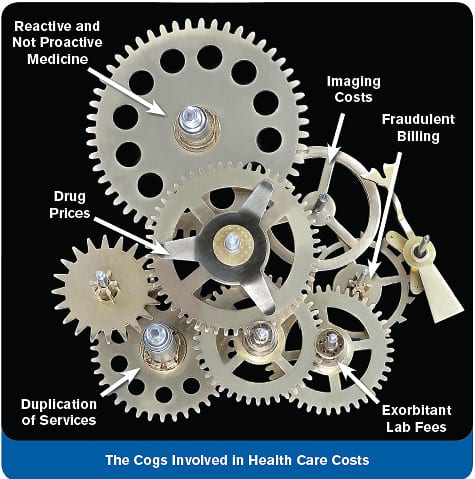 Depraved Behavior Sinks to New LevelAs unproductive as these generic drug wars are, a Senate investigative panel has uncovered misdeeds that brings this depraved behavior down to a level of immorality that may shock even ardent supporters of today’s broken health care system. Lovenox® is the brand name of a low-molecular weight heparin drug used to treat venous blood clots, pulmonary embolism, and various forms of arterial thrombosis. Back in 2002, ® suggested that its anticoagulant properties could benefit cancer patients by preventing acute thrombotic attacks, while reducing metastatic potential by blocking pro-angiogenic factors.17,18 As a result of its efficacy against a wide range of ailments, global sales of Lovenox® topped $4 billion in 2009.19 Its maker, Sanofi SA, desperately wanted to stifle generic competition as it was about to lose patent protection. Sanofi first tried to hold off generic competition by filing a suit in federal court intended to block FDA approval of generic versions made by other companies. After this delay tactic failed in two court cases, Sanofi then filed a safety petition with the FDA alleging that it was against the public interest to allow a low-cost generic to be sold.20 To further bolster its case, Sanofi encouraged doctors and medical associations to contact the FDA and express concern about the safety of generic versions of Lovenox®.21,22 While these underhanded maneuvers have become commonplace amongst pharmaceutical giants, Sanofi was caught red-handed acting at a level of unprecedented decadence. Sanofi Pays Fees to Third Parties to Mislead FDARealizing FDA was not capitulating to its intensive lobbying efforts, Sanofi used payments of almost $5 million to induce influential groups to contact the FDA and support its petition to deny other companies the right to sell generic Lovenox®.23 Sanofi made the following “donations” to third parties24:
In exchange for these and other payments, the FDA was lobbied with seemingly impartial concerns about the safety of generic Lovenox®. The purpose of these FDA contacts, of course, was to argue against approval of generic alternatives to Lovenox®. If successful, Sanofi would continue to earn billions of dollars from a drug that long ago lost patent status. What upset the Senate investigative panel, however, was that none of these ostensibly unbiased third parties had disclosed to the FDA that they had been paid by Sanofi to support the lucrative Lovenox monopoly. The FDA was misled into believing that these parties had legitimate safety concerns, when in reality they functioned as paid shills whose mission was to assist Sanofi in maintaining its multi-billion gusher at the expense of Medicare, Medicaid, health insurance premium payers and ultimately, the American consumer. The Duke University professor denied the monies he received from Sanofi had anything to do with him contacting the FDA, but admitted he should have reminded the FDA of his financial ties to Sanofi in his letter arguing against approval of generic Lovenox®.25 According to the Senate investigative panel conclusions, Sanofi “planned a coordinated strategy to delay generic alternatives to its blockbuster blood-thinner drug Lovenox.®”26 Calls for Mandatory Transparency
Pharmaceutical influence within the FDA is common knowledge, but Sanofi’s financial contributions to third parties and other actions raised red flags as to the scientific integrity of the generic drug approval process. These payments made by Sanofi to third parties provided these supposedly independent groups incentive to contact the FDA to express what appeared to be unbiased concerns about generic alternatives to Lovenox®. The strategy took advantage of the FDA’s citizen petition process, which allows individuals and experts to raise these kinds of safety concerns. While the process is intended as a channel for gathering public input into important agency decisions, the Senate investigative committee was infuriated by this egregious abuse of process. “If the FDA isn’t asking for disclosure of financial relationships, it’s operating from an uninformed standpoint,” said one of the Senate committee members that exposed this deceit. This Senator went on to state that “The FDA has a responsibility to conduct due diligence in this area in order to make sure its reviews have credibility.”26 The chairman of the Senate committee took it a step further and stated, “This report uncovers evidence that paying off doctors to lobby the FDA against generics was a drug company strategy—and that’s wholly unacceptable.”26 While the Senate investigative committee expressed outrage over Sanofi’s shady behavior, Life Extension members have long been informed about how these kinds of insidious scandals are bankrupting this nation’s health care system.16,27-30 The harsh reality is that conflicts of interest within the FDA preclude the agency from making scientific decisions about products that affect whether Americans live or die.
