Life Extension Magazine®

When people think about osteoporosis, they assume it refers only to decreased bone density and increased fracture risk.
The reality is far worse.
As skeletal mass is lost, bone-derived growth factors are released that may contribute to atherosclerosis,1,2 inflammation,3,4 calcification,5,6 and cancer.7-9
Osteoporosis is mostly associated with women, but it also occurs in men. In both men and women, it lays the groundwork for chronic age-related illnesses. These pathologies almost always develop before bone loss is detected.
Maintaining skeletal integrity is a lifelong process that involves the dissolving of old bone and precise restoration by laying down minerals such as calcium.
In the absence of bone-building nutrients and hormones, growth factors that should remain in bone are instead released into the blood where they create systemic havoc.
Osteoporosis in men is underestimated. In the setting of newly diagnosed prostate cancer, evidence of significant bone loss has been observed virtually 100% of the time.10-15 Prostate cancer-cell propagation increases in response to growth factors released as bone remodels.7,16
When osteoporotic fractures occur in older men, death rates are higher than women with fractures.17,18
Awareness of osteoporosis risk motivates health-conscious women (and some men) to proactively protect against bone loss.
For those who want to reduce their intake of encapsulated minerals, a chewable tablet now provides optimal potencies with a natural chocolate-flavor taste.
This issue of Life Extension Magazine® describes hidden causes of bone loss that can be rectified with lifestyle changes, hormone/mineral balance, and avoidance of bone-depleting drugs.
People may lose bone mass at any age, and it is a common part of normal degenerative processes.
Documented methods exist to slow and reverse bone loss.
As bone density declines, inflammatory cytokines are released into the blood such as interleukin-6, tumor necrosis factor-alpha, and interleukin 1.19-21
These pro-inflammatory factors may accelerate vascular disease and dementia while shortening overall longevity.22-26 It is important to note that inflammation, aging, and cancer are interrelated.
Bone contains growth factors that are needed to maintain skeletal density. When bone breaks down, these growth factors are released into the blood where they can fuel cancer cell propagation.10,11
Testosterone plays an important role in maintaining bone density in men.27,28 Women rely more on estrogen and progesterone.29,30
Both sexes can benefit from DHEA, a hormone that converts to estrogen and testosterone in a highly individualistic manner.31
What’s important to understand is that bone is not static. Our skeleton comprises energized tissues that are rich in bone-derived growth factors.
Keeping these growth factors in bone and out of soft tissues is essential for healthy longevity.
Overlooked Role of Magnesium
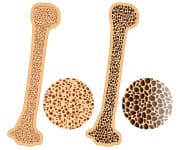
Bone with Osteoporosis
(reduced density &
quality of bone — right)
When Life Extension® was founded 37 years ago, most dietary supplements designed to prevent osteoporosis contained only calcium and a little vitamin D.
We argued that magnesium was also important to maintain aging bones.
A large volume of evidence now substantiates the role magnesium plays in bone health.32-36
A study published in 2017 produced compelling findings showing that low serum magnesium concentration is independently associated with an increased risk of fractures in middle-aged Caucasian men.37
For this study, researchers evaluated 2,200 men over a median follow-up of 25.6 years. They found that men in the lowest quartile of serum magnesium had a two-fold increased risk of bone fractures, compared to those in the highest quartile. This study found that 63.4% of those fractures involved the femur—the major weight-bearing bone of the lower extremity.
Of 22 men in this study with above-normal magnesium levels at baseline, none experienced a fracture.
What’s revealing here is that only 1% of men had above-normal magnesium serum levels. This may indicate how widespread magnesium deficiency is in the general population.
The researchers who conducted this study commented that it would be difficult for aging people to obtain optimal blood magnesium levels via diet. They suggested using supplements to ensure higher magnesium blood levels.
When looking at the data showing magnesium’s multiple benefits, it makes sense to ensure one is obtaining optimal amounts of this mineral that is deficient in most American diets.
Osteoporosis Runs Rampant
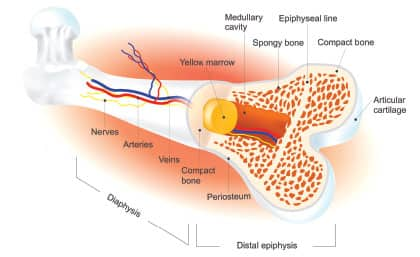
Bone fractures are a leading cause of disability and ill health among the aging population.37 Fracture incidence worsens as more people are prescribed bone-robbing drugs in the face of epidemic nutrient deficiencies.
