
Pancreatic Cancer
Pancreatic Cancer
Last Section Update: 06/2021
Contributor(s): Jennifer Pryor, M.D.; Maureen Williams, ND; Shayna Sandhaus, PhD
1 Introduction
Pancreatic cancer is less common than many other cancers but has a high mortality rate and poor prognosis. It accounts for only about 3% of all cancers in the United States (US) but roughly 7% of US cancer deaths are attributable to pancreatic cancer.1 There were 57,600 estimated new cases in the US in 2020.2 Over a 5-year period from diagnosis, the expected survival rate is less than 10%.2,3 Globally, pancreatic cancer is ranked as the 7th most common cause of death due to cancer; it ranks 4th in the US.4,5 The incidence of pancreatic cancer increases with age, with a median age at diagnosis of 70 years, and it is slightly more common in men than women.2
The incidence of pancreatic cancer is rising in industrialized countries—the National Cancer Institute reported that the incidence in the US increased at a rate of approximately 1% per year from 1974 to 2017.2 This increase in incidence is thought to attributable largely to lifestyle factors—particularly higher rates of obesity and diabetes—and to overall population aging.4
A major challenge with pancreatic cancer is that surgical resection remains the only potentially curative therapy, but only 15‒20% of patients are eligible for surgery at the time of presentation.6 The reason so few patients are eligible for surgery is multi-factorial. Due to the vague and non-specific nature of symptoms associated with pancreatic cancer, delays in rendering a correct diagnosis are quite common, and it is often diagnosed later in the course of the disease. Pancreatic cancer also has a high tendency toward vascular invasion due to the presence of major blood vessels adjacent to the pancreas. This means that spread via the blood and metastasis often occur relatively early in tumor growth. Sadly, by the time many patients are diagnosed with pancreatic cancer, they have either locally advanced or metastatic disease, rendering them ineligible for surgical resection.4
Although surgical resection is the only potentially curative therapy for pancreatic cancer, chemotherapy and radiotherapy may be a part of the treatment plan as well. Indications for their use are dependent upon whether a cancer is resectable (has potential for complete removal), borderline-resectable (is at high risk for local recurrence), or locally advanced (unresectable). Preoperatively, these additional therapies may reduce the tumor burden sufficiently to render a locally advanced cancer surgically resectable or allow for a borderline-resectable cancer to be completely resected. As postoperative therapy, they can improve chances for a cure or reduce the risk of recurrence.7 Despite advances in treatment approaches, however, the median survival of patients with surgically resected pancreatic cancer who received adjuvant therapy in clinical trials was only 28 months.8
Lifestyle factors play a significant role in the risk and development of pancreatic cancer, which may explain the variation in incidence seen globally. Specifically, cigarette smoking, obesity, physical inactivity, a typical “Western” diet high in red meats and sugar and low in fruits and vegetables, and heavy alcohol consumption have all been identified as risk factors.6 Smoking in particular has been shown worldwide to infer a significantly increased risk of pancreatic cancer compared with those who never smoked.9 These findings lend strong support to the role of a healthy diet and lifestyle in reducing the incidence of pancreatic cancer.
Evidence also strongly supports a hereditary risk for the development of pancreatic cancer. Cases with a hereditary component account for 5‒10% of total pancreatic cancer. The incidence increases with number of first-degree relatives (parent, sibling, or child) who have been affected.4 Thus, people with a family history of pancreatic cancer should take extra care to implement the healthy dietary and lifestyle habits described in the “Prevention” section of this protocol.
Due in part to the poor prognosis for most patients with pancreatic cancer, investigations have continued in the search for new treatment options for these patients. One area of research has been repurposing drugs previously used for other indications. For instance, statin drugs and angiotensin II receptor blockers, among others, are undergoing clinical trials in pancreatic cancer.10 This area of research has great potential to improve treatment options for these patients.
New and innovative pancreatic cancer treatment options are being developed as well. A promising advance is in gene therapy; this approach involves transferring DNA of one or more therapeutic genes into patients—either into the bloodstream or directly into the tumor—using a vector for delivery. The DNA transferred can replace a cancer-fighting gene that the patient is lacking, add a new therapeutic gene, or decrease the activity of a cancer-causing gene like an oncogene. Another strategy bypasses DNA and uses RNA (microRNAs or interfering RNAs). One particular technology, called CRISPR-Cas9, allows a specific portion of DNA within the patient to be targeted and modified using gene transfer. Oncolytic virotherapy uses viruses to target cancer cells and destroy them. Finally, immunotherapy utilizes the patient’s own immune system to combat the cancer; for example, by increasing anti-tumor messenger molecules or blocking a receptor that increases the growth of cells.11
In this protocol you will learn about pancreatic cancer, its potential causes and known risk factors, and signs and symptoms associated with the disease. Diagnostic approaches, including screening, blood testing, imaging, and staging, will be covered. We will provide a thorough review of conventional treatment methods as well as new and emerging modalities in the treatment of pancreatic cancer. We will conclude with a discussion of dietary and lifestyle considerations, including preventative steps you can take to lower your risk of developing pancreatic cancer, approaches for supportive care during treatment, and nutrients that may reduce pancreatic cancer risk and support treatment.
2 Background
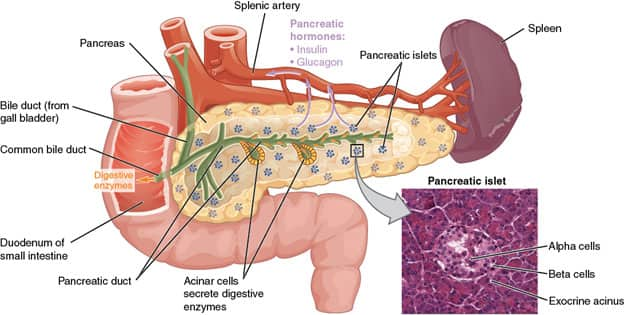
The pancreas is an organ in the upper abdominal cavity that has both endocrine and exocrine functions. Its exocrine functions are to make digestive enzymes, and its endocrine role is to make insulin and glucagon for glucose metabolism. The pancreas is surrounded by the stomach, small intestine, liver, and spleen, and is subdivided into a head, body, and tail. The head is very close to the small intestine and connects to it via the pancreatic duct, and the tail is near the spleen. The cell types that make up the pancreas are primarily ductal cells, acinar cells, and islet cells—any of which can give rise to a tumor, as well as primitive cells.12
Pancreatic cancer develops from previously normal cells through a build-up of genetic mutations, either inherited or arising when cells are dividing.4 As the cancer evolves, these genetic mutations can alter the appearance of cells. Several precursor lesions (discussed below) are recognized which, if left untreated, would be expected to develop into pancreatic cancer. These precursor lesions may also be present within or near pancreatic cancer and can be identified on biopsy and surgical resection.
Exocrine Pancreatic Cancers
As in other organs of the body, pancreatic neoplasms, or abnormal tissue masses, can be benign or malignant. Of all pancreatic neoplasms, approximately 85% are ductal adenocarcinoma and its subtypes.13 Ductal adenocarcinoma forms from ductal cells which, along with acinar cells, make up the exocrine pancreas. Over 95% of pancreatic cancers originate from the exocrine pancreas.12 Because of its frequency, the term “pancreatic cancer” generally refers to ductal adenocarcinoma; this meaning will be used throughout this text, and ductal adenocarcinoma is the main topic of this protocol.
