Life Extension Magazine®
Longtime members of the Life Extension Foundation® have heard our warnings against synthetic alpha tocopherol many times. In 1997, we reported that taking only the alpha tocopherol form of vitamin E displaces critically important gamma tocopherol in the body. By displacing gamma tocopherol, we feared that high doses of alpha tocopherol could increase cancer risks. In fact, three years after Life Extension’s first warning, the Johns Hopkins School of Public Health released the results of a huge study (10,456 men). The findings showed that men with the highest gamma tocopherol blood levels had a fivefold reduction in prostate cancer risk. This same study showed that selenium and alpha tocopherol also reduced prostate cancer risk, but only when gamma tocopherol levels were high.1 Confirmatory studies document higher levels of gamma tocopherol to be strongly associated with reduced cancer risks.2-5 While both alpha and gamma tocopherol are potent antioxidants, gamma tocopherol has a unique function. Because of its different chemical structure, gamma tocopherol scavenges reactive nitrogen species, which can damage proteins, lipids, and DNA.6-8 Cancer is the end result of damage inflicted upon critical DNA genes that help regulate cellular growth and maturation. The fact that supplementation with isolated, synthetic alpha tocopherol depletes plasma gamma tocopherol levels means that the researchers who designed a study using only high-dose alpha tocopherol (the SELECT trial) created a biological catastrophe. The result of their ignorance is that men randomized to receive only synthetic alpha tocopherol suffered significant gamma tocopherol depletion and consequently, DNA damage from reactive nitrogen species. The fact that higher prostate cancer rates were observed in the group overloaded with synthetic alpha-tocopherol in the SELECT trial was predictable and expected based upon fundamental facts Life Extension understood as far back as 1997. This costly government-funded study was initiated in the year 2001. Life Extension was highly critical of this study design because it exposed healthy men to relatively high doses of synthetic alpha tocopherol without any supplemental gamma tocopherol to compensate. The results confirmed Life Extension’s worst fears. Compared to placebo, men taking synthetic alpha tocopherol had a 17% greater incidence of prostate cancer.9 This is a tragedy from the standpoint of the study participants who should not have been given alpha tocopherol by itself. It was also a waste of tax dollars used to fund a study that was designed to fail from the outset. In another arm of the study where selenium was given in addition to alpha tocopherol, there was not a statistically significant increase in prostate cancer.9 The study’s authors commented on the protective effect of selenium, but never mentioned the damage they inflicted by failing to include gamma tocopherol in their study. Selenium boosts antioxidant defenses in the body (such as glutathione peroxidase) that would help compensate for the displacement of gamma tocopherol by alpha tocopherol.10 The doctors involved in the design of the study comprise a “who’s who” of the conventional cancer establishment. Virtually every major cancer center was involved in the study conception, and it seemed that virtually every pharmaceutical company had made generous payments to the study’s overseers. If there was ever a greater financial conflict of interest, we have yet to see it. It was in the economic interests of the drug companies, the cancer centers, and the mainstream doctors to see this study fail, and the harsh comments against dietary supplements by the mainstream doctors reveal a strong bias against over-the-counter dietary supplements. While the study’s authors claim that there is not a “biological explanation” from their data to explain the increase in prostate cancer in the alpha tocopherol-only group, Life Extension® long ago predicted in writing this study would fail. As we expected, the men in this study that received alpha tocopherol experienced a 45% depletion of vital gamma tocopherol during the initial 5.5-year median study period.11 Myriad reports now point to the urgent need for Americans to obtain sufficient gamma tocopherol.8,12-22 Yet the vast majority of human clinical research focuses only on alpha tocopherol—as if it were the only form of vitamin E people require. This article explains the mechanisms involved in development of prostate cancer and why no single supplement can be counted on alone to prevent it. Based on reports showing antioxidants reduce incidence of prostate cancer, the federal government spent over $114 million to see if synthetic alpha tocopherol and/or a single-sourced selenium supplement would prevent prostate cancer in a large placebo-controlled trial conducted at major cancer centers throughout the United States.11 The long-term follow-up of the SELECT study was published in the Journal of the American Medical Association on October 12, 2011.11 Long before this study’s findings were released, Life Extension® predicted that it would fail and warned that men taking high doses of alpha tocopherol without also taking gamma tocopherol faced increased disease risk. In the initial results of the SELECT study over a median 5.5-year period, men supplemented with synthetic alpha tocopherol experienced significant gamma tocopherol depletion of 45%. Men supplemented with alpha tocopherol plus selenium experienced a 48% depletion of gamma tocopherol. These gamma tocopherol depletions occurred by 6 months and were sustained during the course of a median trial period of 5.5 years.11 It should be noted that serious supplement users choose natural alpha tocopherol because it has been shown to exert superior biological effects in the body.23,24 The synthetic form of alpha tocopherol is most often used in brand-name multivitamins made by pharmaceutical companies that are widely advertised on national television. Organizations like Life Extension have resisted the cheap price of synthetic vitamin E and use the more expensive natural form of alpha tocopherol in nutrient formulations. It should also be noted that the only form of selenium used in this study was L-selenomethionine (200 mcg). Yet scientific studies dating back to the 1970s show that other forms of selenium might provide greater protection against cancer. That’s why most Life Extension members obtain their selenium from more than one source that includes Se-methyl L-selenocysteine and/or, other anti-cancer forms of selenium.25-30 Life Extension has conducted a thorough review of the SELECT study that is now being used as a basis to attack dietary supplements. The more of this article you read, the more you will understand why the SELECT study was designed to fail from the outset. Initial SELECT Report Showed No Risk or Benefit
