Life Extension Magazine®
Aging individuals suffer progressive frailty, cognitive dysfunction, and a diminished sense of well-being. These conditions correlate with declining dopamine levels in the aging brain.1-3
Dopamine functions as a neurotransmitter, where it signals nerve cells to regulate mood, cognition, and bodily function.4
From the time a person is born, dopamine plays a major role in reward behavior.5 So much so that dopamine response can dictate cravings for sugary foods, recreational drugs, ethanol, tobacco, gambling, and/or sex.6
All of these behaviors can prompt a dopamine release in the brain that creates individualistic pleasure addictions.7-13
The dilemma is that as we age, dopamine depletion causes us to lose neurological functions that enable us to enjoy and function in everyday life.
Restoring dopamine to more youthful ranges is vital for healthy longevity.
Role of Dopamine in Addictive Behaviors

Narcotics like cocaine have long been associated with intense dopamine-fueled euphoria.14 Less recognized is the impact that alcohol, nicotine, and activities like gambling play in the brain’s dopamine release.
This can be seen in the form of “binge drinking,” where ethanol initially prompts dopamine release, which motivates the drinker to imbibe increasing quantities of ethanol to enhance their dopamine high.15
Compulsive gamblers feel a surge of dopamine every time a card is flipped, causing gambling binges to go on for days in some cases without regard to sleep or food. This is especially true when gambling is combined with nicotine, which prompts even greater flows of euphoric dopamine.
Perhaps one of the most common dopamine-fueled cravings people have today is for sugary foods. Despite knowing the toxic effect of glucose overload, people excessively consume sweets because of the dopamine release it generates in the brain.
Activities that promote the rapid release of dopamine are often dangerous and addicting.16 Yet some very intelligent people succumb to these euphoric effects even though their lives are adversely impacted.
Clearly, safer methods are needed to restore brain dopamine to a more youthful “feel-good” range.
Dopamine and Brain Aging
Dopamine levels decline as we age past 45 years.17 When this happens, people no longer “feel” as young as they used to. This sometimes manifests as clinical depression.18,19 For the most part, however, aging people find that their motivation, their cognitive function, and their ability to enjoy pleasurable events all diminish.
Loss of dopamine also causes people to lose their sense of well-being, i.e. they don’t feel the same as they once did.20 This is often accompanied by reduced bodily coordination that may progress to frailty.21,22
If we did not know what caused the age-related decline in brain dopamine, this would be a depressing situation for us all.
How to Boost Brain Dopamine
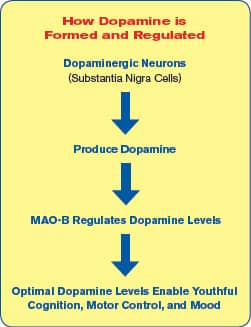
Fortunately, a prime reason for dopamine depletion was long ago discovered. It involves a dopamine-degrading enzyme in the brain called MAO-B, which stands for monoamine oxidase-B.
In youth, MAO-B is crucial in regulating just the right amount of dopamine in the brain.23 As humans age, however, MAO-B levels increase too much.24,25 The result of excess MAO-B is a dopamine deficit.
A natural method has been discovered to inhibit excess MAO-B in the brain. Placebo-controlled human studies show meaningful improvements in response to this novel dopamine restoration approach.
Dopamine is known as the “feel-good” neurotransmitter and it is a key factor in preserving a range of cognitive functions and promoting longevity. 26-29
By age 45, the brain’s levels of dopamine begin to diminish.28 This not only makes people feel older, but is also involved in accelerated brain aging.
Dopamine depletion is largely caused by rising levels of the MAO-B enzyme. The ensuing dopamine deficiency strikes the brain’s signaling system. The tragic result is cognitive decline, destruction of brain cells, reduction of youthful vigor/sexual desire, progression toward Parkinson’s/neurological disorders, and a decrease in life span.25,30-34
Laboratory studies have shown that the inhibition of MAO-B not only preserves brain function by protecting dopamine, but it also has longevity benefits.34
In a search for a safe method to block the insidious MAO-B enzyme, scientists have identified a bioactive extract of wild green oat that not only inhibits MAO-B and the resulting breakdown of dopamine, but enhances dopaminergic neurotransmission that normally declines with aging. These protective actions enable more dopamine availability for use by brain cells.
In human studies, the effects of wild green oat extract resulted in increased focus and concentration, processing speed, executive function, and working memory as well as other parameters of enhanced dopaminergic transmission.35,36
The discovery of the specific actions of wild green oat extract represents a significant advance in the technology of age management. It provides a method for halting some of the most destructive aspects of neurological aging, thus helping improve cognitive function and enhancing the quality of life.37
What You Need to Know
 |
Restore Youthful Cognition
- Deprenyl is a MAO-B inhibiting drug with powerful life-extending properties. Deprenyl has been well known and thoroughly researched for more than 30 years.
- Studies reveal that deprenyl extends life span in animal models by as much as 34%.
- Antiquated regulations in the United States have kept this potential life span-enhancer from the American public.
- Only people who already show symptoms of Parkinson’s disease are permitted access to deprenyl and its benefits.