In American white women over age 50, roughly four in ten will experience a hip, spine or wrist fracture sometime during the remainder of their lives. Lifetime risk of fractures will increase for all ethnic groups as people live longer.38
By 2020, one of every two Americans over age 50 is expected to have, or be at risk of developing, osteoporosis of the hip. Even more will be at risk of developing osteoporosis at any skeletal site.38
The high prevalence of osteoporosis in women has caused many men to be complacent about their bone health.
We at Life Extension have observed clinical data indicating that bone loss is associated with coronary artery blockage, carotid artery/aortic valve stenosis and/or prostate cancer.39-47
In other words, as bone mass breaks down and releases its contents into the blood, there is systemic ossification (bone formation) of soft tissues and widespread inflammation, along with bone-derived growth factors acting as biological fuel for cancer cell proliferation.
So in a nutshell, maintaining and restoring youthful bone density reduces not only fractures but also risk of the most common degenerative disorders.
Bone Is Living Tissue
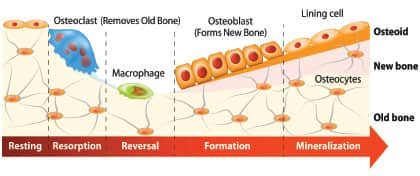
The Bone Remodeling Process
|
Lay people often mistakenly think of bone as calcified rock that slowly erodes with aging.
In reality, your bones “remodel” through a lifelong process where old bone tissue is removed from the skeleton and new bone tissue formed.70,71
An imbalance in the regulation of bone “remodeling” results in disorders such as osteoporosis.71
Bone tissue is removed by osteoclasts, and new bone tissue is formed by osteoblasts. Both processes utilize the signaling cytokines transforming growth factor-beta (TGF-β) and insulin-like growth factor (IGF).72,73
When bone breaks down, it releases TGF-B and IGF into the blood where these growth factors can fuel cancer cell proliferation.74,75
You Don’t Have to be a Victim!

Data on osteoporosis incidence is frightening. One should find comfort that they have the ability to improve bone health at any age.
Bone loss is exacerbated in those who ingest excess phosphates (such as those found in soda beverages), consume too much alcohol, and/or smoke cigarettes.48-54
Thyroid or parathyroid disorders can cause bone density loss,55-57 as can sex hormone deficits (estrogen, testosterone, progesterone, DHEA).29,58-61
Loss of bone mass is attributed to mineral deficiencies such as boron, magnesium and calcium.37,62-64
Vitamin K2 is essential to keep calcium in bone and out of soft tissues like arteries and heart valves.65-69
Those taking any drug that causes bone mass loss should be particularly vigilant. We describe common bone-depleting drugs in the osteoporosis article appearing on page 34 of this month’s issue.
New Way to Take Bone-Building Minerals

Chewable Mineral Tablets
There are a number of well-designed formulas that provide calcium, magnesium and other nutrients for bone health. To obtain optimal potencies, consumers usually have to swallow about four capsules a day.
Minerals don’t taste bad the way B-vitamins and some amino acids do.
That prompted us to combine optimal potencies of calcium, magnesium, boron and other nutrients to create a pleasant-tasting chewable chocolate lozenge.
I was surprised when I tried this mineral lozenge because it tasted as good as unsweetened chocolate I’ve used in the past.
Just two of these nice-tasting lozenges provide the same potencies of nutrients as are found in four capsules of popular bone-building formulas.
No one should risk complications of osteoporosis when there are low-cost options available, with robust published scientific data to support efficacy.
For longer life,
William Faloon, Co-Founder
Life Extension Buyers Club
References
- Hamerman D. Osteoporosis and atherosclerosis: biological linkages and the emergence of dual-purpose therapies. Qjm. 2005;98(7):467-84.
- Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347(1):155-75.
- Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85(7):596-607.
- Bussard KM, Venzon DJ, Mastro AM. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J Cell Biochem. 2010;111(5): 1138-48.
- Danilevicius CF, Lopes JB, Pereira RM. Bone metabolism and vascular calcification. Braz J Med Biol Res. 2007;40(4):435-42.