When data was first reported from the SELECT trial on December 9, 2008, it found no reduction in prostate cancer incidence in men taking alpha tocopherol or selenium over a median period of 5.5 years.11 This was not surprising since we have known for the past 14 years that when alpha tocopherol is taken by itself, it displaces critically important gamma tocopherol in our cells.31-34 An abundance of evidence points to the gamma tocopherol form of vitamin E as the most protective against prostate cancer.2,35-37 By supplementing aging men with only alpha tocopherol, doctors increased these men’s prostate cancer risk by depriving prostate cells of critical gamma tocopherol. This is only a tiny part of the real story behind this flawed study. The American Medical Association used the initial finding of no benefit to discredit vitamin E and selenium supplements. An editorial by the American Medical Association concluded by advising:“… physicians should not recommend selenium or vitamin E—or any other antioxidant supplements—to their patients for preventing prostate cancer.”38 In January 2008, as part of our article titled “Merv Griffin’s Tragic Death from Prostate Cancer,”39 we predicted that the SELECT trial would fail. We also stated that this faulty SELECT study would be misused by the medical establishment to discredit by extrapolation, other low-cost efficacious nutrients like vitamin D and fish oil. How Gamma Tocopherol Protects Against CancerGamma tocopherol exerts anticancer effects through a variety of important mechanisms, giving it an especially broad spectrum of action against a host of tumor types. At the very beginning of the cancer development process, gamma tocopherol traps reactive nitrogen species and other free radicals that cause mutations in DNA strands and render cells vulnerable to malignant transformation.6-8 This is a crucial step in the prevention of cancer. Gamma tocopherol inhibits cancer cell growth in culture through a number of different mechanisms.37 It downregulates control molecules known as cyclins, which traps cancer cells in the midst of their reproduction cycle and prevent them from reproducing and spreading.36 This anticancer effect appears to be based on a mechanism separate from the vitamin’s well-known antioxidant powers. A cell membrane receptor called PPAR-gamma (peroxisome proliferator-activated receptor-gamma) is a promising target for anticancer therapies because it affects genes that control cancer cell growth and death.40 This is why PPAR-activating drugs are being researched and developed by pharmaceutical companies as anticancer drugs. Gamma tocopherol is more powerful than alpha tocopherol at stimulating PPAR-gamma activity, especially in colon cancer cells.12,41 In prostate cancer cells, PPAR-gamma stimulation by gamma tocopherol resulted in a complete cessation of cancer cell growth.12 Once cancerous transformation has taken place, there are still biological opportunities to prevent full-blown tumor development. One of these ways is the induction of deliberate cell death through built-in genetic programs, a process called apoptosis. In a variety of cancer tissues, gamma tocopherol has been found to be superior to alpha tocopherol at inducing apoptosis, triggering a number of desirable cell disposal-pathways.2,42 In prostate cancer cells, gamma tocopherol induced cell death by blocking synthesis of important cell membrane components.43 Gamma tocopherol also reduces the development of new blood vessel formation in tumors, depriving them of the nutrients they need to thrive.44 To date, all of these mechanisms have been shown to inhibit cancers of the colon, prostate, breast, and lung in animal models, with many more under active investigation.45 A study found that women who consumed most vitamin E from food sources had a 60% reduction in the risk of breast cancer, compared to women with the lowest consumption. The form of vitamin E that strongly predominates in food sources is gamma tocopherol.46 In the December 2000 issue of the Journal of the National Cancer Institute, researchers at the Johns Hopkins School of Public Health published results of a huge study of 10,456 men showing that those with the highest gamma tocopherol blood levels had a fivefold reduction in prostate cancer risk. This same study showed that selenium and alpha tocopherol also reduced prostate cancer risk, but only when gamma tocopherol levels were high.1 These findings should have been glaringly apparent to those involved in the SELECT human clinical trial that began one year later (in 2001), yet the SELECT study design called for men to be given a high dose of synthetic alpha tocopherol that resulted in depletion of vital gamma tocopherol by 45% during the initial 5.5-year median trial period! This is one reason why we believe the SELECT study was “designed to fail.” We know that free radical-induced damage to DNA genes can cause cancer, but there are other risk factors beyond oxidative stress to blame for most prostate tumors.
Prostate Cancer Is Initiated Early in LifeWhile prostate cancer is not usually diagnosed until men reach older ages, it can be initiated 15-25 years prior to clinical manifestation. In fact, there is convincing evidence that the initiating DNA damage inflicted by estrogen to prostate cells can occur before a man is even born.47 Studies show that as early as the second and third trimester of life, exposure to elevated estrogens in the womb can initiate prostate cancer that may not manifest for 80 years.48-53 A man’s lifetime exposure to higher-than-normal estrogen may be a contributing factor to prostate cancer development. There is no evidence that antioxidants like alpha tocopherol and selenium would protect against this kind of prostate cancer induced by prolonged excess estrogen exposure. Please don’t feel helpless about this, as it requires more than mere initiation for cancer to fully develop. Dietary and other lifestyle factors have an enormous impact on whether men will develop prostate cancer, even if they are genetically predisposed. The Cause of All Cancers