- Innovative researchers have, however, discovered a safe and natural alternative that operates by the same mechanism as deprenyl—inhibition of the dopamine-degrading MAO-B enzyme.
- Studies with the extract of a wild green oat strain show both MAO-B-inhibition and the improvements in brain activity and function that such inhibition produces.
- Human studies with wild green oat extract confirm superior cognitive and memory function in both healthy people and those with early loss of cognition.
- There is reason to anticipate that longer life spans may arise from this modest MAO-B inhibition, as has been shown with deprenyl.
A Drug that Safely Inhibits MAO-B
Until recently, one of the best solutions to combat dopamine depletion was a prescription drug called deprenyl. Discovered in Hungary, deprenyl boosts dopamine levels by decreasing the damaging MAO-B enzyme while increasing life span in animals by a stunning 34%.38-42
For the past 27 years, Life Extension® has been reporting on the longevity benefits of deprenyl.
Despite impressive laboratory and clinical data, deprenyl has been denied to the general public due to FDA restrictions that limit deprenyl prescriptions only for those afflicted with Parkinson’s disease. The tragedy of this restriction is that people over age 45 are deprived of this drug’s unique anti-aging mechanisms.43 Deprenyl is the only drug compound ever discovered that both prolongs life and preserves cognition in higher animals.42,44,45
Searching for an alternative MAO-B inhibitor that could be made available without prescription, researchers identified a patented extract from wild green oat that works along the same pathways as deprenyl.
Unlike the commercial oats that are eaten for breakfast, wild green oat extract is derived from the green, unripe part of the oat plant. Like deprenyl, wild green oat extract inhibits the damaging MAO-B enzyme.46
In a series of human studies, this special variety of green oat extract significantly improved concentration and cognitive functions, both in healthy people and in those whose brains are just beginning to fail.35,36 By preserving the “feel-good” aspects of dopamine, green oat extract also shows evidence of effectiveness as a tool in smoking cessation, consistent with the role that dopamine plays in addictive behaviors.47
MAO-B in the Aging Brain
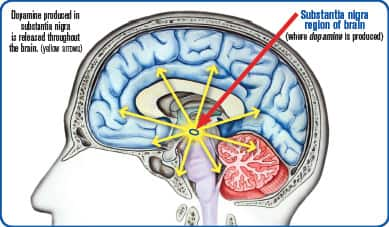
Brain cells communicate with one another and with those in the rest of the body through the use of chemical signals called neurotransmitters.48
Dopamine is one of the brain’s most important neurotransmitters. Dopamine is the signal your brain uses to transmit information related to movement control, cognition, concentration, and rewards (such as those involved in accomplishments, sexual activity, and addictions).49
Sadly, dopamine levels in the human brain begin to diminish between the ages of 45 and 60.50-52 This produces many of the subtle early changes seen in cognitive dysfunction. If left unchecked, this can eventually lead to dementia and early death by Parkinson’s disease.50,53
Why do we lose dopamine as we age?
Part of the answer lays in mitochondrial damage that occurs in the region of the brain (substantia nigra) that produces dopamine.54,55Life Extension consumers long ago gained mitochondrial protection by using nutrients like coenzyme Q10 and PQQ (pyrroloquinoline quinone).
A more insidious cause of dopamine depletion are rising levels of MAO-B in the aging brain. Excess MAO-B enzyme activity relentlessly destroys dopamine with advancing age, causing a progressive dopamine loss that falls dramatically over age 60.50-52,56,57
The age-related increase in MAO-B occurs in key areas throughout the brain, which explains why a “dopamine deficiency” manifests as so many different outward symptoms such as frailty, cognitive impairment, and loss of youthful sense of well-being. Collectively, this decline of dopamine contributes to cerebral senescence.
Age-related symptoms of dopamine deficit include high rates of depression, loss of sexual vigor, and motor control problems resembling (and sometimes progressing to) Parkinson’s disease.50
Rising levels of MAO-B also increase mitochondrial damage and destruction of brain cells.25,58 That’s because in addition to MAO-B breaking down dopamine, it also produces hydrogen peroxide, an unstable molecule that is a massive source of destructive oxygen free radicals.59,60 Because the MAO-B molecule resides on the membranes of mitochondria, the outflow of reactive oxygen species can directly damage mitochondrial membranes, leading to inefficient energy use, additional cellular damage, and eventually, death of the cell.61,62
Deprenyl Extends Life Span via MAO-B Inhibition
 |
Deprenyl has been intensely studied from 1988 to the present, with particular focus on its long-term effects in animals.28 A surprising outcome of these studies has been the observation that, time after time, in a wide range of animal models, deprenyl is not only neuroprotective, but significantly increases life span, when given at the proper dose level.
To date, deprenyl remains the only drug that has been shown to increase longevity in at least four different mammalian species.41,42
In rats, deprenyl treatment:
- Extended average life span to 198 weeks. This is remarkable in that this average exceeds the estimated maximum age at death of a normal rat, 182 weeks.91,92 In this study, the longest-living control rat died at 164 weeks, while the longest-living deprenyl-treated rat lived to 226 weeks. That’s a nearly 25% increase over the typical maximum age at death.