The majority (60‒70%) of ductal adenocarcinomas arise in the head of the pancreas, with about 15% in the body and 15% in the tail.4 Those arising in the head of the pancreas tend to present earlier because they block a main duct via which bile leaves, resulting in jaundice and epigastric pain. When surgically removed, ductal adenocarcinomas average 3-5 cm in size and show invasion of surrounding tissue. The cancer can also invade nerves, blood vessels, and lymphatics within the pancreas. Direct extension to nearby organs and larger blood vessels outside of the pancreas is common.12
There are several types of pancreatic cancer that look and behave differently but are still considered to be ductal in origin. Adenosquamous carcinoma has at least 30% squamous cells and has a worse prognosis than ductal adenocarcinoma. Undifferentiated/anaplastic carcinoma has little to no resemblance to normal ductal cells and is a highly aggressive cancer with extremely poor survival rates. A type with a better prognosis is colloid/mucinous carcinoma. In this form, large amounts of mucin—a gel-like fluid that is made by normal glands and some adenocarcinomas—are secreted into the surrounding tissue. Most colloid/mucinous carcinomas form in association with intraductal papillary mucinous neoplasms (described below).4
Precursor Lesions
Pancreatic intraepithelial neoplasia (PanIN) is a non-invasive lesion composed of abnormal ductal cells. The lesions arise in small pancreatic ducts and are too small to be detected by imaging studies. As a non-invasive lesion, PanIN cannot spread to other parts of the body, but may progress over time and eventually become ductal adenocarcinoma. The changes in the appearance of cells can be graded into low-grade PanIN and high-grade PanIN.12
Intraductal papillary mucinous neoplasms (IPMNs) are another category of precursor lesion. Unlike PanIN, which are very small lesions in small ducts, IPMNs are larger and grow in the main pancreatic duct or its smaller branches. The location is relevant because co-existing cancer is found in about 70% of main pancreatic duct IPMNs versus only 25% of those arising inside branches.4
Mucinous cystic neoplasms (MCNs) are precursor lesions that appear as cysts and so must be distinguished from benign pancreatic cysts. One study found that about 25% of surgically resected pancreatic cysts are MCNs.14 Of MCNs resected, approximately 18% have been found to have co-existing cancer.15 Diagnosis of MCN requires surgical excision with examination by a pathologist.12 Recognition of pancreatic cysts on abdominal imaging offers one of the few opportunities for early intervention in the prevention and early diagnosis of pancreatic cancer.4
Non-Ductal Exocrine Pancreatic Cancers
A few non-ductal cancers can arise in the exocrine pancreas and are much less common than ductal cancers.
As previously described, the exocrine pancreas includes not just ductal cells but also acinar cells. Cancers arising from this cell type are calledacinar cell carcinoma and mixed acinar carcinomas. These are rare cancers, representing only 1‒2% of all adult pancreatic neoplasms, and 15% of pancreatic neoplasms in the pediatric population.16
Pancreatoblastoma arises from primitive cells and can have different appearances. Although rare in adults, it is the most common pancreatic neoplasm in children, comprising about 25% of all cases.16
Finally, solid pseudopapillary neoplasm is a low-grade cancer that represents 2‒5% of all pancreatic cancers; it is not well-understood what cell it arises from.16
Endocrine Pancreatic Cancers
Cancers of the endocrine pancreas fall under the general category of pancreatic endocrine neoplasms (PENs), which include insulinomas and glucagonomas. Due to their unpredictable behavior, prognosis can be difficult to determine. They may be functioning (secreting hormones and thus giving rise to related symptoms) or non-functioning. While many small PENs can be cured with surgical resection, larger (>2 cm) tumors have a worse prognosis; of this group, 50‒80% ultimately recur or spread to other parts of the body.16
3 Causes and Risk Factors
Cancer develops when genetic mutations form in genes that encode proteins involved in cellular function, particularly those with roles in growth and division. Mutations present at birth are considered inherited (germline mutations) and are present in every cell, whereas those that occur throughout one’s life are considered acquired (somatic mutations) and occur initially in only some cells.17 Somatic mutations are caused either by damage to the cellular DNA by environmental factors, intrinsic conditions within the individual, or occur by random chance during cellular division. Because there are two copies of every gene—one from the mother and one from the father—an inherited mutation in one of the copies infers an increase in cancer risk but does not necessarily cause it, since there is one healthy copy of the gene remaining.18
Non-Modifiable Risk Factors
Non-modifiable risk factors include those that an individual is born with and factors that cannot be prevented or changed by behavioral modification. Within this category are both inherited cancer-associated mutations and biological traits that are intrinsic to individuals, specifically age, sex, ethnicity, and blood type.4,19
Genetics and family history. Cases of pancreatic cancer with genetic susceptibility comprise 5‒15% of total cases and are due to a known genetic predisposition syndrome or are familial in nature.4,20 These individuals are identified by their positive family history, with one or more close relatives having the disease. There is not one specific gene that causes pancreatic cancer, but rather several genes that are known and likely more that have not yet been identified.18 Since the mutations in these genes are inherited, they are considered non-modifiable risk factors. A positive family history in a first-degree relative is identified in approximately 5‒10% of individuals with pancreatic cancer.4,20
Familial pancreatic cancer (FPC) refers to pancreatic cancer occurring in at least a pair of immediate relatives who do not meet criteria for one of the genetic predisposition syndromes associated with pancreatic cancer. Definitive genetic determinants of FPC have not been determined, but mutations in BRCA1 and BRCA2, as well as PALB2, which encodes a protein that interacts with the BRCA2 protein, occur in some familial cases of FPC.20,21
Of genetic predisposition syndromes, hereditary pancreatitis confers the highest lifetime risk (ie, 25–40%) of developing pancreatic cancer. Typically, this is associated with mutations in the serine protease 1 (PRSS1) gene. These individuals develop chronic pancreatitis at a very young age, typically by 20 years, and have a 25‒44% increased risk of pancreatic cancer.20
A number of genes are associated with pancreatic cancer even outside the context of FPC, including BRCA1 and BRCA2, the genes which are well-known for the role they play in hereditary breast and ovarian cancers.21 BRCA2 plays the most significant role, being found in 5‒17% of individuals with pancreatic cancer.20 Even in the absence of a positive family history, germline mutations in genes associated with pancreatic cancer were identified in 31 of 854 (3.6%) patients who underwent surgical resection for pancreatic cancer.6
Individuals with a family history of pancreatic cancer or another condition associated with increased risk of pancreatic cancer can undergo genetic consultation with possible genetic screening. Companies that offer hereditary testing for pancreatic cancer include Myriad Genetics (https://myriad.com) and Invitae (https://www.invitae.com).