Cancer can be defined in one sentence as follows: “Cancer is the accumulation of mutations in genes that regulate cellular proliferation.”54 All cancers are caused by gene mutations. Every time a cell divides, there are slight mutations to one’s genes. Oxidative stress accelerates gene mutation, but is by no means the primary factor. While selenium and vitamin E reduce some types of oxidative stress, the aged men in the SELECT study had already sustained considerable genetic mutations that are not reversible by taking antioxidants. Fortunately, there are nutrients that have been shown to favorably reverse the gene alterations involved in cancer initiation and progression. One promising nutrient is vitamin D, which has been shown to slash prostate cancer risk in some studies.55 Serum levels of vitamin D were not assessed in the SELECT study, so it was not possible to know which men had protective levels of vitamin D and those who had insufficient or even deficient levels. If men in the placebo group had even slightly higher vitamin D status, they should have been less likely to contract prostate cancer. What researchers fail to comprehend is that giving aged men a single antioxidant like alpha tocopherol is not going to reverse seven decades of genetic damage to prostate DNA. Fortunately, we know of other mechanisms that fuel prostate cancer progression that can be mitigated. Eating Your Way to Prostate CancerCancer cells lurk in the prostate glands of most aging men, yet only one in six men are ever diagnosed with prostate cancer. If one looks at what is required for a single cancer cell to develop into a detectable tumor, it becomes obvious that natural barriers exist to protect people against full-blown cancer.2,43-46 Unfortunately, the dietary choices of most men living in the modern Western world circumvent the body’s natural protective barriers. The end result is that most men unwittingly provide biological fuel for existing prostate cancer cells to propagate and metastasize. Fortunately, an understanding of the biological roles of diet and specific nutrients can enable aging men to achieve a considerable amount of control over whether isolated cancer cells in their prostate gland will ever show up as a clinically diagnosed disease. The impact of the food we ingest on cell growth and death is so pronounced that in some ways it can be identical to the effects displayed by some anticancer drugs. As it relates to the SELECT study on alpha tocopherol and/or selenium, the study participants’ diet was not taken into consideration. This fact alone could have rendered the findings highly suspect. Read on to see what we mean. 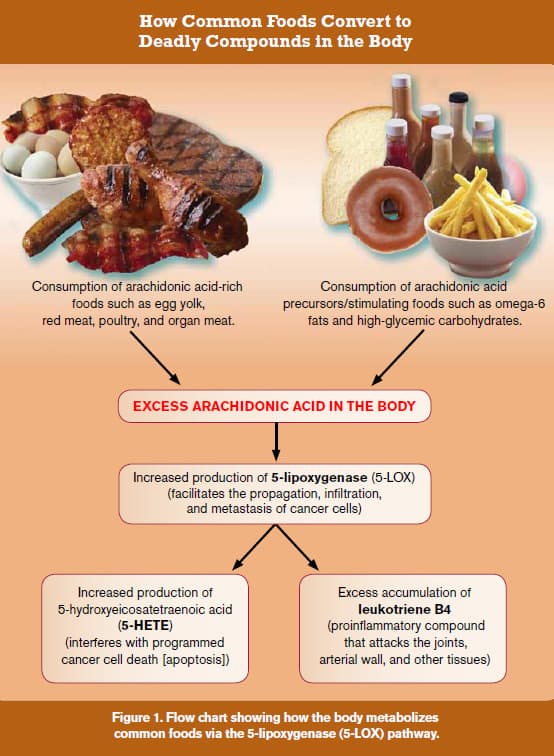 |
The First Line of Defense: Omega-3 Fatty AcidsDiets high in omega-6 fats and saturated fats are associated with greater prostate cancer risk, whereas increased intake of omega-3 fats from fish has been shown to reduce risk. Based on consistent epidemiological findings across a wide range of human populations, scientists have sought to understand why eating the wrong kinds of fat (saturated and omega-6 fats) provokes a stimulatory effect on prostate cancer.56-58 To ascertain what happens after we eat bad fats, all one has to do is look at the metabolic breakdown pathways that these fats follow in the body, as shown in the chart on the previous page (Figure 1). For example, let us assume that for dinner, you eat a steak (a source of saturated fat) and a salad, along with a typical salad dressing of soybean and/or safflower oils (sources of omega-6 fats). As can be seen in the flow chart (Figure 1), saturated and omega-6 fats convert to arachidonic acid in the body. The meat itself contains arachidonic acid. One way that the body rids itself of excess arachidonic acid is provoking a dangerous metabolizing pathway through 5-lipoxygenase (5-LOX). Studies show that 5-LOX products directly stimulate prostate cancer cell proliferation via several well-defined mechanisms.59-64 Arachidonic acid is metabolized by 5-LOX to 5-hydroxyeicosatetraenoic acid (5-HETE), a potent survival factor that prostate cancer cells use to escape destruction.65,66 Figure 1 clearly demonstrates how consuming a diet of foods rich in arachidonic acid directly provokes the production of dangerous 5-LOX products, which can promote the progression of prostate cancer. In addition to 5-HETE, 5-LOX also metabolizes arachidonic acid into leukotriene B4, a potent pro-inflammatory agent that causes destructive reactions throughout the body and inflicts severe damage to the arterial wall.67-73
One reason that fish oil supplements have become so popular is that their beneficial EPA/DHA fatty acids can help reduce the production of arachidonic acid-derived eicosanoids in the body.74-79 As shown in Figure 1, if arachidonic acid levels are reduced, there would be a corresponding suppression of the 5-LOX products 5-HETE and leukotriene B4. Once one understands the lethal metabolic cascades that occur in response to poor dietary choices, it is easy to see why people who excessively consume foods rich in arachidonic acid, and/or those who do not reduce the production of excessive arachidonic acid metabolites, are setting themselves up for prostate cancer and a host of inflammatory diseases (including atherosclerosis). A chart appearing later in this article clearly shows the destructive cascade initiated by excess arachidonic acid. Men in the SELECT study who took alpha tocopherol-selenium supplements, but consumed foods high in arachidonic acid and not enough omega-3s, would be more likely to develop prostate cancer. The researchers who designed this study should have known to correct for this critical confounding factor, i.e. dietary patterns.