- Extended average life spans by up to 34% when started at 24 months (late middle age for a rat).40,93
- Extended life span significantly even when started late (at 23 to 25 months of age).94
- Restored sexual activity in 64 of 66 2-year-old (elderly) rats (Untreated animals did not have full scale sexual activity past age 2).92
- Decreased brain MAO-B activity by 85%.95
In mice, deprenyl treatment increased survival in immunocompromised animals by about 200% compared with controls,96,97 while in hamsters, deprenyl produced a three-month delay in the onset of long-term memory impairment, compared with controls.98
And in elderly dogs, deprenyl treatment started while the animals were in late mid-life led to 80% survival of treated animals to the end of a 26-month study, while just 39% of placebo dogs were alive. Interestingly, when the first dopamine-treated dog died, 12.2% of the placebo animals had already succumbed.78
The Vicious Cycle of Increasing Levels of MAO-B
As the MAO-B enzyme begins to invade our brain and devour our precious reserves of dopamine, our life span begins to shorten.50,63-66 Why?
It turns out that excess MAO-B not only destroys functioning brain cells, but replaces these healthy cells with non-neuronal “zombie cells” (called glia cells) that continue to manufacture even more MAO-B.25,67,68
You can see that once started, this becomes a vicious cycle that increasingly depletes dopamine production and destroys functioning brain cells and progressively leads to a shortened life span. Without intervention, the aggression of the MAO-B enzyme can result in an early death.
Wild Green Oat Extract Inhibits MAO-B

A patented extract from wild green oat has shown potent MAO-B-inhibiting properties. A series of human studies have demonstrated neurological-enhancing effects.
Wild green oats are younger plants (different) than those used for cereal grains. They have a long history of traditional therapeutic use in supporting mental health and cognitive function.47,69 Extracts of this “oat herb” are now widely used for indications that include anxiety, tension, stress, excitation, and other neurologic problems.69
Given the brain protective and longevity benefits of deprenyl, European scientists turned to wild green oats in search of a natural MAO-B inhibitor. A patented extract of wild green oat was developed after researchers screened 36 different varieties of wild oats for their ability to inhibit MAO-B.46
Once scientists identified the specific strain of wild green oat with the most potent biological activity, laboratory assays were conducted using various concentrations of the extract (based on standardized concentrations of isovitexin, a bioactive flavone molecule).
What they found was a 50% inhibition of the MAO-B enzyme by wild green oat extract at small concentrations.46 These findings show that this specific strain of wild green oat extract, by inhibiting the MAO-B enzyme, could have the potential of raising dopamine levels in the brain.
Demonstration of significant MAO-B inhibition by wild green oat extract would not only be a safe and inexpensive means to improve cognitive function in aging adults, it would also be likely to have favorable impacts on life span itself.
Wild Green Oat Extract in Lab Studies
Once the potency of wild green oat extract had been standardized, a study of laboratory rats was conducted to examine actual changes in behavior as a result of MAO-B inhibition from ingestion of the extract.
Animals were fed for seven weeks with either a normal diet or one supplemented with two different dosages (low and high) of the wild green oat extract. All three groups then underwent a series of behavioral tests.69
In this study, the animals showed impressive gains in learning and memory formation. Animals supplemented with the lower dose made significantly fewer mistakes in learning, learned tasks faster, and demonstrated accelerated memory formation, compared with either control or high-dose rats. As an important added benefit, low-dose supplemented rats showed increased social interest in other animals and improvement of their reactions to social signals from others.
This response highlights the essential value of raising the “feel-good” aspects of dopamine levels by properly inhibiting MAO-B. These results are consistent with findings in the literature on the use of pharmaceutical MAO-B inhibitors in Parkinson’s disease patients.69
In another study in rats, scientists used an electroencephalogram (EEG) to evaluate the impact of wild green oat extract supplementation on specific areas of brain activity. The researchers were looking to measure changes in neurotransmitters as a result of ingesting wild green oat extract.70 These changes can be “mapped” and compared to those produced by known drugs that would produce similar behavioral outcomes.
Within the first hour of oral administration, the supplemented animals demonstrated positive changes in their electrical brain activity.70 The most impressive changes were seen in electrical frequencies that are known to be controlled by dopamine.
This confirmed previous studies showing that the wild green oat extract activates the dopamine-signaling system. In fact, the EEG signatures induced by the extract closely resembled those of known antidementia drugs.
Human Study Reveals Similar Finding
Scaling up from the animal studies, a similar investigation into EEG changes resulting from ingestion of wild green oat extract has now been conducted on humans. This enabled researchers to further validate the dopamine-promoting effects of wild green oat extract.36
In a human study testing concentration skills, a group of healthy males and females, aged 30 to 60 years, took a single dose of wild green oat extract at 1,250 or 2,500 mg or placebo. Using a specialized EEG brain mapping, positive changes were shown in the green oat groups during concentration tests in an area of the brain essential for cognitive function (the left frontotemporal lobe).