Age. The incidence of pancreatic cancer increases with age, with diagnosis rarely made before age 45, and 90% of patients diagnosed over age 55.4,6 The peak age at diagnosis is 65–69 years for men and 75–79 years for women. The association of age with pancreatic cancer can be attributed to accumulation of genetic mutations over time. This may be one of the reasons incidence of pancreatic cancer is rising: people with chronic health conditions are living longer due to improved treatments and thus have more time to accumulate genetic mutations that may give rise to pancreatic cancer.22
Sex. Worldwide, pancreatic cancer occurs more commonly in men than women, with variability in incidence rates by region. The age-standardized incidence rate per 100,000 in North America is 8.7 in males compared with 6.5 in females; for comparison, in South Central Asia it is 1.1 in males compared with 1.0 in females.4 Sex differences in incidence are greater in more developed countries, which could potentially be explained by environmental and genetic factors.23
Ethnicity. The incidence of pancreatic cancer between 1973 and 2014 in Whites was almost 7% versus nearly 10% in Blacks. Among other ethnicities, including American Indian/Alaskan Native and Asian/Pacific Islander, the incidence is almost 6%.5 Whether these differences were due to lifestyle, body mass index (BMI), presence of diabetes mellitus, or underlying genetic variations is poorly understood.4 Higher rates of pancreatic cancer among individuals with Ashkenazi Jewish ancestry are believed to be at least partially genetic, due to germline mutations in cancer-causing genes, including BRCA2 and BRCA1.24
Blood type. Blood type has been associated with risk of several gastrointestinal (GI) cancers.6 Its role in risk of pancreatic cancer was investigated in two large cohort studies, which found that type A, AB, or B blood was significantly associated with risk of pancreatic cancer, and 17% of pancreatic cancer cases were attributable to having non-type O blood. The association between blood type and pancreatic cancer risk was not significantly modified by any of the other risk factors studied, including age, sex, smoking status, BMI, or level of physical activity. The mechanism for blood type’s role in pancreatic cancer risk is not well understood, though it is known that ABO antigens are expressed not just on red blood cells but also on some epithelial cells, including in the GI tract. It is postulated that these antigens could play a role in intercellular adhesion and membrane signaling in pancreatic cancer tumorigenesis or may alter the host inflammatory state.25
Modifiable Risk Factors
Somatic mutations in pancreatic cancer that are caused by environmental factors are primarily due to lifestyle and behavior choices and, consequently, many can be prevented.18 These modifiable risk factors have been extensively studied, with evidence supporting smoking, diabetes, obesity, and exposure to toxic substances each conferring an increased risk of pancreatic cancer, while alcohol and dietary factors may also play a role.26,27
Smoking. Cigarette smoking is by far the most important non-hereditary risk factor for pancreatic cancer development.4 Eleven to 32% of pancreatic cancer cases can be attributed to cigarette smoking alone.6 Worldwide, the 2017 Global Burden of Disease Study found that 21% of pancreatic cancer-related deaths were likely caused by smoking. A meta-analysis of 82 studies found a 74% increased risk of pancreatic cancer in current smokers, while another study showed risk increases with number of cigarettes smoked.4 There is a particularly high risk for pancreatic cancer among individuals who both smoke heavily and have homozygous deletions of the gene that encodes glutathione S-transferase theta 1 (GSTT1), an enzyme that metabolizes carcinogens.6 Cigarette smoking influences the prognosis for diagnosed patients; those who continue to smoke are at higher risk for developing other primary cancers.9 Promisingly, smoking cessation decreases pancreatic cancer risk, achieving the risk level of non-smokers 10‒15 years after stopping. After only two years of smoking cessation, the risk of pancreatic cancer decreases 48%.6
Diabetes. A number of studies of diabetes mellitus (including types 1 and 2) have linked abnormal glucose metabolism, insulin resistance, and hyperinsulinemia with risk of pancreatic cancer.6 In fact, there is a 30% increased risk of developing pancreatic cancer in the 20-year period after a diagnosis of diabetes. It has been suggested that new onset diabetes could potentially be used as a marker of pancreatic cancer risk.26 While the mechanisms behind the link between diabetes and pancreatic cancer are multifactorial and not well-understood, some data support a role for the hormone adiponectin, which has insulin-sensitizing and anti-inflammatory properties.6
Promisingly, there is research supporting that type 2 diabetes mellitus can be treated and even reversed by bariatric surgery, low-calorie diets, and carbohydrate restriction.31 Adoption of these strategies may have a role in prevention of pancreatic cancer, particularly for those individuals at increased risk of its development. Moreover, adjuvant treatment with metformin, a first-line antidiabetic drug, has been associated with improved pancreatic cancer outcomes in preliminary and observational studies.32 More rigorous randomized controlled trials are needed to further assess the potential utility of metformin as an adjuvant therapy for pancreatic cancer.
Obesity and lack of physical activity. Several studies have identified a link between high BMI, lack of physical activity, and risk of pancreatic cancer. In the Health Professionals Follow-up Study and Nurses’ Health Study, a positive association was found in individuals with a BMI > 30 kg/m2.6 A meta-analysis of 19 studies identified by the World Cancer Research Fund as showing an increased risk of pancreatic cancer in obese patients found a 10% increased risk with every 5 BMI units.4 Obesity is known to be strongly associated with type 2 diabetes; both of these conditions contribute to a pro-inflammatory state with insulin resistance.9
Environmental toxins. Several environmental and synthetic toxins may increase the risk of pancreatic cancer. Individuals may encounter Bis(2-ethylhexyl) phthalate (DEHP), present in polyvinyl chloride (PVC), in many products—for instance, floor coverings and toys. DEHP causes several cancer-associated changes in human tumor cell lines, including increased cellular proliferation. The toxic metal cadmium (Cd) is used in battery production, fertilizers, and sewage, but most individuals are exposed to it in foods. Increased urinary Cd concentrations were found to show a significant association with pancreatic cancer. Several other toxins have also been reported to increase risk of pancreatic cancer; these include arsenic, lead, benzene, asbestos, and chlorinated hydrocarbons.9
Chronic pancreatitis. Chronic pancreatitis manifests as an inflammatory state in which the pancreatic ducts and acini are gradually replaced by fibrosis.4 Although chronic pancreatitis can arise within a genetic predisposition syndrome, it is more commonly due to lifestyle factors. The International Pancreatitis Study Group calculated country-specific incidence data, revealing the ratio of observed cases of pancreatic cancer in subjects having chronic pancreatitis to expected cases of pancreatic cancer in the general population to be about 26:1 over a mean follow-up period of 7.4 years, with a cumulative risk of 4% for its development at 20 years.6 However, since overall only 1.3% of pancreatic cancer cases can be attributed to chronic pancreatitis, prevention of this inflammatory condition will only reduce pancreatic cancer by a small number of cases.6
Chronic infections. Some infections may also modestly increase the risk of pancreatic cancer, with several mechanisms postulated for this finding. Helicobacter pylori is a bacterium that commonly infects the lining of the stomach and can cause gastritis; however, people also can be asymptomatic carriers. One meta-analysis showed that H. pylori could confer a 45% increased risk of developing pancreatic cancer; however, due to the small number of cases studied, further studies were required.4 The same meta-analysis indicated that it was specifically theH. pylori CagA-positive strain that conferred this increased risk.33 Other studies have been mixed, yet overall there appears to be a small but significant association between H. pylori infection and pancreatic cancer.6 Due to the high prevalence of H. pylori in Western countries, the infection could potentially contribute to 4‒25% of pancreatic cancer cases.9 This suggests that treating H. pylori infection (even when asymptomatic) is a possible avenue for reducing cancer risk, though data so far are insufficient to make definite conclusions.26
Other infections have also shown an association with pancreatic cancer. Hepatitis C virus (HCV) and hepatitis B virus (HBV) have been reported to mildly increase the cancer risk.6 Given that about 170 million and 350 million individuals worldwide have HCV and HBV infection, respectively, the number of pancreatic cancer cases potentially associated with these viruses is significant.34
Dysbiosis (microbiome imbalance). The microbial population and associated local environment (microbiome) of the oral cavity, and in particular the lower GI tract (gut), has increasingly been shown to play a role in tumor development and growth and in the response to cancer treatment. A growing number of studies have shown that the gut microbiome has an influence on response to chemotherapy and immunotherapy in cancer patients. There are complex interactions between the pancreas and an individual’s microbiome that shape immune regulation and influence the effects of therapy. These interactions are in part facilitated by the close proximity and direct link of the pancreas with the small intestine via the main pancreatic duct. In mouse models, the gut microbiome colonizes pancreatic tumors, influencing its bacterial composition.19
A prospective cohort study of over 200,000 individuals found that those with periodontal disease had 1.6-fold higher risk for pancreatic cancer compared with no disease.9 This finding is independent of other risks for pancreatic cancer, including diabetes, pancreatitis, and viral hepatitis, and it is primarily seen in individuals over age 65.35