5-LOX Is Overexpressed in Prostate CancerBased on studies showing that consumption of foods rich in arachidonic acid is greatest in regions with high incidences of prostate cancer,59,60,64,84 scientists sought to determine how much of the 5-LOX enzyme is present in malignant versus benign prostate tissues.85 Using biopsy samples taken from living human patients, the researchers found that 5-LOX mRNA levels were an astounding sixfold greater in malignant prostate tissues compared with benign tissues. This study also found that levels of 5-HETE were 2.2-fold greater in malignant versus benign prostate tissues.85 The scientists concluded this study by stating that selective inhibitors of 5-LOX may be useful in the prevention or treatment of patients with prostate cancer. 5-LOX Promotes Tumor Growth FactorsAs the evidence mounts that ingesting “bad fats” increases prostate cancer risk, scientists are evaluating the effects of 5-LOX on various growth factors involved in the progression, angiogenesis, and metastasis of cancer cells. One study found that 5-LOX activity is required to stimulate prostate cancer cell growth by epidermal growth factor (EGF) and other cancer cell-proliferating factors produced in the body. When 5-LOX levels were reduced, the cancer cell-stimulatory effect of EGF and other growth factors was diminished.59 In a mouse study, an increase in 5-LOX resulted in a corresponding increase in vascular endothelial growth factor, a key growth factor that tumor cells use to stimulate new blood vessel formation (angiogenesis) into the tumor. 5-lipoxygenase inhibitors were shown to reduce tumor angiogenesis along with a host of other growth factors.86 In both androgen-dependent and androgen-independent human prostate cancer cell lines, the inhibition of 5-LOX has consistently been shown to induce rapid and massive apoptosis (cancer cell destruction).60,84,87,88 Suppressing Arachidonic Acid Byproducts
Health-conscious people take nutrients like fish oil, curcumin, and lycopene that help to lower 5-LOX activity in the body.84,89-96 A rat study showed that gamma tocopherol, but not alpha tocopherol, exhibited potent reduction of PGE2 and leukotriene B4, powerful pro-inflammatory end products of the COX-2 and 5-LOX pathways, respectively.97 A review of several studies indicates that combinations of alpha and gamma tocopherol optimally reduce end products (such as PGE2 and leukotriene B4) of arachidonic acid breakdown in the body.18,98,99 Extracts from the boswellia plant selectively inhibit 5-lipoxygenase (5-LOX).100,101 A novel boswellia extract has been developed that is 52% more bioavailable compared to standard boswellia extracts102 thus providing a greater opportunity to suppress deadly 5-LOX and other cancer-promoting byproducts of arachidonic acid. As humans age, overexpression of the enzymes 5-LOX and COX-2 typically occurs. For maturing males, excess levels of these pro-inflammatory enzymes may contribute to the epidemic of prostate cancer observed after the age of 60.103 Based on the cumulative knowledge that 5-LOX, COX-2, and their breakdown products can promote the invasion and metastasis of prostate cancer cells, it would appear advantageous to take aggressive steps to suppress these lethal enzymes. For the unfortunate men who received only alpha tocopherol in the SELECT study, the suppression of gamma tocopherol that occurred in their bodies presumably exposed them to higher levels of cancer-promoting byproducts of arachidonic acid. Interestingly, selenium has shown 5-LOX-inhibiting effects, which may partially explain why men receiving selenium and alpha tocopherol-alone did not show a statistically significant increase in prostate cancer. Soy, Lignans, and Cruciferous VegetablesMen who regularly consume certain plant foods have sharply lower rates of prostate cancer. Studies show that cauliflower, broccoli, flax lignans, and soy isoflavones141-150 protect against a host of diseases, including prostate cancer. If the men in the SELECT placebo group ate an even slightly healthier diet, then they would be expected to enjoy a lower rate of prostate cancer compared with men who took the alpha tocopherol-selenium supplements but ate fewer cancer-preventing plant foods. Low Testosterone Increases Prostate Cancer RiskIn a book authored by Harvard University experts titled Testosterone for Life, detailed findings are presented that dispel a misleading notion about testosterone causing prostate cancer.151 These researchers meticulously document their observations that men with low levels of testosterone have higher prostate cancer risks. This finding provides another confounding factor that skews the results of the SELECT trial that only used alpha tocopherol and/or selenium. If men receiving the supplements had lower testosterone levels, they would conceivably have a higher rate of prostate cancer. Too Many Factors Involved in Prostate Cancer CausationThe SELECT study was designed based on prior studies showing sharply lower risks of prostate cancer in men who consumed vitamin E and selenium.152-158 It was also based on the premise that protecting genes against oxidative stress would reduce prostate cancer incidence in aged men. We now know of dozens of factors involved in the development of full-blown prostate cancer. One could not expect that taking just one or two nutrients would result in less prostate cancer developing in these older study subjects. There are too many other causes that should have been factored in when the SELECT study was originally designed. It is encouraging that a plethora of new research findings have identified definitive ways for aging men to drastically slash their risk of developing prostate and other cancers. 