This EEG brain mapping also showed significant increases in the power of theta brainwaves that are involved in focusing attention and detection ofsignals.71
Signal detection, as measured by theta wave power, is an essential component of the process by which we recognize familiar faces and objects, skills that are all too often lost to neurodegenerative diseases.72,73 Theta brain activity is an important marker of cognitive health. It is well known that Alzheimer’s patients have heartbreaking difficulties recognizing the faces of loved ones.74,75
Wild Green Oat Reduces Mental Errors

A study was conducted among a group of elderly people, some of whom showed signs of mild cognitive impairment while others in the group showed no cognitive impairment. The researchers wanted to look for measurements of cognitive performance after taking wild green oat .35
Subjects were randomly assigned to receive single doses of the oat extract once a week. The three doses in the study were 0 mg (control group), 1,600 mg, or 2,400 mg. This was a “crossover” design, so that each subject was rotated through all three dosages and received each treatment arm on separate occasions, allowing each participant to function as a control.
Using the Stroop Color-Word Test, subjects were tested for a range of cognitive functions including memory, executive function, catching errors, appropriate response, and attention. It focuses on two key areas of the brain’s cortex. In the test, participants must look at a card covered with words that name colors. The difficulty is that each word is printed in a color that does not match the word (e.g., the word “RED” printed in blue). They are first asked to read all the words as quickly and accurately as possible. They are then asked to name the color of each word, disregarding the word name itself.76,77 While the test sounds simple, it is highly diagnostic with regard to cognitive abilities and brain health.
In this study, those not given the wild green oat extract made an average of 3.39 errors on the test. The study subjects receiving 1,600 mg of wild green oat extract group made only 1.2 errors, a significant improvement of 65%.
In the group of subjects that had no diagnosis of mild cognitive impairment, the improvement was even more dramatic, with patients taking the wild green oat extract averaging just 0.55 errors compared to placebo recipients, who averaged 2.13 errors. This represented a significant 74% improvement in this test of mental acuity for those taking wild green oat extract.
In this study, even those patients with mild cognitive impairment had a 26% reduction in a score that measured how distraction affects mental performance and the ability to concentrate (lower scores reflect better performance).
The conclusion from this study is analogous to lab experiments involving deprenyl that suggest that the earlier MAO-B inhibition is initiated the better chances of preserving brain function during aging.78
Interestingly, the higher dose (2,400 mg) of green oat extract did not show this cognitive improvement, indicating that excessively supressing MAO-B does not elicit desired effects.76 This is analogous to deprenyl, where lower doses are shown to be more beneficial in some studies.79
Boosting Cognitive Function
Wild green oat extract has been associated with improving mental processing time and speed.
In a soon-to-be published study, 42 healthy middle-to-older aged adults (mean age 59), with self-reported age-related cognitive decline, were given a single dose (800 mg) of wild green oat extract. Before and after the dose, they were given a battery of neuro-cognitive tests administered by computer.80
Supplemented subjects showed significantly faster responses to stimuli compared with placebo recipients, taking less time to plan and complete specific tasks. Supplemented subjects also performed significantly better on memory-related tasks, making fewer errors in recalling words after a delay, and achieving greater accuracy in remembering spatial arrangements of objects.
Those who were given wild green oat extract showed a 180% increase in speed in the “global speed of performance test” compared to those given only the placebo.
These human trials represent a promising advance in the preservation of brain health.
MAO-B and Brain Disease
 |
Both Parkinson’s and Alzheimer’s diseases have now been found to be associated with elevated MAO-B activity, even greater than those found in healthy people of the same age. This suggests a potential causative effect, due to the decreased dopamine availability, or the increased oxidant stress caused by MAO-B or both.53,60,68
One study found that MAO-B activity in Alzheimer’s patients was higher than in controls in all brain regions studied: up to 70% higher in white matter, and 20-50% higher in gray matter regions.99 Another study showed that Alzheimer’s brains had up to 3-fold increased MAO-B activity compared with controls, with the bulk of the excess located in precisely the areas of greatest loss to Alzheimer’s patients, areas concerned with emotional processing, cognition, and personality.100
Aging people even without clear-cut diagnoses of neurodegenerative diseases also show evidence of substantial loss of brain cells, which may contribute to loss of cognitive performance and premature mortality.101-104 Given the cell-killing effects of excessive MAO-B, it seems likely that this brain shrinkage is related to increased activity of that enzyme, suggesting a potential mechanism for the life span-increasing effects of compounds that inhibit MAO-B.105-107
Improved Blood Flow to the Brain
Wild green oat extract has demonstrated improvements in tests of systemic endothelial function and cerebral vascular responsiveness, as shown in a recent human study.
The study evaluated healthy adults older than 60 years, providing them with 1,500 mg a day of wild green oat extract or placebo.81
By the end of the 12-week study period, the group receiving wild green oat extract had a significant 41% increase in measurement of flow mediated dilation, indicating an improvement in endothelial function.
The study also showed that cerebral vascular responsiveness increased significantly by 42%, suggesting the middle cerebral artery’s ability to dilate in response to stress increased.
The ability of wild green oat extract to improve endothelial function as well as cerebral vasodilator function is a property that should capture the attention of anyone wishing to protect and improve their brain health and cognitive function.