Pfizer Conceals Suicidal Side EffectsA side effect of many FDA-approved drugs on the market today is their propensity to sharply increase suicide risk. For those who don’t know, suicide is the 8th leading cause of death (for persons aged 55-64) in the United States.31 Depression is a leading underlying medical cause of suicide. The fact that many anti-depressant drugs now require black box warnings of increased risks of suicide is a testament to horrific side effects these drugs inflict. Many users of anti-depressant drugs report they feel more depressed after taking them (or encounter other side effects), which begs the question as to how these drugs ever gained FDA approval in the first place.32-37 In many instances, the increased risk of suicide was later found to be covered up by pharmaceutical companies during the initial approval process.38,39 Chantix® is the brand name of a drug made by Pfizer and approved by the FDA to help people stop smoking cigarettes. Chantix® works by altering brain chemistry to reduce nicotine craving. A side effect of this brain chemical alteration is an increased propensity to commit suicide, along with other psychological impairments.40-42 Not wanting to interfere with the lucrative sales of this drug, Pfizer devised a method to conceal the reports of suicide—reporting that is a mandatory requirement of the FDA. Rather than identifying the reports of suicides, suicide attempts, depression, aggression, and hostility in users of Chantix®, Pfizer instead mixed them among some 26,000 records of non-serious side effects such as nausea and rashes.42-46 This prevented the FDA from identifying a trend of serious adverse effects that were occurring in Chantix® users. Pfizer was accused by the FDA of submitting these adverse reports through “improper channels” that made it impossible for the agency to determine that so many serious adverse reactions to Chantix® were occurring.45,47 Thomas J. Moore, an official at the non-profit Institute for Safe Medication Practices stated “We’ve had a major breakdown in safety surveillance.”45 In sworn testimony, Chantix® was described as causing twice as many reported fatalities as any other drug. Pfizer responded by stating there is no proof that Chantix® causes suicides or other side effects.45,46
|
FDA's Response to Pfizer's MisdeedThe FDA’s response to this latest scandal was to force Pfizer to resubmit all reports of adverse reactions through the “proper channel” that would enable the agency to identify serious problems such as suicide, depression, and other psychiatric disorders induced by Chantix®. FDA officials said these new reports did not change the agency’s position on the risks and benefit of the controversial drug, which received a black box warning that included suicide—the strongest caution possible—in 2009. In other words, other than warning of the lethal side effects a user of Chantix® faces, the FDA has no plans to withdraw it from the market. FDA officials did say they are considering changing regulations to allow expedited reports of suicides and other serious problems. This change was first proposed by the FDA in 2003, but is still pending. This is just another example of the glacial pace at which the FDA moves even as body counts pile up from side effects inflicted by prescription drugs the agency erroneously approves and then allows to remain on the market far too long. A one-month supply of Chantix® costs about $179, which is typical of what patented drugs now sell for.