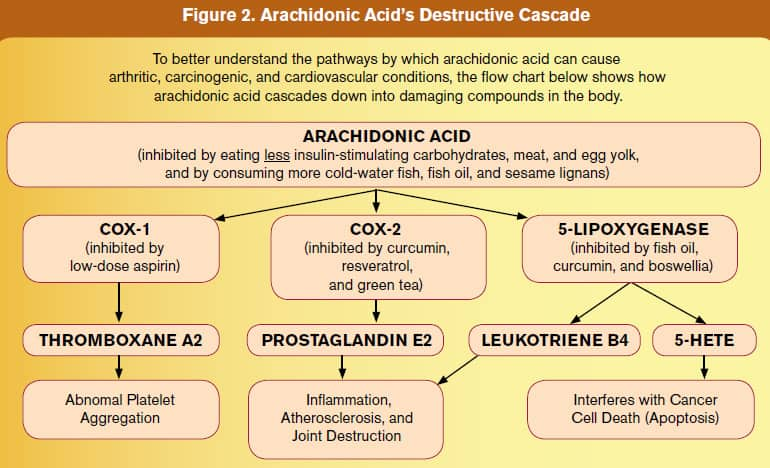 ConclusionLife Extension long ago warned of increased disease risk in those who only took alpha tocopherol supplements without also taking gamma tocopherol. Leaving out gamma tocopherol is not the only flaw in the SELECT study. It is rather conspicuous, however, since men supplemented with alpha tocopherol experienced a 45-48% depletion in gamma tocopherol levels by six months that was sustained during the course of the initial 5.5-year median trial period.11 The facts revealed in this rebuttal to the SELECT study identify a fundamental problem confronting researchers who seek to “prove” whether a certain supplement prevents a disease. There are too many “other” factors involved in the development and progression of prostate cancer, including low levels of testosterone, increased levels of estrogen, coexisting diabetes or metabolic syndrome, low vitamin D intake, and increased dietary saturated fats.159 These confounding factors therefore make it difficult to study just one or two compounds and expect to come up with a valid finding. To emphasize today’s sense of urgency, the aging population will contract prostate cancer at epidemic levels unless aggressive changes are implemented immediately. That’s because mutated cells in the prostate glands of aging males are already on the verge of maturing into full-blown cancer. This is why we encourage Life Extension members to consume healthy diets and supplements that have been shown to sharply reduce prostate cancer incidence. There is not enough time left in our generation’s projected life spans to withstand the kind of scientific design flaws seen in the SELECT study, the medical establishment’s bias against supplements, and arbitrary standards set by the pharmaceutical monopoly. If you have any questions on the scientific content of this article, please call a Life Extension® Health Advisor at 1-866-864-3027. | |||||||
| References | |||||||
| 1. Helzlsouer KJ, Huang HY, Alberg AJ, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000 Dec 20;92(24):2018-23. 2. Jiang Q, Wong J, Ames BN. Gamma-tocopherol induces apoptosis in androgen-responsive LNCaP prostate cancer cells via caspase-dependent and independent mechanisms. Ann N Y Acad Sci. 2004 Dec;1031:399-400. 3. Barve A, Khor TO, Nair S, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009 Apr 1;124(7):1693-9. 4. Jeong NH, Song ES, Lee JM, et al. Plasma carotenoids, retinol and tocopherol levels and the risk of ovarian cancer. Acta Obstet Gynecol Scand. 2009;88(4):457-62. 5. Yu W, Park SK, Jia L, et al. RRR-gamma-tocopherol induces human breast cancer cells to undergo apoptosis via death receptor 5 (DR5)-mediated apoptotic signaling. Cancer Lett. 2008 Feb 8;259(2):165-76. 6. Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1771-5. 7. Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. Gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3217-22. 8. Johansson C, Rytter E, Nygren J, Vessby B, Basu S, Möller L. Down-regulation of oxidative DNA lesions in human mononuclear cells after antioxidant supplementation correlates to increase of gamma-tocopherol. Int J Vitam Nutr Res. 2008 Jul-Sep;78(4-5):183-94. 9. Klein EA, Thompson IM Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011 Oct 12;306(14):1549-56. 10. Al-Taie OH, Seufert J, Karvar S, et al. Selenium supplementation enhances low selenium levels and stimulates glutathione peroxidase activity in peripheral blood and distal colon mucosa in past and present carriers of colon adenomas. Nutr Cancer. 2003;46(2):125-30. 11. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009 Jan 7;301(1):39-51. 12. Campbell SE, Musich PR, Whaley SG, et al. Gamma tocopherol upregulates the expression of 15-S-HETE and induces growth arrest through a PPAR gamma-dependent mechanism in PC-3 human prostate cancer cells. Nutr Cancer. 2009 61(5):649-62. 13. Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med. 2008 Mar 15;44(6):1203-8. 14. Giannini C, Lombardo F, Currò F, et al. Effects of high-dose vitamin E supplementation on oxidative stress and microalbuminuria in young adult patients with childhood onset type 1 diabetes mellitus. Diabetes Metab Res Rev. 2007 Oct;23(7):539-46. 15. Jiang Q, Moreland M, Ames BN, Yin X. A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. J Nutr Biochem. 2009 Nov;20(11):894-900. 16. Masterjohn C, Mah E, Guo Y, Koo SI, Bruno RS. γ-Tocopherol abolishes postprandial increases in plasma methylglyoxal following an oral dose of glucose in healthy, college-aged men. J Nutr Biochem. 2011 May 2. 17. Oláh G, Módis K, Gero D, et al. Cytoprotective effect of γ-tocopherol against tumor necrosis factor α induced cell dysfunction in L929 cells. Int J Mol Med. 2011 Nov;28(5):711-20. 18. Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol Aspects Med. 2007 Oct-Dec;28(5-6):668-91. 19. Singh I, Turner AH, Sinclair AJ, Li D, Hawley JA. Effects of gamma-tocopherol supplementation on thrombotic risk factors. Asia Pac J Clin Nutr. 2007 16(3):422-8. 20. Vucinic L, Singh I, Spargo FJ, Hawley JA, et al. Gamma tocopherol supplementation prevents exercise induced coagulation and platelet aggregation. Thromb Res. 2010 Feb;125(2):196-9. 21. Wagner JG, Jiang Q, Harkema JR, et al. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2008 Mar;38(3):501-11. 22. Wiser J, Alexis NE, Jiang Q, et al. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic Biol Med. 2008 Jul 1;45(1):40-9. 23. Burton GW, Traber MG, Acuff RV, et al. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998 Apr;67(4):669-84. 24. Traber MG, Elsner A, Brigelius-Flohé R. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 1998 Oct 16;437(1-2):145-8. 25. Dong Y, Lisk D, Block E, Ip C. Characterization of the biological activity of gamma-glutamyl-Se-methylselenocysteine: a novel, naturally occurring anticancer agent from garlic. Cancer Res. 2001 Apr 1;61(7):2923-8. 26. Kim T, Jung U, Cho DY, Chung AS. Se-methylselenocysteine induces apoptosis through caspase activation in HL-60 cells. Carcinogenesis. 2001 Apr;22(4):559-65. 27. Medina D, Thompson H, Ganther H, Ip C. Se-methylselenocysteine: a new compound for chemoprevention of breast cancer. Nutr Cancer. 2001;40(1):12-7. 28. Yeo JK, Cha SD, Cho CH, et al. Se-methylselenocysteine induces apoptosis through caspase activation and Bax cleavage mediated by calpain in SKOV-3 ovarian cancer cells. Cancer Lett. 2002 Aug 8;182(1):83-92. 29. Yang Y, Huang F, Ren Y, et al. The anticancer effects of sodium selenite and selenomethionine on human colorectal carcinoma cell lines in nude mice. Oncol Res. 2009;18(1):1-8. 