“This is the first study in humans to show sustained improvement of cerebral vasodilator function following a chronic dietary supplementation,” researchers state.
Impairments of blood flow to the heart and brain are common ailments of aging.82,83
Compromised cerebral vasodilator function has been reported as an independent predictor of stroke and transient ischemic attack.84
Impaired endothelial function is a well-established biomarker for future cardiovascular disease and cardiac events. 85-87
The tests used in this study assessed blood flow markers to the brain and other parts of the body. Wild green oat extract supplementation improved outcomes in both of these tests of vascular function.
Green Oats and Smoking Cessation
Dopamine controls our sense of accomplishment and the cycle of addiction.
When a tobacco user smokes or ingests nicotine, it binds to brain receptors that results in the release of dopamine, producing a satisfying, even euphoric, initial sensation.47,88,89
But once the stimulus goes away, the user is left with a relative dopamine deficiency, which produces cravings and eventually dependence on yet another dose of nicotine.88,90
Japanese scientists realized that this sequence of events was ripe for intervention with a product that worked along dopamine pathways. Wild green oat extract was selected as a test product.47
A group of male smokers, who all used different cigarette brands, was given 300 mg wild green oat extract taken after each meal ( 900 mg per day) for 28 days. Average cigarette consumption prior to supplementation was almost 20 cigarettes (one pack) per day, but after the end of the study, average consumption fell to just 8.9 cigarettes per day. This was an astounding 54% decrease in tobacco intake versus baseline.
Scientists are aware that dopamine is involved in both addiction and reward stimulus in the brain. This study demonstrated how wild green oat extract could significantly reduce smoking by improving dopaminergic transmission.
The discovery that the dopamine-enhancing effects of wild green oat extract can reduce cigarette consumption may be a revolutionary advance in the science of smoking cessation.
Summary
Aging exposes our brain to increased MAO-B, which results in dopamine deficit, loss of brain cells, cognitive decline, and risk for early death.
Deprenyl, a selective inhibitor of MAO-B, has been proven to ameliorate dopamine deficiency and improve cognitive functions. Numerous animal studies demonstrate that regular deprenyl administration produces increases in life span.
The FDA does not allow Americans the use of deprenyl for the treatment of any longevity-related disease, or any disease for that matter except for Parkinson’s.
Fortunately, diligent research has yielded an exciting alternative. An extract from a specific strain of wild green oat has demonstrated MAO-B-inhibiting activity. Animal studies have established improvements in dopamine-related behavior and brain activity.
Human studies confirm significantly better performance on tasks of cognition and memory in response to this wild green oat extract, as would be expected from an MAO-B-inhibiting compound.
Improved indicators of blood flow to the brain and other parts of the body, along with a reduction in addictive behavior are among the quality-of-life enhancing properties of this natural extract from select wild green oats.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
References
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995 May;15(5 Pt 1):3429-39.
- Greenwood PM, Lin MK, Sundararajan R, Fryxell KJ, Parasuraman R. Healthy aging increases the cognitive effects of two genes that influence extracellular dopamine. Psychol Aging. 2014 Jun;29(2):363-73.
- Klostermann EC, Braskie MN, Landau SM, O’Neil JP, Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiol Aging. 2012 Mar;33(3):623 e615-24.
- Burke SM, van de Giessen E, de Win M, et al. Serotonin and dopamine transporters in relation to neuropsychological functioning, personality traits and mood in young adult healthy subjects. Psychol Med. 2011 Feb;41(2):419-29.
- Arias-Carrion O, Poppel E. Dopamine, learning, and reward-seeking behavior. Acta Neurobiol Exp. 2007;67(4):481-8.
- Blum K, Liu Y, Shriner R, Gold MS. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des. 2011;17(12):1158-67.
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737-44.
- Yeh SH, Lin MH, Kong FL, et al. Evaluation of inhibitory effect of recreational drugs on dopaminergic terminal neuron by PET and whole-body autoradiography. BioMed Res Int. 2014;2014:157923.
- Kleitz-Nelson HK, Dominguez JM, Cornil CA, Ball GF. Is sexual motivational state linked to dopamine release in the medial preoptic area? Behav Neurosci. 2010 Apr;124(2):300-4.
- Joutsa J, Johansson J, Niemela S, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage. 2012 May 1;60(4):1992-9.
- Wing VC, Payer DE, Houle S, George TP, Boileau I. Measuring cigarette smoking-induced cortical dopamine release: A [(1)(1)C]FLB-457 PET study. Neuropsychopharmacology. 2015 May;40(6):1417-27.
- Di Chiara G. Alcohol and dopamine. Alcohol Health Res World. 1997;21(2):108-14.
- Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002 Jul;1(1):13-20.
- Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005 Dec;3(1):4-10.
- Rice OV, Patrick J, Schonhar CD, Ning H, Ashby CR, Jr. The effects of the preferential dopamine D(3) receptor antagonist S33138 on ethanol binge drinking in C57BL/6J mice. Synapse. 2012 Nov;66(11):975-8.
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29-71.
- Knoll J. Deprenyl (selegiline): the history of its development and pharmacological action. Acta Neurol Scand Suppl. 1983;95:57-80.