Unhealthy diet. A poor diet can promote development of cancer —a high-calorie diet with excess fat and sugar is associated with obesity, one of the risk factors for pancreatic cancer, while high red meat and processed meat consumption is independently linked with pancreatic cancer. The mechanisms linking red meat and pancreatic cancer are debated and require further study.9,36 A Western diet, along with aging, obesity, diabetes, and smoking, can contribute to accumulation of advanced glycation end products, which are proteins or fats combined with sugar; these compounds are abnormal and can enhance tumor growth.9 Conversely, a diet rich in fruits, vegetables, nuts, and whole grains can play a preventative role in cancer, including pancreatic cancer. These foods contain dietary fibers along with bioactive phytochemicals that can protect against several chronic diseases and cancer, although the mechanisms are not fully understood.36
Coffee has a more controversial association with pancreatic cancer; a meta-analysis concluded there is about a 1.1 relative risk of pancreatic cancer with higher levels of consumption compared with lower levels; however, the association was not significant and therefore not likely to be appreciably related to pancreatic cancer risk.6
Alcohol. Data regarding the role of alcohol consumption on development of pancreatic cancer have been conflicting.6 Any role at all is likely to be small and only present in heavier drinkers (>30 grams of alcohol per day).4,6 Risk is greatest for men and those who drink >60 grams of alcohol per day, particularly spirits (hard liquors).9 A meta-analysis showed that occasional to moderate levels of drinking conferred no increased risk of pancreatic cancer.4 It should be noted that regular intake of alcohol at high doses can cause chronic pancreatitis, which is a known risk factor for pancreatic cancer.4
Chronic psychological stress. The relationship between stress and health status has been debated and is complicated by individual variation in genes, epigenetic effects, and physiology. Some studies found an association between severe, repeated psychological stresses and several diseases, including neoplasms. A large study conducted in Sweden identified a link between severe emotional stress (eg, loss of a parent) with an increased risk of early onset (<40 years) pancreatic cancer. Animal studies demonstrated that the neurotransmitters released during stress negatively influence outcomes of pancreatic cancer treatment.9
4 Signs and Symptoms
Initial signs and symptoms of pancreatic cancer vary depending upon the location of the tumor within the pancreas. For instance, most tumors arise in the head of the pancreas, where they will be more likely to show signs and symptoms related to obstruction of the pancreatic or bile ducts than tumors in the tail.13 Jaundice can be seen earlier in cancer progression in these individuals due to compression or obstruction of the common bile duct and, therefore, jaundice could be an early sign of the disease.37 In tumors of the body or tail, jaundice occurs in locally advanced disease or when the cancer has metastasized to the liver. Fatty stools (steatorrhea) is more common with pancreatic cancer of the head of the pancreas; this can be attributed to impaired secretion of digestive enzymes.13
Overall, the most common symptoms experienced by people with pancreatic cancer are weakness and loss of energy (asthenia), weight loss, a diminished appetite or aversion to food (anorexia), and abdominal pain. These four symptoms will be experienced by a majority of individuals with pancreatic cancer. It should be noted that asthenia and weight loss are experienced by many people with cancer—and even for some with infectious or inflammatory diseases, and are in no way specific to pancreatic cancer. Likewise, anorexia and abdominal pain can occur in several other conditions. For individuals with pancreatic cancer, pain in the abdomen is most common, but it also often occurs in the epigastric area and sometimes in the back. Pain can be present even for small (<2 cm) tumors but is insidious in onset, may not be considered particularly worrisome to the individual at first, and is usually present for 1‒2 months before seeking medical attention.13
On physical examination of a patient with pancreatic cancer, findings may suggest an abnormality involving the GI system, but none are specific to the pancreas. The most frequent findings are jaundice, enlargement of the liver (hepatomegaly), a right upper quadrant or epigastric mass, weight loss with muscle wasting(cachexia), a swollen but non-tender gallbladder (Courvoisier’s sign), and fluid accumulation in the abdominal cavity (ascites). Jaundice is usually progressive in pancreatic cancer and can be caused by any condition that raises blood bilirubin levels, including liver disease, gallbladder stones, and blood conditions (eg, hemolytic anemia). Jaundice is typically accompanied by itching (pruritus), darkening of the urine, and pale stool.13 Cachexia, meanwhile, can occur in people with other cancers, infections, and inflammatory conditions.
A study that included 185 patients diagnosed with pancreatic cancer over a three-year period identified the following symptoms at presentation, in decreasing order of prevalence: asthenia, weight loss, anorexia, abdominal pain, epigastric pain, dark urine, jaundice, nausea, back pain, diarrhea, vomiting, steatorrhea, and clotting and inflammation of veins (thrombophlebitis). Of these patients, 62% had cancer in the head of the pancreas, 10% in the body, and 6% in the tail, with the remaining cases not determined. Signs identified in these patients in decreasing order of prevalence were jaundice, hepatomegaly, right upper quadrant mass, cachexia, Courvoisier’s sign, epigastric mass, and ascites.13
5 Diagnosis
Diagnosis of pancreatic cancer is complicated by the non-specific nature of its symptoms and short window of time available to start treatment before the disease spreads.4 Individuals may be unaware the symptoms they are having are being caused by pancreatic cancer and do not seek treatment quickly enough, or their healthcare provider does not recognize that the symptoms and physical exam findings are due to pancreatic cancer. Given these difficulties, prevention and early detection of pancreatic cancer are of utmost importance.
Screening
There is no single laboratory or imaging test to diagnose or screen for pancreatic cancer. Screening of the general population is also not recommended because only a very small percentage of people will develop pancreatic cancer in their lifetime. Although screening of the general population is not done, there is a potential role for screening of certain high-risk populations. For instance, individuals with a family history of FPC were recommended as a potential target for screening by the International Cancer of the Pancreas Screening Consortium, though there was disagreement about what age screening should begin.4
In terms of the most appropriate test for screening purposes, imaging studies can be used, with a combination of secretin-enhanced magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) recommended by the consortium.4 Other methods for screening at-risk individuals have also been developed. One method called PancPRO uses an individual’s family history to determine their probability of having a genetic mutation associated with pancreatic cancer risk. In 2018, the American Society of Clinical Oncology provisionally recommended that patients diagnosed with pancreatic cancer be tested for hereditary syndromes that are associated with increased risk for pancreatic cancer.20 Identifying patients with hereditary forms of pancreatic cancer can help identify their close family members who may also be at risk.
Biomarkers
Identifying novel biomarkers is an area of interest for treatment of pancreatic cancer as it could allow for individualized cancer therapy, but their role in screening is limited. As of mid-2021, the only biomarker approved by the US Food and Drug Administration (FDA) for pancreatic cancer is serum cancer antigen 19-9 (CA 19-9), but it has no role for general screening of asymptomatic patients.4
Given the link between diabetes mellitus and pancreatic cancer, hemoglobin A1c (HbA1c) has been considered a potential biomarker for early detection of cancer.4 HbA1c is a form of hemoglobin coated with sugar that is increased in individuals with untreated or poorly-controlled type 2 diabetes. One study found that the risk of pancreatic cancer in patients with levels of HbA1c in the highest quartile (7.7–15.4%) was two-fold higher than in the lowest (4.6–6.3%). This pattern was seen if the elevated HbA1c was detected within the five years prior to diagnosis of pancreatic cancer. These findings suggest a role for HbA1c in screening of patients with diabetes mellitus for development of pancreatic cancer.38
Another possible biomarker for early detection that is under investigation utilizes a non-invasive test measuring the concentration of volatile organic compounds (VOC) in breath exhalations. A study found that patients with pancreatic cancer had elevated levels of VOC compared with healthy patients. Another possible biomarker being studied is the presence of mutated DNA in pancreatic juice. Gene mutations associated with pancreatic cancer and its precursor lesions were shown to be more common in DNA in pancreatic juice among pancreatic cancer patients than in health controls.4 Finally, glypican-1 (GPC-1), a modulator of growth factor signaling, is abnormally expressed in some cancers and is detectable in blood.39
Laboratory testing for some of these biomarkers will be discussed in a later section.
Signs and Symptoms
Because of the vague and non-specific nature of the signs and symptoms of pancreatic cancer, including variation caused by location of the tumor within the pancreas, the differential diagnosis is fairly broad.13 It can be helpful to note the signs and symptoms present in a particular patient and determine the differential diagnosis from there. Age of the patient should also be taken into consideration.