30. Pei Z, Li H, Guo Y, Jin Y, Lin D. Sodium selenite inhibits the expression of VEGF, TGFbeta(1) and IL-6 induced by LPS in human PC3 cells via TLR4-NF-(K)B signaling blockage. Int Immunopharmacol. 2010 Jan;10(1):50-6. 31. Huang HY, Appel LJ. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J Nutr. 2003 Oct;133(10):3137-40. 32. Yu W, Jia L, Park SK, et al. Anticancer actions of natural and synthetic vitamin E forms: RRR-alpha-tocopherol blocks the anticancer actions of gamma-tocopherol. Mol Nutr Food Res. 2009 Dec;53(12):1573-81. 33. Campbell SE, Stone WL, Lee S, et al. Comparative effects of RRR-alpha- and RRR-gamma-tocopherol on proliferation and apoptosis in human colon cancer cell lines. BMC Cancer. 2006 Jan 17;6:13. 34. Handelman GJ, Epstei WL, Peerson J, et al. Human adipose alpha-tocopherol and gamma-tocopherol kinetics during and after 1 year of alpha-tocopherol supplementation. Am J Clin Nutr. 1994 May;59(5):1025-32. 35. Galli F, Stabile AM, Betti M, et al. The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch Biochem Biophys. 2004 Mar 1;423(1):97-102. 36. Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002 Dec;16(14):1952-4. 37. Moyad MA, Brumfield SK, Pienta KJ. Vitamin E, alpha- and gamma-tocopherol, and prostate cancer. Semin Urol Oncol. 1999 May;17(2):85-90. 38. Gann PH. Randomized trials of antioxidant supplementation for cancer prevention: first bias, now chance--next, cause. JAMA. 2009 Jan 7;301(1):102-3. 39. Faloon W. Merv Griffin’s tragic death from prostate cancer. Life Extension. 2008 Jan;14(1):7-11. 40. Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003 Oct 1;3:25. 41. Stone WL, Krishnan K, Campbell SE, Qui M, Whaley SG, Yang H. Tocopherols and the treatment of colon cancer. Ann N Y Acad Sci. 2004 Dec;1031:223-33. 42. Takahashi S, Takeshita K, Seeni A, et al. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Prostate. 2009 May 1;69(6):644-51. 43. Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. Gamma-tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17825-30. 44. Wells SR, Jennings MH, Rome C, Hadjivassiliou V, Papas KA, Alexander JS. Alpha-, gamma- and delta-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J Nutr Biochem. 2010 Jul;21(7):589-97. 45. Ju J, Picinich SC, Yang Z, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010 Apr;31(4):533-42. 46. London SJ, Stein EA, Henderson IC, et al. Carotenoids, retinol, and vitamin E and risk of proliferative benign breast disease and breast cancer. Cancer Causes Control. 1992 Nov;3(6):503-12. 47. Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008 Feb;102(2):134-8. 48. Singh PB, Matanhelia SS, Martin FL. A potential paradox in prostate adenocarcinoma progression: oestrogen as the initiating driver. Eur J Cancer. 2008 May;44(7):928-36. 49. Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007 Apr;23(3):374-82. 50. Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006 Jun 1;66(11):5624-32. 51. Prins GS, Birch L, Couse JF, et al. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001 Aug 15;61(16):6089-97. 52. Development of the prostate. In: Haseltine F, Paulsen C, Wang C, eds. Reproductive Issues and the Aging Male. Washington, D.C: American Association for the Advancement of Science; 1993:15-34. 53. Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. Br J Cancer. 1988 Feb;57(2):216-8. 54. Haber D. Roads leading to breast cancer. N Engl J Med. 2000 Nov 23;343(21):1566-8. 55. Holick MF. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol. 2008 Sep;3(5):1548-54. 56. Newcomer LM, King IB, Wicklund KG, Stanford JL. The association of fatty acids with prostate cancer risk. Prostate. 2001 Jun 1;47(4):262-8. 57. Leitzmann MF, Stampfer MJ, Michaud DS, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004 Jul;80(1):204-16. 58. Niclis C, Díaz MD, Eynard AR, Román MD, Vecchia CL. Dietary habits and prostate cancer prevention: A review of observational studies by focusing on South America. Nutr Cancer. 2011 Dec 2. 59. Hassan S, Carraway RE. Involvement of arachidonic acid metabolism and EGF receptor in neurotensin-induced prostate cancer PC3 cell growth. Regul Pept. 2006 Jan 15;133(1-3):105-14. 60. Moretti RM, Montagnani Marelli M, Sala A, Motta M, Limonta P. Activation of the orphan nuclear receptor RORalpha counteracts the proliferative effect of fatty acids on prostate cancer cells: crucial role of 5-lipoxygenase. Int J Cancer. 2004 Oct 20;112(1):87-93. 61. Matsuyama M, Yoshimura R, Mitsuhashi M, et al. Expression of lipoxygenase in human prostate cancer and growth reduction by its inhibitors. Int J Oncol. 2004 Apr;24(4):821-7. 62. Gupta S, Srivastava M, Ahmad N, et al. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001 Feb 15;91(4):737-43. 63. Ghosh J, Myers CE. Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem Biophys Res Commun. 1997 Jun 18;235(2):418-23. 64. Gao X, Grignon DJ, Chbihi T, et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995 Aug;46(2):227-37. 65. Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem BiophysRes Commun. 2006 Jan 6;339(1):93-8. 66. Myers CE, Ghosh J. Lipoxygenase inhibition in prostate cancer. Eur Urol. 1999 35(5-6):395-8. 67. Helgadottir A, Manolescu A, Thorleifsson G, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004 Mar;36(3):233-9. 68. Poff CD, Balazy M. Drugs that target lipoxygenases and leukotrienes as emerging therapies for asthma and cancer. Curr Drug Targets Inflamm Allergy. 2004 Mar;3(1):19-33. 69. Crooks SW, Bayley DL, Hill SL, Stockley RA. Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4. Eur Respir J. 2000 Feb;15(2):274-80. 70. Tabata T, Ono S, Song C, et al. Role of leukotriene B4 in monocrotaline-induced pulmonary hypertension. Nihon Kyobu Shikkan Gakkai Zasshi. 1997 Feb;35(2):160-6. 71. Gadaleta D, Fantini GA, Silane MF, Davis JM. Neutrophil leukotriene generation and pulmonary dysfunction after abdominal aortic aneurysm repair. Surgery. 1994 Nov;116(5):847-52. 72. Shindo K, Miyakawa K, Fukumura M. Plasma levels of leukotriene B4 in asthmatic patients. Int J Tissue React. 1993 15(5):181-4. 73. Mullane K, Hatala MA, Kraemer R, Sessa W, Westlin W. Myocardial salvage induced by REV-5901: an inhibitor and antagonist of the leukotrienes. J Cardiovasc Pharmacol. 