- Brown AS, Gershon S. Dopamine and depression. J Neural Transm Gen Sect. 1993;91(2-3):75-109.
- Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry. 1992 Jul 1;32(1):1-17.
- Blum K, Chen AL, Braverman ER, et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr Dis Treat. 2008 Oct;4(5):893-918.
- Mizrahi R, Mamo D, Rusjan P, Graff A, Houle S, Kapur S. The relationship between subjective well-being and dopamine D2 receptors in patients treated with a dopamine partial agonist and full antagonist antipsychotics. Int J Neuropsychop. 2009 Jun;12(5):715-21.
- Andersson DR, Nissbrandt H, Bergquist F. Partial depletion of dopamine in substantia nigra impairs motor performance without altering striatal dopamine neurotransmission. Eur J Neurosci. 2006 Jul;24(2):617-24.
- Zhou Q, Chen J, Yi S, Lou Y, Tang W, Liu Y, Zhang P. Zhichan powder regulates nigrostriatal dopamine synthesis and metabolism in Parkinson’s disease rats. Neural Regen Res. 2012 Sep 25;7(27):2107-14.
- Mazzio E, Deiab S, Park K, Soliman KF. High throughput screening to identify natural human monoamine oxidase B inhibitors. Phytother Res. 2013 Jun;27(6):818-28.
- Mallajosyula JK, Kaur D, Chinta SJ, et al. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PloS One. 2008;3(2):e1616.
- Downs B, Oscar-Berman M, Waite R, et al. Have we hatched the addiction dgg: reward deficiency syndrome solution system. J Genet Syndrom Gene. 2013 Jun 3;4(136):14318.
- Yamaguchi Y, Lee YA, Goto Y. Dopamine in socioecological and evolutionary perspectives: implications for psychiatric disorders. Front Neurosci. 2015;9:219.
- Grady DL, Thanos PK, Corrada MM, et al. DRD4 genotype predicts longevity in mouse and human. J Neurosci. 2013 Jan 2;33(1):286-91.
- Knoll J. The striatal dopamine dependency of life span inmale rats. Longevity study with (-)deprenyl.
Mech Aging Dev.
1988 Dec;46(1-3):237-62. - Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psy. 1998 Mar;155(3):344-49.
- Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann Neurol. 1998 Sep;44(3 Suppl 1):S175-88.
- Montgomery KA. Sexual desire disorders. Psychiatry (Edgmont). 2008 Jun;5(6):50-5.
- Chen L, Zhuang X. Transgenic mouse models of dopamine deficiency. Ann Neurol. 2003;54 Suppl 6:S91-102.
- Knoll J, Dallo J, Yen TT. Striatal dopamine, sexual activity and lifespan. Longevity of rats treated with (-)deprenyl. Life Sci. 1989;45(6):525-31.
- Berry NM, Robinson MJ, Bryan J, Buckley JD, Murphy KJ, Howe PR. Acute effects of an Avena sativa herb extract on responses to the Stroop Color-Word test. J Altern Complement Med. 2011 Jul;17(7):635-7.
- Dimpfel W, Storni C, Verbruggen M. Ingested oat herb extract (Avena sativa) changes EEG spectral frequencies in healthy subjects. J Altern Complement Med. 2011 May;17(5):427-34.
- Wong RH, Howe PR, Bryan J, Coates AM, Buckley JD, Berry NM. Chronic effects of a wild green oat extract supplementation on cognitive performance in older adults: a randomised, double-blind, placebo-controlled, crossover trial. Nutrients. 2012 May;4(5):331-42.
- Magyar K, Szende B. (-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology. Jan 2004;25(1-2):233-42.
- Magyar K, Szende B, Jenei V, Tabi T, Palfi M, Szoko E. R-deprenyl: pharmacological spectrum of its activity. Neurochemi Res. 2010 Dec;35(12):1922-32.
- Kitani K, Kanai S, Sato Y, Ohta M, Ivy GO, Carrillo MC. Chronic treatment of (-)deprenyl prolongs the life span of male Fischer 344 rats. Further evidence. Life Sci. 1993;52(3):281-8.
- Kitani K, Kanai S, Ivy GO, Carrillo MC. Assessing the effects of deprenyl on longevity and antioxidant defenses in different animal models. Ann N Y Acad Sci. 1998 Nov 20;854:291-306.
- Kitani K, Minami C, Isobe K, et al. Why (--)deprenyl prolongs survivals of experimental animals: increase of anti-oxidant enzymes in brain and other body tissues as well as mobilization of various humoral factors may lead to systemic anti-aging effects. Mech Ageing Dev. 2002 Apr 30;123(8):1087-100.
- Available at: www.nlm.nih.gov/medlineplus/druginfo/meds/a697046.html. Accessed May 27, 2015.
- Lees AJ. Selegiline hydrochloride and cognition. Acta Neurol Scand Suppl. 1991;136:91-94.
- Agnoli A, Fabbrini G, Fioravanti M, Martucci N. CBF and cognitive evaluation of Alzheimer type patients before and after IMAO-B treatment: a pilot study. Eur Neuropsychopharmacol. 1992 Mar;2(1):31-5.