Jaundice, one of the most common signs in pancreatic cancer, is caused by elevated bilirubin due to obstruction of one or more bile ducts. There are many other causes of elevated bilirubin besides pancreatic cancer, and exclusion of one of the other causes of the abnormality is necessary in the clinical work-up. The age of a patient with jaundice is relevant; jaundice is more likely to predict pancreatic cancer in patients over 60 years of age.13
A patient presenting with epigastric pain could have any number of conditions, not just from the pancreas or GI system but also potentially from a cardiac or vascular cause (eg, aortic dissection). Additionally, gastritis and peptic ulcer disease are significantly more common causes of epigastric pain than pancreatic cancer.40
Unintentional weight loss can occur with not only pancreatic cancer but also other cancers, endocrinopathies, and some psychiatric conditions.13 A swollen and non-tender gallbladder can be seen with benign disorders of the gallbladder.
Laboratory Studies
While biomarkers might aid in screening for pancreatic cancer, laboratory studies are also performed in patients presenting with possible cancer to evaluate the underlying condition and guide decision-making. Those presenting with jaundice should have liver function tests, and those with epigastric pain should have lipase tested to evaluate for acute pancreatitis, in addition to liver function tests.13 Although a complete blood count (CBC) and general chemistry studies are routinely performed, their use in evaluating pancreatic cancer is limited.
The CA 19-9 biomarker previously described can help determine the likelihood of cancer for those who present with suspicious signs and symptoms. Studies have shown high sensitivity and specificity of CA 19-9 for detection of pancreatic cancer, with very high levels uncommon in benign conditions. With that said, some benign conditions can also cause elevations in the biomarker, complicating diagnosis.41
Measurement of serum IgG4 is performed for the work-up of IgG4-related disease (IgG4-RD), a systemic fibro-inflammatory disease suspected to be autoimmune in nature. IgG4-RD can involve almost any organ system, with a level greater than 135 mg/dL proposed as a diagnostic criterion. Patients with this condition typically present with a mass or enlargement of the involved organ, which can be the pancreas. For individuals with a pancreatic mass who are suspected to have pancreatic cancer but for whom pancreatic involvement by IgG4-RD must be excluded, this test has potential utility in helping distinguish the two.42
Imaging Studies
The diagnostic work-up for patients with signs and symptoms suspicious for pancreatic cancer includes abdominal imaging studies. This is particularly important for those with risk factors for its development, for whom a more aggressive approach is recommended. For those with jaundice, the most common initial study is a transabdominal ultrasound; however, if they are suspected to have a gallstone in a bile duct, an endoscopic retrograde cholangiopancreatography (ERCP) or MRCP might be utilized instead. ERCP is diagnostic but can also be therapeutic by allowing for drainage of small gallstones, bile, or pancreatic juice, placement of a stent, or removal of large gallstones. Biopsy of a tumor can also be performed during an ERCP, though this is a suboptimal method compared with endoscopic ultrasound-guided biopsy.13
For patients unable to have an ERCP (eg, those with gastric outlet obstruction), MRCP is a viable alternative. Although it lacks the therapeutic capabilities of ERCP, MRCP is preferred by some because it does not require the use of contrast agent and therefore potentially has fewer complications.13
For patients without jaundice who are experiencing epigastric pain and weight loss, abdominal computed tomography (CT) is the preferred study. With that said, ultrasound is often used for these patients because of cost and the fact that it is more widely available. Ultrasound also has high sensitivity for detecting tumors greater than 3 cm. Regrettably, ultrasound has difficulty detecting tumors smaller than 3 cm or when there is acute pancreatitis or necrosis of the pancreas is present. Dual-phase abdominal CT with intravenous contrast is typically best—the results obtained may even be sufficient to warrant surgical resection without additional testing if the appearance is typical and the patient is fit for surgery.13,43
It should be noted that lesions in the pancreas may appear solid or cystic on imaging.13 While ductal adenocarcinoma usually appears as a solid mass, other cancers and benign conditions of the pancreas can appear similarly, including IgG4-RD.13,42,44
After initial imaging studies are done, for a patient suspected of having pancreatic cancer who still does not have a confirmed diagnosis, the next step in the work-up is usually staging rather than obtaining a biopsy. For those cases in which a biopsy before surgical excision is recommended, it is performed either through the skin or via endoscopic ultrasound.
Staging
Staging provides important information about how locally advanced a pancreatic cancer is and whether and how far it has metastasized, guides treatment decisions, and helps establish prognosis. The American Joint Committee on Cancer (AJCC) staging system is used; it incorporates size and spread of the primary tumor (T), involvement of lymph nodes (N), and spread to distant sites (M). Cancer staging is a complex process, integrating information from clinical history, laboratory tests, imaging studies, and the pathology report. Imaging studies, particularly CT, provide information about whether a cancer is likely resectable, borderline resectable, locally advanced, or has metastasized. Complete staging requires examination of tissue by a pathologist to confirm the size and extent of the primary tumor and confirm presence of tumor in lymph nodes or distant sites. When no pathology report is available—such as when no biopsy has been performed—a clinical stage can be determined to help plan treatment.45
The AJCC stages are determined from the T, N, and M components described above. Size of the tumor (T) comes from the pathology report of the resection specimen.46 Examination of any regional lymph nodes (N) received with the surgical resection will determine how many are involved.45 Finally, if there is tissue confirmation of spread to a distant (non-regional) lymph node or organ, M1 is given, otherwise the designation used is M0.46 Table 1 lists the AJCC stages and describes the elements of each stage.
| Table 1. American Joint Committee on Cancer Stages of Pancreatic Cancer | ||||
|
AJCC Stage |
Tumor |
Lymph nodes |
Metastasis |
Description |
|
IA |
T1 |
N0 |
M0 |
The primary cancer is 2 cm or less and there are no positive regional lymph nodes or metastasis |
|
IB |
T2 |
N0 |
M0 |
The primary cancer is >2 cm and ≤4 cm and there are no positive regional lymph nodes or metastasis |
|
IIA |
T3 |
N0 |
M0 |
The primary cancer is >4 cm and there are no positive regional lymph nodes or metastasis |
|
IIB |
T1 |
N1 |
M0 |
The primary cancer is 2 cm or less, one to three regional lymph nodes are involved, and there is no metastasis |
|
|
T2 |
N1 |
M0 |
The primary cancer is >2 cm and ≤ 4 cm, one to three regional lymph nodes are involved, and there is no metastasis |
|
|
T3 |
N1 |
M0 |
The primary cancer is >4 cm, one to three regional lymph nodes are involved, and there is no metastasis |
|
III |
T1 |
N2 |
M0 |
The primary cancer is <2 cm, four or more regional lymph nodes are involved, and there is no metastasis |
|
|
T2 |
N2 |
M0 |
The primary cancer is >2 cm and ≤4 cm, four or more regional lymph nodes are involved, and there is no metastasis |
|
|
T3 |
N2 |
M0 |
The primary cancer is >4 cm, four or more regional lymph nodes are involved, and there is no metastasis |
|
|
T4 |
Any N |
M0 |
The primary cancer involves the celiac axis, superior mesenteric artery or common hepatic artery, regardless of size, and there is no metastasis |
|
IV |
Any T |
Any N |
M1 |
Distant metastasis is present |
6 Treatment
Surgery
Despite advances in novel treatment strategies, surgical resection remains the only option with the potential to cure pancreatic cancer.4 In the past, only patients with pancreatic cancer confined to the pancreas (“localized”) were considered candidates for surgery, but this changed in 2003 when the National Comprehensive Cancer Network (NCCN) recognized the category “borderline resectable.” Borderline resectable cancers involve nearby structures, with uncertainty regarding whether they can be fully resected. Aggressive chemoradiotherapy before surgery (“neoadjuvant therapy”) in patients with borderline resectable cancers has allowed some patients to become eligible for surgery, giving patients who might otherwise have no hope the potential for a cure.14
Expectations for surgery depend upon location of the tumor within the pancreas. Naturally, the goal of surgery is to completely remove the cancer and have all margins in the specimen be negative for cancer (R0). Patients who successfully have R0 resections have significantly improved survival compared with those in which cancer is found at or near the resection margins (R1).4 Since a majority of pancreatic cancers are located in the head of the pancreas, surgical resection usually requires removal of not just the head of the pancreas but also part of the small intestine (pancreaticoduodenectomy or Whipple procedure). This is a major surgery with potential for serious complications.47 Removal of some or all of the pancreas also destroys the body’s ability to produce insulin and glucagon, causing patients to be insulin-dependent for the rest of their lives.48