- Pal SN, Clancy P, Golledge J. Circulating concentrations of stem-cell-mobilizing cytokines are associated with levels of osteoprogenitor cells and aortic calcification severity. Circ J. 2011;75(5):1227-34.
- Reis ST, Pontes-Junior J, Antunes AA, et al. Tgf-beta1 expression as a biomarker of poor prognosis in prostate cancer. Clinics (Sao Paulo). 2011;66(7):1143-7.
- Kingsley LA, Fournier PGJ, Chirgwin JM, et al. Molecular Biology of Bone Metastasis. Molecular Cancer Therapeutics. 2007;6(10):2609-17.
- Yin JJ, Selander K, Chirgwin JM, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103(2):197-206.
- Roodman GD. Mechanisms of bone metastasis. Discov Med. 2004;4(22):144-8.
- Farhat GN, Taioli E, Cauley JA, et al. The association of bone mineral density with prostate cancer risk in the Osteoporotic Fractures in Men (MrOS) Study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):148-54.
- Israeli RS. Managing bone loss and bone metastases in prostate cancer patients: a focus on bisphosphonate therapy. Rev Urol. 2008;10(2):99-110.
- Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167(6):2361-7; discussion 7.
- So A, Chin J, Fleshner N, et al. Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: Incorporating new agents into clinical practice. Can Urol Assoc J. 2012;6(6):465-70.
- Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur Urol. 2004;46(6):731-39; discussion 9-40.
- Available at: https://s3.amazonaws.com/curetoday/media/u02/data/htdocs/media/downloads/documents/BoneHealth_PG_rev_b.pdf. Accessed September 12, 2017.
- Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878-82.
- Kamel HK. Male osteoporosis: new trends in diagnosis and therapy. Drugs Aging. 2005;22(9):741-8.
- Lacativa PG, Farias ML. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol. 2010;54(2):123-32.
- Omoigui S. The Interleukin-6 inflammation pathway from cholesterol to aging – Role of statins, bisphosphonates and plant polyphenols in aging and age-related diseases. Immunity & Ageing. 2007;4(1):1.
- Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immunity & Ageing. 2005;2(1):14.
- Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61(5):668-72.
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539-52.
- Sundelof J, Kilander L, Helmersson J, et al. Systemic inflammation and the risk of Alzheimer’s disease and dementia: a prospective population-based study. J Alzheimers Dis. 2009;18(1):79-87.
- Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92-105.
- Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69(2):165-73.
- Spry NA, Galvao DA, Davies R, et al. Long-term effects of intermittent androgen suppression on testosterone recovery and bone mineral density: results of a 33-month observational study. BJU Int. 2009;104(6):806-12.
- Available at: http://www.medscape.com/viewarticle/846022. Accessed September 12, 2017.
- Scheven BA, Damen CA, Hamilton NJ, et al. Stimulatory effects of estrogen and progesterone on proliferation and differentiation of normal human osteoblast-like cells in vitro. Biochem Biophys Res Commun. 1992;186(1):54-60.
- Seifert-Klauss V, Prior JC. Progesterone and bone: actions promoting bone health in women. J Osteoporos. 2010;2010:845180.
- Labrie F, Luu-The V, Labrie C, et al. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22(3):185-212.
- Tucker KL, Hannan MT, Chen H, et al. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69(4):727-36.
- Carpenter TO, DeLucia MC, Zhang JH, et al. A randomized controlled study of effects of dietary magnesium oxide supplementation on bone mineral content in healthy girls. J Clin Endocrinol Metab. 2006;91(12):4866-72.
- Matias CN, Santos DA, Monteiro CP, et al. Magnesium intake mediates the association between bone mineral density and lean soft tissue in elite swimmers. Magnes Res. 2012;25(3):120-5.
- Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009;28(2):131-41.
- Saito N, Tabata N, Saito S, et al. Bone mineral density, serum albumin and serum magnesium. J Am Coll Nutr. 2004;23(6):701s-3s.
- Kunutsor SK, Whitehouse MR, Blom AW, et al. Low serum magnesium levels are associated with increased risk of fractures: a long-term prospective cohort study. Eur J Epidemiol. 2017.
- Available at: https://www.ncbi.nlm.nih.gov/books/NBK45515/. Accessed September 13, 2017.
- Farhat GN, Strotmeyer ES, Newman AB, et al. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcif Tissue Int. 2006;79(2):102-11.
- Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73(1):15-20.
- Kiel DP, Kauppila LI, Cupples LA, et al. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68(5):271-6.
- Broussard DL, Magnus JH. Coronary heart disease risk and bone mineral density among U.S. women and men. J Womens Health (Larchmt). 2008;17(3):479-90.
- Barengolts EI, Berman M, Kukreja SC, et al. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62(3): 209-13.
- Pfister R, Michels G, Sharp SJ, et al. Inverse association between bone mineral density and risk of aortic stenosis in men and women in EPIC-Norfolk prospective study. Int J Cardiol. 2015;178:29-30.
- Galvao DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-7.
- Neubecker K, Adams-Huet B, Farukhi IM, et al. Predictors of fracture risk and bone mineral density in men with prostate cancer on androgen deprivation therapy. J Osteoporos. 2011;2011:924595.
- Kim SH, Kim YM, Cho MA, et al. Echogenic carotid artery plaques are associated with vertebral fractures in postmenopausal women with low bone mass. Calcif Tissue Int. 2008;82(6):411-7.
- Fung TT, Arasaratnam MH, Grodstein F, et al. Soda consumption and risk of hip fractures in postmenopausal women in the Nurses’ Health Study. Am J Clin Nutr. 2014;100(3):953-8.
- Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97(4):667-75.
- Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121(5):406-18.
- Porter SE, Hanley EN, Jr. The musculoskeletal effects of smoking. J Am Acad Orthop Surg. 2001;9(1):9-17.
- Wyshak G, Frisch RE. Carbonated beverages, dietary calcium, the dietary calcium/phosphorus ratio, and bone fractures in girls and boys. J Adolesc Health. 1994;15(3):210-5.
- Mazariegos-Ramos E, Guerrero-Romero F, Rodriguez-Moran M, et al. Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in children: a case-control study. J Pediatr. 1995;126(6):940-2.
- Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15(4):710-20.
- Lewiecki EM, Miller PD. Skeletal effects of primary hyperparathyroidism: bone mineral density and fracture risk. J Clin Densitom. 2013;16(1):28-32.
- Williams GR. Actions of thyroid hormones in bone. Endokrynol Pol. 2009;60(5):380-8.
- Zaidi M, Davies TF, Zallone A, et al. Thyroid-stimulating hormone, thyroid hormones, and bone loss. Curr Osteoporos Rep. 2009;7(2):47-52.
- Fink HA, Ewing SK, Ensrud KE, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91(10):3908-15.
- Kung AW. Androgen and bone mass in men. Asian J Androl. 2003;5(2):148-54.
- Riggs BL, Khosla S, Melton LJ, 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13(5):763-73.
- Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf). 2000;53(5):561-8.
- Pizzorno L. Nothing Boring About Boron. Integr Med (Encinitas). 2015;14(4):35-48.
- Zofkova I, Nemcikova P, Matucha P. Trace elements and bone health. Clin Chem Lab Med. 2013;51(8):1555-61.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-36.
- Wallin R, Schurgers L, Wajih N. Effects of the blood coagulation vitamin K as an inhibitor of arterial calcification. Thromb Res. 2008;122(3):411-7.
- Zittermann A. Effects of vitamin K on calcium and bone metabolism. Curr Opin Clin Nutr Metab Care. 2001;4(6):483-7.
- Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134(11):3100-5.
- Jie KS, Bots ML, Vermeer C, et al. Vitamin K intake and osteocalcin levels in women with and without aortic atherosclerosis: a population-based study. Atherosclerosis. 1995;116(1):117-23.
- Beulens JW, Bots ML, Atsma F, et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203(2):489-93.
- Hadjidakis DJ, Androulakis, II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385-96.
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-45.
- Xiao W, Wang Y, Pacios S, et al. Cellular and Molecular Aspects of Bone Remodeling. Front Oral Biol. 2016;18:9-16.
- Allori AC, Sailon AM, Warren SM. Biological basis of bone formation, remodeling, and repair-part I: biochemical signaling molecules. Tissue Eng Part B Rev. 2008;14(3):259-73.
- Hiraga T, Myoui A, Hashimoto N, et al. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Res. 2012;72(16):4238-49.
- Chiechi A, Waning DL, Stayrook KR, et al. Role of TGF-beta in breast cancer bone metastases. Adv Biosci Biotechnol. 2013;4(10c):15-30.