- Moccetti T, Wullschleger C, Aydogan C, Keruter M. Bioactivity-based development of a wild green oat (Avena sativa L.) extract in support of mental health disorders. Kongressband Phytopharmaka Phytotherapie. 2006 Sep.
- Fujii F, Hashimoto T, Suzuki N, Suzuki R, Mohri K. Pilot study of the standardized oatsh extract for smoking reduction. Pharmacometrics. 2008;75(3/4):47-53.
- Lovinger DM. Communication networks in the brain: neurons, receptors, neurotransmitters, and alcohol. J Natl Instit Alcohol. 2008;31(3):196-214.
- Available at: http://www.ncbi.nlm.nih.gov/books/nbk64328. Accessed July 25, 2015.
- Knoll J. The facilitation of dopaminergic activity in the aged brain by (-)deprenyl. A proposal for a strategy to improve the quality of life in senescence. Mech Ageing Dev. 1985 May 13;30(2):109-22.
- Galva MD, Bondiolotti GP, Olasmaa M, Picotti GB. Effect of aging on lazabemide binding, monoamine oxidase activity and monoamine metabolites in human frontal cortex. J Neural Transm Gen Sect. 1995;101(1-3):83-94.
- Saura J, Andres N, Andrade C, Ojuel J, Eriksson K, Mahy N. Biphasic and region-specific MAO-B response to aging in normal human brain. Neurobiol Aging. 1997 Sep-Oct;18(5):497-507.
- Nagatsu T, Sawada M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implications of glial cells. J Neural Transm Suppl. 2006 (71):53-65.
- Venkateshappa C, Harish G, Mythri RB, Mahadevan A, Bharath MM, Shankar SK. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson’s disease. Neurochem Res. 2012 Feb;37(2):358-69.
- Simon DK, Lin MT, Zheng L, et al. Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson’s disease. Neurobiol Aging. 2004 Jan;25(1):71-81.
- Kumar P, Taha A, Kale RK, Cowsik SM, Baquer NZ. Physiological and biochemical effects of 17beta estradiol in aging female rat brain. Exp Gerontol. 2011 Jul;46(7):597-605.
- Kumar MJ, Andersen JK. Perspectives on MAO-B in aging and neurological disease: where do we go from here? Mol Neurobiol. 2004 Aug;30(1):77-89.
- Siddiqui A, Hanson I, Andersen JK. Mao-B elevation decreases parkin’s ability to efficiently clear damaged mitochondria: protective effects of rapamycin. Free Rad Res. 2012 Aug;46(8):1011-8.
- Pritchett S, Green D, Rossos P. Accidental ingestion of 35% hydrogen peroxide. Can J Gastroen. 2007 Oct;21(10):665-7.
- Mallajosyula JK, Chinta SJ, Rajagopalan S, Nicholls DG, Andersen JK. Metabolic control analysis in a cellular model of elevated MAO-B: relevance to Parkinson’s disease. Neurotox Res. 2009 Oct;16(3):186-93.
- Binda C, Hubalek F, Li M, Castagnoli N, Edmondson DE, Mattevi A. Structure of the human mitochondrial monoamine oxidase B: new chemical implications for neuroprotectant drug design. Neurology. 2006. Oct 10;67(7 Suppl 2):S5-7.
- Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev. 2007;39(2-3):443-55.
- Miyasaki JM, Kluger B. Palliative care for Parkinson’s disease: has the time come? Curr Neurol Neurosci Rep. 2015 May;15(5):26.
- Xiao L, Saiki C, Ide R. Stem cell therapy for central nerve system injuries: glial cells hold the key. Neural Reg Res. 2014 Jul 1;9(13):1253-60.
- Poewe W. The natural history of Parkinson’s disease. J Neurol. 2006 Dec;253 Suppl 7:VII2-6.
- Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013 Nov;28(11):1109-24.
- Ulusu NN. Glucose-6-phosphate dehydrogenase deficiency and Alzheimer’s disease: Partners in crime? The hypothesis. Med Hypotheses. 2015 May 11.
- Gulyas B, Pavlova E, Kasa P, et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int. 2011 Jan;58(1):60-8.
- Schellekens C, Perrinjaquet-Moccetti T, Wullschleger C, et al. An extract from wild green oat improves rat behaviour. Phytother Res. 2009 Oct;23(10):1371-7.
- Effect of Neuravena™ on the brain: continuous in-vivo analysis of electrical activity in freely moving rats. Unpublished data on file. Frutarom 2015.
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Reb. 1999 Apr;29(2-3):169-95.
- Brenner CA, Rumak SP, Burns AM, Kieffaber PD. The role of encoding and attention in facial emotion memory: an EEG investigation. Int J Psychophysiol. 2014 Sep;93(3):398-410.
- Crespo-Garcia M, Cantero JL, Atienza M. Effects of semantic relatedness on age-related associative memory deficits: the role of theta oscillations. Neuroimage. 2012 Jul 16;61(4):1235-48.
- Kawamura M, Sugimoto A, Kobayakawa M, Tsuruya N. Neurological disease and facial recognition. Brain Nerve. 2012 Jul;64(7):799-813.