Some cancers are located in the body or tail of the pancreas and do not require resection of the duodenum. Removal of the tail of the pancreas (distal pancreatectomy) preserves the head, though may require removal of the spleen, while a central pancreatectomy (rarely performed) preserves both some of the head and tail.48 The distal pancreatectomy can be performed laparoscopically, a minimally invasive approach that reduces complications from surgery.14 Removal of just the body or tail of the pancreas for localized cancers can also preserve the patient’s ability to make insulin and glucagon.14,48 It is doubtful that further improvements in surgical treatment of patients with pancreatic cancer will significantly improve survival going forward because rates of death due to surgery are already low, and more aggressive resections have yielded little improvement in clinical outcomes.49
Neoadjuvant Therapy
Patients with borderline resectable, locally advanced, or unresectable pancreatic cancer represent 30‒40% of all cases and include those with cancer involving critical structures like the superior mesenteric arteries. Management of those with borderline resectable tumors generally involves efforts to reduce the tumor burden in or near critical structures with neoadjuvant (pre-surgery) therapy. While a small proportion of these patients are successfully downstaged to operable disease, they unfortunately get only a modest increase in survival.49 Initial management of locally advanced, unresectable cancers is controversial and may include radiation therapy, chemotherapy, or a combination of both (chemoradiotherapy). Reasons to administer neoadjuvant chemotherapy include to determine which patients will not benefit from surgical resection, increase the numbers of patients who can get an R0 resection, and treat any cancer that has begun to spread.50
Patients with locally advanced pancreatic cancer should have genetic testing done on both healthy and tumor tissue soon after diagnosis to evaluate for both germline (inherited) and somatic (acquired) genetic mutations. Genetic testing can identify mutations such as those in BRCA or PALB2, which are found in about 10% of pancreatic cancers. Of these, about half will be germline and the remainder somatic. Genetic testing is essential since it guides treatment choices. For instance, BRCA-associated cancers have an improved response with platinum-based chemotherapies.50
A meta-analysis that studied the effect of neoadjuvant therapy on survival for patients with pancreatic cancer found a modest improvement in overall survival of about 19 months in the group treated with neoadjuvant therapy compared with about 15 months in the group without. For those who underwent surgery, the improvement in survival was higher, with about 26 months for the neoadjuvant group compared with 15 months for the group without. Although these data suggest some benefit, neoadjuvant therapy can cause complications in some patients. Some may have to delay or even cancel surgery, some tumors may have a decreased response to chemoradiotherapy, and neoadjuvant therapy may cause fibrosis.4
Adjuvant Therapy
Adjuvant therapy is additional treatment given after surgical resection and includes systemic chemotherapy. Due to the poor prognosis of patients with pancreatic cancer even after neoadjuvant therapy and surgery, there is an urgent need to improve systemic therapy for these patients.49 In fact, most research of systemic therapy for resectable and borderline resectable cases has been done on patients who had surgery. Data support the use of adjuvant chemotherapy to improve long-term outcomes in these patients.43
The main treatment for locally advanced and metastatic disease are a combination of FOLFIRINOX (5-fluorouracil [5-FU], folinic acid [leucovorin], irinotecan, and oxaliplatin) or gemcitabine plus nab-paclitaxel (Abraxane).43 Data from the landmark PRODIGE-24 trial in 2018 supported use of six months of adjuvant modified FOLFIRINOX (mFOLFIRINOX) for patients with a good performance status after surgical resection.43,51 Median disease-free survival was 21.6 months in the mFOLFIRINOX group versus 12.8 months in the gemcitabine group, and median overall survival was 54.4 months versus 35 months in the mFOLFIRINOX and gemcitabine groups, respectively. A downside of the treatment, however, was a higher risk of severe and potentially life-threatening side effects, including gastrointestinal symptoms, peripheral neuropathy, and an increase in the gamma-glutamyl transferase (GGT) enzyme level, among others. Although side effects of treatment with mFOLFIRINOX were less favorable than that of gemcitabine, they were manageable, and the significantly improved outcomes with mFOLFIRINOX still supported its use over gemcitabine.51
Second-Line and Palliative Therapy
Systemic chemotherapy for patients with metastatic pancreatic cancer is generally palliative to relieve symptoms, not curative; however, there can be improved survival compared with supportive care alone. A meta-analysis of trials between 1974 and 2001 examined chemotherapy versus supportive care alone for patients with advanced disease; it found an improvement in one-year mortality in the patients that received chemotherapy.52 Gemcitabine has been the palliative therapy of choice since the 1990s, though multiple other therapies have been studied.14 More recently, studies have shown an improvement in survival when palliative therapy with a combination of drugs is used instead of gemcitabine alone.52
There is limited evidence to support the use of second-line chemotherapy over supportive care alone for patients with locally advanced or metastatic pancreatic cancer whose cancers have progressed despite initial adjuvant chemotherapy. Decisions regarding second-line chemotherapy should be individualized and dependent upon patient preferences, any clinical factors that affect prognosis, and presence or absence of germline or somatic mutations. Some patients with BRCA- or PALB2-associated pancreatic cancer could potentially benefit from a PARP inhibitor as second-line therapy. Pembrolizumab (Keytruda) is approved for pancreatic cancer with deficient mismatch repair (dMMR) or high levels of microsatellite instability (MSI-H) that has progressed following treatment.53
Patients who progressed after initial treatment and who do not have a known genetic mutation have few options for second-line therapy. It is generally recommended that these patients, when eligible, be enrolled in clinical trials. Treatment choice is dependent upon the regimen used for first-line treatment as well as patient’s performance status and bilirubin level.53
Maintenance Therapy
Maintenance therapy for selected patients with pancreatic cancer is becoming standard practice. Maintenance therapy is given after initial therapy to prevent relapse for those who went into remission, slow cancer growth, or potentially reduce its size. A clinical trial showed moderately improved progression-free survival for patients with metastatic pancreatic cancer who received four months of FOLFIRINOX followed by maintenance therapy with 5-FU and folinic acid, compared with patients who received six months of FOLFIRINOX alone or alternating gemcitabine and FOLFIRI (folinic acid, 5-FU, and irinotecan) every two months. However, the maintenance therapy group experienced higher rates of severe neurotoxicity.54,55
The drug olaparib (Lynparza) given by mouth is a viable option for maintenance therapy in patients with BRCA-associated metastatic pancreatic cancer; olaparib was FDA approved for maintenance therapy in 2019.54 Patients with germline BRCA or PALB2 mutations will likely benefit from stopping chemotherapy and starting maintenance therapy using olaparib after at least 16 weeks of platinum-based chemotherapy, assuming there has been no continued growth of the cancer.52
Regardless of decisions regarding systemic chemotherapy, all patients with advanced pancreatic cancer should be offered supportive care, including aggressive pain management and addressing other cancer-associated symptoms.50 A palliative care consultation should be considered early in the treatment process, particularly for patients with advanced disease.52 Patient preferences will guide decisions regarding treatment, and some with locally advanced or metastatic disease may choose pain relief and supportive care alone.
7 Novel and Emerging Strategies
Promisingly, 10% of patients with pancreatic cancer now survive for five years compared with 6% in 2014. Unfortunately, a majority (53%) of patients presenting with pancreatic cancer already have metastasis, and only 3% of those with metastatic disease will survive for five years. Improvements in supportive care, procedures like ERCP, treatment of infections, systemic and local therapies, and the delivery of treatments have all improved survival. Identification of germline and somatic mutations, and improved knowledge about the tumor microenvironment and role of the immune system in tumor growth and development have also played roles.54 Management of pancreatic cancer continues to evolve through re-examination of established treatments and investigation of novel and emerging strategies.