Pfizer Seeks to Delay Sales of Generic Lipitor®According to the New York Times, the largest introduction of a generic drug in history is being impeded in another devious scheme perpetrated by Big Pharma. Lipitor® is the best-selling drug of all time, generating sales of $106 billion over the last decade. Pfizer has told financial analysts it is preparing for the loss of Lipitor®’s patent with a variety of business moves to preserve market share. In a letter to pharmacists, Pfizer is asking many drugstores to block prescriptions for a generic version of Lipitor®. This cholesterol-lowering drug lost patent protection on November 30, 2011.50 One of the nation’s largest pharmacy benefit managers issued instructions seeking to have pharmacists keep filling prescriptions with the more expensive Lipitor® for six months. Pfizer will provide large discounts to benefit managers that block the use of generic versions of Lipitor®. This tactic will benefit Pfizer and benefit managers at the expense of employers and taxpayers, who are on the hook for paying more than they should for an off-patent drug. Pharmacy benefit managers function as middlemen between drug companies and insurers and employers that sponsor health insurance plans. In too many cases, these “pharmacy benefit managers” function as shills for pharmaceutical companies to cause more expensive name-brand drugs to be used in place of low-cost generics. How Generic Price Gouging Impacts ConsumersWe at Life Extension are constantly interacting with patients who utilize off-label drugs to better control their disease. Since insurance does not fully cover these medications, patients are finding it difficult to afford the cost of their medications. Here is an email excerpt from a prostate cancer patient regarding a generic medication he is using: “Cabergoline is very costly, around $600 for 60 days’ supply. This has eaten up my insurance allowance for drugs. Your latest email indicates necessity, but is it possible to lessen amounts taken without hindrance to outcomes?” Cabergoline is a generic drug that suppresses prolactin release from the pituitary gland. Life Extension has long recommended this drug to certain cancer patients, though it is not recognized by the FDA for this purpose.55 Prolactin makes prostate cells more sensitive to the growth-promoting effects of testosterone. By reducing prolactin levels, cabergoline has a potential role in prostate cancer treatment. In addition, lowering prolactin results in a reflex increase in dopamine, which has the beneficial effect of inhibiting angiogenesis—the formation of new blood vessels that facilitate the growth and spread of most cancers. Furthermore, increased dopamine enhances neurotransmission which improves clarity of thinking or cognition. Therefore, cabergoline is a true multi-tasking pharmaceutical agent that increases the therapeutic index or ratio. The patient who sent us this email concerned about the high cost of cabergoline may risk his life by taking a lower-than-recommended dose because he can no longer afford it. Yet this is an off-patent generic drug that should cost only a fraction of what is being charged. Generic drug prices change daily, so by the time this article is published, generic cabergoline might once again be affordable, or it could cost even more. A physician on the Life Extension’s Scientific Advisory Board wrote us about eye drops he needs for glaucoma that will cost $99.20 a month for the rest of his life, despite having Medicare and AARP. That’s $1,200 a year just for eye drops. This physician wonders how our health care system can tolerate such blatant price gouging. A calculation of the price for the generic version of this eye drug is $2.50 per drop, which prompted the dispensing pharmacist to say to the doctor, “This is a license to steal.” Life Extension® has published over the past four decades about how generic drug prices are artificially inflated because of FDA over-regulation and pharmaceutical industry corruption.
What We Are Doing to Fight BackThe October 2011 edition of Life Extension Magazine launched a new book titled Pharmocracy. This book exposes how pharmaceutical companies collude with their allies in the FDA and Congress to cause inflated drug prices. Copies of Pharmocracy have been sent to the president, all presidential candidates, and every member of Congress. The purpose is to enlighten political leaders about free-market approaches that can resolve today’s health care cost crisis that will otherwise put Medicare, Medicaid, and the private sector on the chopping block of bankruptcy. We initiated a massive promotion to get this book into the hands of policy-making think tanks and to the public. We need to alert them to the harsh reality that it is mathematically impossible to resolve the health care cost crisis by increasing taxes, slashing payments to doctors, or asking Medicare recipients to pay more toward their own medical expenses. The only solution to overpriced medicine is to abolish all restrictive bureaucratic regulations and let the free market do for medical practice what it did for computers and cell phones, i.e. enable scientific breakthroughs of unparalleled magnitude to occur at rapid speed while slashing consumer costs to comparatively nothing.