- Mendez MF, Martin RJ, Smyth KA, Whitehouse PJ. Disturbances of person identification in Alzheimer’s disease. A retrospective study. J Nerv Ment Dis. 1992 Feb;180(2):94-6.
- Berry NM, Robinson MJ, Bryan J, Buckley JD, Murphy KJ, Howe PR. Acute effects of an Avena sativa herb extract on responses to the Stroop Color-Word test. J Altern Complement Med. 2011 Jul;17(7):635-7.
- Jensen AR, Rohwer WD, Jr. The Stroop color-word test: a review. Acta Psychol (Amst). 1966;25(1):36-93.
- Ruehl WW, Entriken TL, Muggenburg BA, Bruyette DS, Griffith WC, Hahn FF. Treatment with L-deprenyl prolongs life in elderly dogs. Life Sci. 1997;61(11):1037-44.
- Available at: http://smart-drugs.net/ias-deprenyljs.htm. Accessed July 25, 2015.
- Neuravena™ improves cognitive function in healthy older adults. Unpublished data on file. Frutarom 2015.
- Wong RH, Howe PR, Coates AM, Buckley JD, Berry NM. Chronic consumption of a wild green oat extract (Neuravena) improves brachial flow-mediated dilatation and cerebrovascular responsiveness in older adults. J Hyperten. 2013 Jan;31(1):192-200.
- Henriksen OM, Jensen LT, Krabbe K, Larsson HB, Rostrup E. Relationship between cardiac function and resting cerebral blood flow: MRI measurements in healthy elderly subjects. Clin Physiol Funct Imaging. 2014 Nov;34(6):471-7.
- Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004 Jan;57(1):6-14.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001 Mar;124(Pt 3):457-67.
- Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183-98.
- Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013;2(6):e000426.
- Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001 Jul 10;104(2):191-6.
- Jiloha RC. Biological basis of tobacco addiction: Implications for smoking-cessation treatment. Indian J Psychiatry. 2010 Oct;52(4):301-7.
- D’Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011 Jul;6(1):4-16.
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Ann Rev Pharmacol Toxicol. 2009;49:57-71.
- Knoll J. The facilitation of dopaminergic activity in the aged brain by (-)deprenyl. A proposal for a strategy to improve the quality of life in senescence. Mech Ageing Dev. 1985 May 13;30(2):109-22.
- Knoll J, Dallo J, Yen TT. Striatal dopamine, sexual activity and lifespan. Longevity of rats treated with (-)deprenyl. Life Sci. 1989;45(6):525-31.
- Kitani K, Kanai S, Miyasaka K, Carrillo MC, Ivy GO. Dose-dependency of life span prolongation of F344/DuCrj rats injected with (-)deprenyl. Biogerontology. 2005;6(5):297-302.
- Milgram NW, Racine RJ, Nellis P, Mendonca A, Ivy GO. Maintenance on L-deprenyl prolongs life in aged male rats. Life Sci. 1990;47(5):415-20.
- Bickford PC, Adams CE, Boyson SJ, et al. Long-term treatment of male F344 rats with deprenyl: assessment of effects on longevity, behavior, and brain function. Neurobiol Aging. 1997 May-Jun;18(3):309-18.
- Freisleben HJ, Lehr F, Fuchs J. Lifespan of immunosuppressed NMRI-mice is increased by deprenyl. J Neural Transm Suppl. 1994;41:231-6.
- Freisleben HJ, Neeb A, Lehr F, Ackermann H. Influence of selegiline and lipoic acid on the life expectancy of immunosuppressed mice. Arzneimittelforschung. 1997 Jun;47(6):776-80.
- Stoll S, Hafner U, Pohl O, Muller WE. Age-related memory decline and longevity under treatment with selegiline. Life Sci. 1994;55(25-26):2155-63.
- Jossan SS, Gillberg PG, Gottfries CG, Karlsson I, Oreland L. Monoamine oxidase B in brains from patients with Alzheimer’s disease: a biochemical and autoradiographical study. Neuroscience. 1991;45(1):1-12.
- Saura J, Luque JM, Cesura AM, et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994 Sep;62(1):15-30.
- Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012 Feb;71(2):258-66.
- Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013 May 28;80(22):2055-61.
- Kovacs GG, Milenkovic I, Wohrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013 Sep;126(3):365-84.
- Tang Y, Lopez I, Baloh RW. Age-related change of the neuronal number in the human medial vestibular nucleus: a stereological investigation. J Vestib Res. 2001;11(6):357-63.
- Maruyama W, Naoi M. Neuroprotection by (-)-deprenyl and related compounds. Mech Ageing Dev. 1999 Nov;111(2-3):189-200.
- Maruyama W, Yamamoto T, Kitani K, Carrillo MC, Youdim M, Naoi M. Mechanism underlying anti-apoptotic activity of a (-)deprenyl-related propargylamine, rasagiline. Mech Ageing Dev. 2000 Jul 31;116(2-3):181-91.
- Knoll J. Sexual performance and longevity. Exp Gerontol. 1997 Jul-Oct;32(4-5):539-52.