1987 Oct;10(4):398-406. 74. Adam O, Beringer C, Kless T, et al. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol Int. 2003 Jan;23(1):27-36. 75. Pizzorno J. Omega 3-fatty acids: a key nutrient in cancer care. Presented at Comprehensive Cancer Care 2001. Arlington, Virginia. October 17-21, 2001. 76. Barham JB, Edens MB, Fonteh AN, et al. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr. 2000 Aug;130(8):1925-31. 77. Akiba S, Murata T, Kitatani K, Sato T. Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull. 2000 Nov;23(11):1293-7. 78. Schwartz J. Role of polyunsaturated fatty acids in lung disease. Am J Clin Nutr. 2000 Jan;71(1 Suppl):393S-6S. 79. Ikeda I, Yoshida H, Tomooka M, et al. Effects of long-term feeding of marine oils with different positional distribution of eicosapentaenoic and docosahexaenoic acids on lipid metabolism, eicosanoid production, and platelet aggregation in hypercholesterolemic rats. Lipids. 1998 Sep;33(9):897-904. 80. St Sauver JL, Jacobson DJ, McGree ME, Lieber MM, Jacobsen SJ. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am J Epidemiol. 2006 Oct 15;164(8):760-8. 81. Egan K, FitzGerald GA. Eicosanoids and the vascular endothelium. Handb Exp Pharmacol. 2006 (176 Pt 1):189-211. 82. Wu KK. Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin Vasc Med. 2003 May;3(2):107-12. 83. Xu S, Gao JP, Zhou WQ. Cyclooxygenase-2 and cyclooxygenase-2 inhibitors in prostate cancer. Zhonghua Nan Ke Xue. 2008 Nov;14(11):1031-4. 84. Ghosh J. Inhibition of arachidonate 5-lipoxygenase triggers prostate cancer cell death through rapid activation of c-Jun N-terminal kinase. Biochem Biophys Res Commun. 2003 Jul 25;307(2):342-9. 85. Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001 Feb 15;91(4):737-43. 86. Ye YN, Liu ES, Shin VY, Wu WK, Cho CH. Contributory role of 5-lipoxygenase and its association with angiogenesis in the promotion of inflammation-associated colonic tumorigenesis by cigarette smoking. Toxicology. 2004 Oct 15;203(1-3):179-88. 87. Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci USA. 1998 Oct 27;95(22):13182-7. 88. Anderson KM, Seed T, Vos M et al. 5-Lipoxygenase inhibitors reduce PC-3 cell proliferation and initiate nonnecrotic cell death. Prostate. 1998 Nov 1;37(3):161-73. 89. Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003 Apr;38(4):343-52. 90. Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2010 Sep;30(5):818-60. 91. Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol.2007;595:213-26. 92. Hong J, Bose M, Ju J, Ryu JHet al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004 Sep;25(9):1671-9. 93. Swamy MV, Citineni B, Patlolla JM, Mohammed A, et al. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr Cancer. 2008;60 Suppl 1:81-9. 94. Saw CL, Huang Y, Kong AN. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: docosahexaenoic acid or eicosapentaenoic acid. Biochem Pharmacol. 2010 Feb 1;79(3):421-30. 95. Hazai E, Bikadi Z, Zsila F, Lockwood SF. Molecular modeling of the non-covalent binding of the dietary tomato carotenoids lycopene and lycophyll, and selected oxidative metabolites with 5-lipoxygenase. Bioorg Med Chem. 2006 Oct 15;14(20):6859-67. 96. Manjunatha H, Srinivasan K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. FEBS J. 2006 Oct;273(19):4528-37. 97. Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003 May;17(8):816-22. 98. Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13’-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunol. 2011 Jan 15;186(2):1173-9. 99. Ju J, Hao X, Lee MJ, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila). 2009 Feb;2(2):143-52. 100. Safayhi H, Rall B, Sailer ER, Ammon HP. Inhibition by boswellic acids of human leukocyte elastase. J Pharmacol Exp Ther. 1997 Apr;281(1):460-3. 101. Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995 Jun;47(6):1212-6. 102. Sengupta K, Kolla JN, Krishnaraju AV, et al. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: a novel Boswellia serrata extract. Mol Cell Biochem. 2011 Aug;354(1-2):189-97. 103. Pommery N, Taverne T, Telliez A, et al. New COX-2/5-LOX inhibitors: apoptosis-inducing agents potentially useful in prostate cancer chemotherapy. J Med Chem. 2004 Dec 2;47(25):6195-206. 104. Bachi AL, Kim FJ, Nonogaki S, et al. Leukotriene B4 creates a favorable microenvironment for murine melanoma growth. Mol Cancer Res. 2009 Sep;7(9):1417-24. 105. Larré S, Tran N, Fan C, et al. PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins Other Lipid Mediat. 2008 Dec;87(1-4):14-9. 106. Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem BiophysRes Commun. 2006 Jan 6;339(1):93-8. 107. Zhi H, Zhang J, Hu G, et al. The deregulation of arachidonic acid metabolism-related genes in human esophageal squamous cell carcinoma. Int J Cancer. 2003 Sep 1;106(3):327-33. 108. Penglis PS, Cleland LG, Demasi M, Caughey GE, James MJ. Differential regulation of prostaglandin E2 and thromboxane A2 production in human monocytes: implications for the use of cyclooxygenase inhibitors. J Immunol. 2000 Aug 1;165(3):1605-11. 109. Rubinsztajn R, Wronska J, Chazan R. Urinary leukotriene E4 concentration in patients with bronchial asthma and intolerance of non-steroids anti-inflammatory drugs before and after oral aspirin challenge. Pol Arch Med Wewn. 2003 Aug;110(2):849-54. 110. Subbarao K, Jala VR, Mathis S, et al. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol. 2004 Feb;24(2):369-75. 111. Laufer S. Role of eicosanoids in structural degradation in osteoarthritis. Curr Opin Rheumatol. 2003 Sep;15(5):623-7. 112. de Leval X, Hanson J, David JL, et al. New developments on thromboxane and prostacyclin modulators part II: prostacyclin modulators. Curr Med Chem. 2004 May;11(10):1243-52. 113. Cheng Y, Austin SC, Rocca B, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002 Apr 19;296(5567):539-41. 114. Catella-Lawson F. Vascular biology of thrombosis: platelet-vessel wall interactions and aspirin effects. Neurology. 2001 57(5 Suppl 2):S5-S7. 115. James MJ, Penglis PS, Caughey GE, Demasi M, Cleland LG. Eicosanoid production by human monocytes: does COX-2 contribute to a self-limiting inflammatory response? Inflamm Res. 2001 May;50(5):249-53. 