I want to thank Life Extension members who purchased four copies of Pharmocracy (at only $8 each) to personally send one each to their two senators and representative. For those who have not done this yet, please do not let apathy stop you from letting your voice be heard in Congress. In Syria, protestors are being murdered in the streets and dragged from their homes, yet they still defy government edicts knowing that snipers are waiting to kill them. For just $32, you can obtain four copies of Pharmocracy and send them to those in our government who have the power to change the corrupt health care system that is bankrupting this country. While it may seem impossible to dislodge the stranglehold that pharmaceutical interests hold over the federal government, I am convinced that unrelenting exposition of the truth will tear down corrupt barriers that cause consumers to be serfs of the pharmaceutical industry. For longer life,
William Faloon | |||||||||
| References | |||||||||
| 1. Available at: http://myhealthcafe.com/generic-drug-news-roche-files-lawsuit-to-block-low-cost-version-of-anti-nausea-drug-aloxi. Accessed November 1, 2011.Generic Drug News: Roche Files Lawsuit To Block Low-Cost Version of Anti-Nausea Drug Aloxi. July 13, 2011. 2. Available at: http://www.gnaipr.com/Articles/Astrazeneca%20files%20patent%20lawsuit%20against%20Torrent%20for%20blockbuster%20drug,%20Crestor.pdf. Accessed November 1, 2011. 3. Available at: http://www.firstwordplus.com/fws.do?articleid=2b6f0583e27545e2baa966606dc76fdf. Accessed November 1, 2011. 4. Available at: http://finance.yahoo.com/news/momenta-sues-3-drugmakers-apf-3561338019.html. Accessed November 1, 2011. 5. Available at: http://www.astellas.com/en/corporate/news/pdf/080722_eg.pdf. Accessed November 1, 2011. 6. Available at: http://www.wellbutrin-lawsuit.net/blog/2010/wellbutrin-lawsuit/wellbutrin-class-action-lawsuit-against-makers-of-generic-wellbutrin-xl/. Accessed November 1, 2011. 7. Available at: http://depatentlaw.morrisjames.com/uploads/file/11%20107%20676.pdf. Accessed November 1, 2011. 8. Available at: http://www.bizjournals.com/philadelphia/stories/2010/08/16/daily28.html. Accessed November 2, 2011. 9. Available at: http://www.atg.wa.gov/pressrelease.aspx?id=19358. Accessed November 2, 2011. 10. Available at: http://www.shire.com/shireplc/en/investors/.../irshirenews?id=467. Accessed November 2, 2011. 11. Available at: http://www.biosciencetechnology.com/news/feedsap/2011/05/j-j-files-new-concerta-lawsuit-against-impax-labs. Accessed November 2, 2011. 12. Available at: http://www.ssab.gov/documents/prepub_theunsustainablecostofhealthcare.pdf. Accessed November 2011. 13. Available at: http://findarticles.com/p/articles/mi_m0fsw/is_5_22/ai_n17207635. Accessed November 2, 2011. 14. Faloon W. Would you tolerate this abuse? Life Extension Magazine®. 2008 Sep; 14(9):7-12. 15. Faloon W. How much more FDA abuse can Americans tolerate? Life Extension Magazine®. 2010 Mar;16(3):12-3. 16. Faloon W. Ending the atrocities. Life Extension Magazine®. 2009 Mar; 15(3):7-12. 17. Greenwell I. Integrative approaches to cancer, cardiovascular disease and diabetes treatment. ACAM Conference, Nashville 2001. Life Extension Magazine®. 2002 Feb;8(2). 18. Cosgrove RH, Zacharski LR, Racine E, et al. Improved cancer mortality with low-molecular-weight heparin treatment: a review of the evidence. Semin Thromb Hemost. 2002 Feb;28(1):79-87. 19. Available at: http://online.wsj.com/article/sb10001424052748703632304575452450498653036.html. Accessed November 3, 2011. 20. Available at: http://www.hpm.com/pdf/lovenox%20-%20sanofi%20msj.pdf. Accessed November 3, 2011. 21. Available at: http://finance.senate.gov/library/prints/?maxrows=25. Accessed November 3, 2011. 22. Available at: http://cardiobrief.org/2011/05/25/senate-report-uncovers-sanofi-attempts-to-stifle-generic-lovenox. Accessed November 3, 2011. 23. Available at: http://online.wsj.com/article/sb10001424052702303654804576343753512740330.html. Accessed November 3, 2011. 24. Available at: http://www.pharmalot.com/2011/05/sanofi-enlisted-third-parties-to-influence-the-fda. Accessed November 21, 2011. 