116. Garcia Rodriguez LA. The effect of NSAIDs on the risk of coronary heart disease: fusion of clinical pharmacology and pharmacoepidemiologic data. Clin Exp Rheumatol. 2001 Nov;19(6 Suppl 25):S41-4. 117. Onguru O, Casey MB, Kajita S, Nakamura N, Lloyd RV. Cyclooxygenase-2 and thromboxane synthase in non-endocrine and endocrine tumors: a review. Endocr Pathol. 2005 16(4):253-77. 118. Mutoh M, Takahashi M, Wakabayashi K. Roles of prostanoids in colon carcinogenesis and their potential targeting for cancer chemoprevention. Curr Pharm Des. 2006 12(19):2375-82. 119. Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006 Jan;55(1):115-22. 120. Wang D, Wang H, Shi Q, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004 Sep;6(3):285-95. 121. Feldman M, Cryer B, Rushin K, Betancourt J. A comparison of every-third-day versus daily low-dose aspirin therapy on serum thromboxane concentrations in healthy men and women. Clin Appl Thromb Hemost. 2001 Jan;7(1):53-7. 122. Sciulli MG, Renda G, Capone ML, et al. Heterogeneity in the suppression of platelet cyclooxygenase-1 activity by aspirin in coronary heart disease. Clin Pharmacol Ther. 2006 Aug;80(2): 115-25. 123. Eidelman RS, Hebert PR, Weisman SM, Hennekens CH. An update on aspirin in the primary prevention of cardiovascular disease. Arch Intern Med. 2003 Sep 22;163(17):2006-10. 124. Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006 Jan 18;295(3):306-13. 125. Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999 Mar;20(3):445-51. 126. Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006 Jan;30(1):45-51. 127. Shah BH, Nawaz Z, Pertani SA, et al. Inhibitory effect of curcumin, a spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol. 1999 Oct 1;58(7):1167-72. 128. Lantz RC, Chen GJ, Solyom AM, Jolad SD, Timmermann BN. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 2005 Jun;12(6-7):445-52. 129. Lev-Ari S, Maimon Y, Strier L, Kazanov D, Arber N. Down-regulation of prostaglandin E2 by curcumin is correlated with inhibition of cell growth and induction of apoptosis in human colon carcinoma cell lines. J Soc Integr Oncol. 2006 4(1):21-6. 130. Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002 8(19):1695-706. 131. Lev-Ari S, Strier L, Kazanov D, et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005 Sep 15;11(18):6738-44. 132. Park C, Kim GY, Kim GD, et al. Induction of G2/M arrest and inhibition of cyclooxygenase-2 activity by curcumin in human bladder cancer T24 cells. Oncol Rep. 2006 May;15(5):1225-31. 133. Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006 34(1-2): 109-15. 134. Lev-Ari S, Zinger H, Kazanov D, et al. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells. Biomed Pharmacother. 2005 Oct;59(Suppl 2):S276-80. 135. Tunstall RG, Sharma RA, Perkins S, et al. Cyclooxygenase-2 expression and oxidative DNA adducts in murine intestinal adenomas: modification by dietary curcumin and implications for clinical trials. Eur J Cancer. 2006 Feb;42(3):415-21. 136. Lee J, Im YH, Jung HH, et al. Curcumin inhibits interferon-alpha induced NF-kappaB and COX-2 in human A549 non-small cell lung cancer cells. Biochem Biophys Res Commun. 2005 Aug 26;334(2):313-8. 137. Noreen Y, Ringbom T, Perera P, Danielson H, Bohlin L. Development of a radiochemical cyclooxygenase-1 and -2 in vitro assay for identification of natural products as inhibitors of prostaglandin biosynthesis. J Nat Prod. 1998 Jan;61(1):2-7. 138. Ahmed S, Rahman A, Hasnain A, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med. 2002 Oct 15;33(8): 1097-105. 139. Yi CO, Jeon BT, Shin HJ, et al. Resveratrol activates AMPK and suppresses LPS-induced NF-κB-dependent COX-2 activation in RAW 264.7 macrophage cells. Anat Cell Biol. 2011 Sep;44(3):194-203. 140. Bishayee A, Waghray A, Barnes KF, et al. Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharm Res. 2010 Jun;27( 6):1080-91. 141. Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br J Nutr. 2007 Aug;98(2):388-96. 142. Hedelin M, Klint A, Chang ET, et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: the cancer prostate Sweden study (Sweden). Cancer Causes Control. 2006 Mar;17(2):169-80. 143. Holzbeierlein JM, McIntosh J, Thrasher JB. The role of soy phytoestrogens in prostate cancer. Curr Opin Urol. 2005 Jan;15(1):17-22. 144. Kumar NB, Cantor A, Allen K, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004 May 1;59(2):141-7. 145. Lee MM, Gomez SL, Chang JS, et al. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003 Jul;12(7):665-8. 146. McCann SE, Ambrosone CB, Moysich KB, et al. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer. 2005;53(1):33-41. 147. Vij U, Kumar A. Phyto-oestrogens and prostatic growth. Natl Med J India. 2004 Jan;17(1):22-6. 148. Sonoda T, Nagata Y, Mori M, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004 Mar;95(3):238-42. 149. Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008 Oct 8;269(2):291-304. 150. Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2004 Oct 20;3:19. 151. Morgentaler A. Testosterone For Life. New York, NY: McGraw-Hill; 2008. 152. Clark LC, Dalkin B, Krongrad A, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998 May;81(5):730-4. 153. Brooks JD, Metter EJ, Chan DW, et al. Plasma selenium level before diagnosis and the risk of prostate cancer development. J Urol. 2001 Dec;166(6):2034-8. 154. Combs GF Jr, Clark LC, Turnbull BW. Reduction of cancer risk with an oral supplement of selenium. Biomed Environ Sci. 1997 Sep;10(2-3):227-34. 155. Venkateswaran V, Klotz LH, Fleshner NE. Selenium modulation of cell proliferation and cell cycle biomarkers in human prostate carcinoma cell lines. Cancer Res. 2002 May 1;62(9):2540-5. 156. Venkateswaran V, Fleshner NE, Klotz LH. Modulation of cell proliferation and cell cycle regulators by vitamin E in human prostate carcinoma cell lines. J Urol. 2002 Oct;168(4 Pt 1):1578-82. 157. No authors listed. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994 Apr 14;330(15):1029-35. 158. Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003 May;91(7):608-12. 159. O’Malley RL, Taneja SS. Obesity and prostate cancer. Can J Urol. 2006 Apr;13 Suppl 2:11-7. |