25. Available at: http://pharmacyblogbuy.com/duke-investigates-professor-over-sanofi-contoversy. Accessed November 3, 2011. 26. Available at: http://finance.senate.gov/newsroom/chairman/release/?id= 53bf8124-5319-4d2c-8c66-f01274a271c7. Accessed November 3, 2011. 27. Faloon W. Drug company pleads guilty to health fraud. Life Extension Magazine®. 2010 Mar;16(3):7-10. 28. Faloon W. The FDA’s most heinous drug approval. Life Extension Magazine®. 2011 Jun;17(6):7-12. 29. Faloon W. Death by regulation. Life Extension Magazine®. 2005 Mar;11(3):7-13. Death by Regulation. 30. Faloon W. Inside the Vioxx® debacle. Life Extension Magazine®. 2005 Apr; 11(4):22-8. 31. Available at: http://www.cdc.gov/injury/wisqars/pdf/death_by_age_2007_bw-a.pdf. Accessed November 4, 2011. 32. Brambilla P, Cipriani A, Hotopf M, Barbui C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005 Mar;38(2):69-77. 33. Preda A, MacLean RW, Mazure CM, Bowers MB Jr. Antidepressant-associated mania and psychosis resulting in psychiatric admissions. J Clin Psychiatry. 2001 Jan;62(1):30-3. 34. Gerber PE, Lynd LD. Selective serotonin-reuptake inhibitor-induced movement disorders. Ann Pharmacother. 1998 Jun;32(6):692-8. 35. Available at: http://www.nytimes.com/2004/09/14/health/14depress.html. Accessed November 4, 2011. 36. Healy D, Aldred G. Antidepressant drug use & the risk of suicide. Int Rev Psychiatry. 2005 Jun;17(3):163-72. 37. Lovrin M. Treatment of major depression in adolescents: weighing the evidence of risk and benefit in light of black box warnings. J Child Adolesc Psychiatr Nurs. 2009 May;22(2):63-8. 38. Available at: http://psychcentral.com/blog/archives/2008/06/16/cover-up-paxil-and-suicide-risk. Accessed November 7, 2011. 39. Available at: http://news.bbc.co.uk/2/hi/health/6308871.stm. Accessed November 7, 2011. 40. Available at: http://www.reuters.com/article/2011/11/02/us-smoking-drug-idustre7a181220111102. Accessed November 7, 2011. 41. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm124818.htm. Accessed November 7, 2011. 42. Available at: http://www.ismp.org/docs/vareniclinestudy.asp. Accessed November 7, 2011. 43. Moore TJ, Glenmullen J, Furberg CD: Prescription drugs associated with reports of violence towards others. PLoS One. 2010 5:e15337. 44. Available at: http://www.fairwarning.org/2011/05/watchdog-group-says-pfizer-played-down-suicides-tied-to-chantix. Accessed November 7, 2011. 45. Available at: http://www.msnbc.msn.com/id/43187290/ns/health-health_care/t/smoking-pill-suicides-overlooked-missing-reports. Accessed November 7, 2011. 46. Available at: http://healthfreedoms.org/2011/06/01/high-suicide-violence-rates-from-chantix-left-out-of-safety-reviews. Accessed November 7, 2011. 47. Available at: http://www.fda.gov/drugs/drugsafety/ucm255918.htm. Accessed November 7, 2011. 48. Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011 6(11):e27016. 49. Available at: http://www.sciencedaily.com/releases/2011/11/111102190026.htm. Accessed November 19, 2011 50. Available at: http://www.nytimes.com/2011/11/12/health/plan-would-delay-sales-of-generic-for-lipitor.html?ref=business. Accessed November 13, 2011. 51. Available at: http://www.nytimes.com/2011/04/09/business/09drug.html?_r=1. Accessed November 7, 2011. 52. Available at: http://www.thehealthyhomeeconomist.com/2011/06/is-your-doctor-getting-drug-kickbacks. Accessed Nov. 7, 2011. 53. Available at: http://www.naturalnews.com/001298.html. Accessed November 2011. 54. Available at: http://www.naturalnews.com/026963_pfizer_bextra.html. Accessed November 7, 2011. 55. Strum SB. Unfounded fears could limit prostate cancer treatment options. Life Extension Magazine®. 2007 Jul;13(7):12. 56. Available at: http://oig.hhs.gov/newsroom/testimony-and-speeches/levinson_051011.asp. Accessed November 7, 2011. 57. Available at: http://www.nytimes.com/2011/05/10/health/policy/10drug.html. Accessed November 7, 2011. 58. Available at: http://www.nytimes.com/2008/03/26/business/26cnd-zyprexa.html?adxnnl=1&adxnnlx=1311116701-eqfl+qz6tj8n5eoh2mntrw. Accessed November 7, 2011. |