Improved Surgical Margin Analysis
As mentioned, the goal of surgical resection of pancreatic cancer is removing the entire tumor and having negative margins—in other words, achieving an R0 resection. This can be difficult because surgeons must rely on visually inspecting and feeling the area in and around the pancreas with their hands to determine where to resect. Cancer cannot be easily distinguished from areas of inflammation or fibrosis in this manner. To date, evaluation of surgical margins for cancer cells has required excision of tissue followed by laboratory processing and examination by a pathologist. A promising diagnostic tool called full-field optical coherence tomography (FF-OCT) is being developed that potentially allows for assessment of margins during surgery without the need for the laboratory.56
FOLFIRINOX Regimens
Although novel treatments are a major focus in current research, there has been continued study of how well different combinations of established chemotherapy drugs work. The mFOLFIRINOX regimen was investigated in metastatic pancreatic cancer that had not previously been treated and was found to provide a survival benefit. In another study, patients with cancer that did not respond to initial treatment with gemcitabine plus nab-paclitaxel who were then treated with mFOLFIRINOX had survival of 13 months. Guidelines now recommend that mFOLFIRINOX be used as a second-line treatment for patients whose disease has progressed after initial treatment with gemcitabine plus nab-paclitaxel.54 In a promising phase 2 clinical trial in patients with locally advanced pancreatic cancer,57 the combination of FOLFIRINOX and losartan (Cozaar) (an angiotensin II receptor blocker) followed by chemoradiotherapy successfully downstaged patients, achieving an R0 resection in 69%.57
Tumor Microenvironment Analysis
Understanding the microenvironment of pancreatic cancer has the potential to lead to new treatment approaches. The characteristics of the tissue and microenvironment surrounding the tumor influence delivery of therapeutic drugs to cancer cells.54 Moreover, the components of the immune system within and surrounding the tumor have a significant impact on tumor growth and development. The local immune activity is typically suppressed by pancreatic cancer tumors.58,59 Subtypes of pancreatic cancer have been recognized, with some that produce an immune response associated with better prognosis.49
Several types of inflammatory cells in the tumor microenvironment could potentially be targeted to boost the immune response.54 Switching the tumor microenvironment from immune-suppressed (“cold”) towards inflammation (“warmed-up”) is a leading approach in clinical oncology (see “Oncolytic Viruses” section below). One method of warming-up the tumor microenvironment is with the rodent H-1 protoparvovirus (H-1PV). The virus is capable of stimulating the immune system via infection-associated events, for instance by release of inflammatory mediators by infected tumor cells.58
Improved Immunotherapies
Early immunotherapy treatments like interleukins and interferon had disappointing results, but checkpoint inhibitors renewed interest in this treatment area. These drugs target immune checkpoints, which are ligand-receptor interactions that can either boost or decrease an immune response. Inhibitory receptors on activated T cells are checkpoint targets for some solid tumors, and inhibition can boost the immune response of these cells against the tumor. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) are two of these inhibitory receptors.49
Monoclonal antibodies that block PD-1 have led to improved long-term survival in many patients with metastatic melanoma and responses in patients with non-small cell lung cancer (NSCLC) who had progressed despite being treated with platinum-based chemotherapy. One PD-1 inhibitor, pembrolizumab, was licensed by the FDA for first-line combination treatment of metastatic NSCLC and for any solid tumors that are MSI-H or dMMR and that have progressed following prior treatment. Although this represents only a small percentage of cases of pancreatic cancer, altering pancreatic cancer tumors to make them more likely to respond to immune checkpoint inhibitors is being explored. A potential method to accomplish this is through immunogenic tumor priming, in which a portion of the tumor is destroyed, for example by stereotactic radiotherapy, in order to increase the amount of antigen being presented to the immune system. Combining checkpoint inhibitors with other therapies, including standard chemotherapy, is also a possibility.49
Advances in technology in the areas of antibodies, small molecules, vaccines, gene therapy, and engineered T cells—and in the understanding of T-cell biology—have boosted development of vaccines and other cellular therapies like chimeric antigen receptor-expressing T-cells (CAR-Ts).60 It has been found that some cytotoxic drugs cause immunogenic cell death with release of tumor antigens, which is a promising area for anti-cancer vaccine development.49 Another approach, utilizing ex vivo stimulation of dendritic cells with various tumor-associated antigens, is being explored as well. For instance, a 2020 study reported the safety and feasibility of a novel cancer vaccine in which dendritic cells were stimulated with antigens commonly found in pancreatic cancer tissue (WT1 and MUC1).61 Larger randomized controlled trials are needed to assess the clinical efficacy of these novel vaccine strategies.
Several small and early-stage clinical trials have recently been completed or are in progress using vaccine therapies in pancreatic cancer with novel targets. Additionally, adoptive cell therapies using administration of tumor-infiltrating lymphocytes or involving T-cell receptor or CAR-T therapies have been used successfully in other cancers and are under development for treatment of pancreatic cancer. Growing understanding of the role of the immune system, and particularly of T cells in pancreatic cancer development and growth, is expected to continue to provide new opportunities for therapies for this aggressive disease.60
Preserving Postoperative Immune Function with Interleukin-2
One reason pancreatic cancer is challenging to treat is that pancreatic tumors directly influence the milieu of cell-signaling molecules that govern immune activity in the body, resulting in dramatic suppression of anti-tumor immunity and unimpeded growth of malignant cells.62
Immunotherapy that enlists the anti-tumor activity of cytotoxic T cells and limits suppression of immunity by regulatory T cells is an emerging area of anti-cancer research. Various types of immunotherapy have shown promising effects in multiple types of cancer, but pancreatic cancer has proven to be relatively resistant to these approaches.62 Nevertheless, researchers continue to explore a promising immune-boosting cytokine called interleukin-2 (IL-2) as a possible neoadjuvant therapy in pancreatic cancer patients. IL-2 is naturally produced in the body and one of its main roles is to promote proliferation of immune cells involved in anti-cancer immunity, namely cytotoxic T cells and natural killer (NK) cells.63
IL-2 is an FDA-approved treatment for metastatic melanoma and renal cell cancer; however, its usefulness in these and other cancer types is limited by its short half-life and toxic side effects at higher doses. In addition, IL-2 preferentially binds to receptors on regulatory T cells, promoting further immune suppression.63,64 Certain IL-2 receptors have even been found be highly expressed on pancreatic cancer cells and may facilitate tumor growth and metastasis, adding complexity to the use of IL-2 in pancreatic cancer treatment.65
Clinical evidence suggests IL-2 may be particularly helpful when used prior to surgery for pancreatic cancer by effectively mitigating the decline in immune function that often compromises outcomes. In a controlled trial involving 19 pancreatic cancer patients scheduled for surgery, those who received IL-2 (9 million IU) for three days preceding surgery had a 2-year survival rate of 33%, while those who underwent the surgery without receiving IL-2 had a 2-year survival rate of 10%. Moreover, postoperative complications occurred more frequently in those who did not receive IL-2.66 In another trial, 30 pancreatic cancer patients were randomized to surgery alone or three days of IL-2 (12 million IU) prior to surgery. Following surgery, T-cell counts were diminished in the surgery-only group but rose significantly in the IL-2 group. After a 3-year follow-up period, both progression-free and overall survival were significantly higher in the IL-2-treated patients.67 In a trial in 31 pancreatic cancer patients undergoing surgery, post-surgery T-cells numbers were better preserved in those who received 12 million IU of IL-2 prior to surgery compared with those who received 9 million IU and those who received surgery only.68 However, in a pilot trial in 13 participants with advanced and previously treated liver or pancreatic cancer, IL-2 in conjunction with chemotherapy did not impact survival.69
Some emerging technologies have been shown to improve the effectiveness of IL-2 against solid tumors, including pancreatic cancer, in preclinical studies. These include63,64,70,71:
- IL-2 mutants. Synthetic IL-2 analogs have been developed that have a longer half-life and bind preferentially to receptors on cytotoxic T cells and NK cells, thereby enhancing promotion of anti-cancer immune activity without over-activating immunosuppressive regulatory T cells.64,70
- Immunocytokines. Immunocytokines are complexes made of natural or synthetic IL-2 bound to antibodies targeting tumor proteins. This allows the IL-2 to target its effects more directly to tumor tissue, possibly reducing adverse side effects.64,70,71
- Adoptive immunotherapy. Exposing specialized tumor-infiltrating T cells, which are retrieved directly from tumor tissue, to IL-2 in the laboratory promotes their activation and expansion. IL-2-treated tumor-infiltrating cells can then be intravenously infused back into the body through a process called adoptive immunotherapy.64,72 Another type of adoptive immunotherapy involves the generation of so-called lymphokine-activated killer cells by exposing immune cells retrieved from the blood to IL-2 and other agents in the laboratory, increasing their anti-cancer activity and promoting their expansion. Lymphokine-activated killer cells can then be infused back into the body as part of cancer treatment. Systemic treatment with IL-2 may be needed for adoptive immunotherapy to be effective.63
- Viral vector. Viruses that have been genetically manipulated can be used to deliver genetic instructions for IL-2 production and can be administered directly into pancreatic tumors. Adenovirus vector delivery, both alone and in conjunction with adoptive immunotherapy, has been found to improve outcomes in animal models of pancreatic cancer.73-75
An observational study included survival data from 86 patients with incurable metastatic pancreatic cancer who were assigned to be treated with a novel adoptive immunotherapy. In this type of therapy, an osteoporosis drug called zoledronate was used along with IL-2 to produce activated killer cells from immune cells retrieved from the participants’ blood. These activated killer cells were returned to the participants up to a maximum of 30 times. Survival was found to be prolonged in those who received the immunotherapy five or more times.76
Oncolytic Viruses
Viruses with natural or engineered cancer-destroying ability (oncolytic viruses) are being utilized as cancer therapeutics with multimodal anti-tumor action. H-1PV, in particular, has a broad range of tumor-suppressive properties, and some approaches using this oncolytic virus have been proven in preclinical and/or clinical studies. One area of study takes advantage of the fact that H-1PV and other parvoviruses induce immunogenic cell death and act as potent triggers for stimulation of an immune response, both directly and indirectly.58
Besides inducing selective tumor cell death, these viruses can potentially reverse the immune suppression found in the tumor, leading to an inflammatory microenvironment. For example, infection of pancreatic tumor cells by H-1PV was shown to stimulate NK cell tumor-killing capacity. H-1PV can also infect immune cells, providing direct immune cell stimulation and potentially exerting multiple immune-stimulating effects within the tumor microenvironment. H-1PV may also impact the microvasculature of the tumor, utilizing endothelial cells as a direct target. H-1PV’s potential for causing a pro-inflammatory microenvironment and directing attention of the body’s immune system to the tumor creates openings for combining the virus with other immune modulators or therapeutic agents. Sensitivity of pancreatic cancer cells to H-1PV-induced oncolysis has been demonstrated in preclinical models. The synergistic effects of gemcitabine and H-1PV have been studied, and administration of H-1PV to animals pre-treated with gemcitabine resulted in a significant prolongation in survival compared with controls treated with gemcitabine alone. In conjunction with other data, H-1PV and gemcitabine appear to complement one another in the induction of immunogenic signals. Besides gemcitabine, H-1PV has also been studied in combination with the histone deacetylase inhibitor valproic acid and separately with the pro-inflammatory cytokine interferon-gamma, among other potential treatments. Overall, use of H-1PV appears to be safe and well-tolerated, but its use is potentially limited by variable patient-dependent factors that can lead to suboptimal treatment results.58
As previously described, the dense tissue in and around the tumor can impair the ability of drugs to reach cancer cells. Recent research has found that vitamin D has the ability to modify this tissue to make it more receptive to delivery of cytotoxic chemotherapy. This offers a potential avenue for increased efficacy of oncolytic virotherapy through combination with vitamin D in the treatment of pancreatic cancer.77
Precision Oncology and Personalized Medicine
Precision oncology is another promising avenue of research, in which potential treatment targets are the genetic mutations and altered molecular pathways seen in the tumors. Genetic profiling is done with the specific mutations identified using next-generation sequencing (NGS) and a treatment is used to selectively target the cancer cells that contain that mutation.78
Given the different genes involved in pancreatic cancer growth and development, there are different approaches for this kind of precision therapy. One approach involves targeting dysregulated oncogenes or their pathways for selective inhibition. Another is to reactivate tumor suppressor genes that have been shut down by mutations. Finally, genes that maintain stability and functioning of normal chromosomes that have been altered by mutations may be targeted for repair.78
Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 (CRISPR/Cas9) has emerged as a powerful gene-editing tool that is driving progress in precision oncology. It is being widely used in research on pancreatic cancer to knock out genes involved in disease progression. There are several current developments in pancreatic cancer gene therapy, including gene-based tumor cell sensitization to chemotherapy, vaccination, and adoptive immunotherapy, but recently CRISPR/Cas9 has been used in several pancreatic cancer studies due to its safety and efficacy. This technology could potentially be used ex vivo, in which cells are isolated and modified outside the body, or in vivo, in which genetic materials are injected directly into the body.
One promising target of gene therapy is the oncogene KRAS, which is mutated in 90‒94% of cases of pancreatic cancer.54 KRAS downstream signaling drives tumor growth in pancreatic cancer and is thought to involve three major pathways: Raf/MEK/ERK, PI3K/PDK1/Akt, and the Ral guanine nucleotide exchange factor pathway.79 Several agents targeting KRAS downstream pathways have been tested alone or in combination with standard cytotoxic therapies and epidermal growth factor receptor inhibitors in advanced pancreatic cancer, though to date there are no positive trials.54 Besides pharmaceutical agents, KRAS downstream signaling pathways are also potential targets for nutrients and other adjuvant cancer therapies.
Immunonutrition
Immunonutrition consists of the feeding of certain nutritional substances known as pharmaconutrients to combat the cachexia that typically accompanies advanced cancer and possibly favorably modulate immune activity. Feeding may be done via the GI system (enterally) or given through a vein (parenterally). These regimens often include omega-3 fatty acids (discussed in “Nutrients”); RNA, and other substances may also be included.80
A systematic review and meta-analysis of immunonutrition and pancreatic cancer found that the intervention significantly decreased the risk of complications from infection as well as the length of hospital stay. Use of immunonutrition, particularly immediately prior to surgery, can improve outcomes in patients with pancreatic cancer.80 More information about the role of nutrition in combatting cachexia is available in Life Extension’s Cachexia protocol.
Radiotherapy
In pancreatic cancer treatment, radiotherapy may be used as part of neoadjuvant therapy, definitive therapy (in other words, replacing surgical resection), or adjuvant therapy. Long-course conventionally fractionated radiotherapy and stereotactic body radiotherapy approaches can be integrated with systemic chemotherapy for pancreatic cancer that has not spread. New technologies and treatment modalities are revolutionizing the field of modern radiotherapy. Two emerging technologies are particle therapy and magnetic resonance-guided radiotherapy. Stereotactic body radiotherapy is a promising technical delivery application, while simultaneous integrated boost intensity-modulation radiotherapy holds promise as a dose delivery technique. Combining radiotherapy with immunotherapy is an opportunity for multimodal integration.7
Intravenous Vitamin C
Vitamin C (ascorbic acid) is needed for synthesis of collagen, which is a protein present throughout the body, particularly in the skin, bones, and soft tissues. In addition to its role in biosynthesis, vitamin C functions as an antioxidant at low concentrations such as those achievable with oral dosing, but functions as a pro-oxidant at doses achievable with intravenous dosing. Vitamin C cannot be made endogenously by the body and must be ingested in the diet. Dietary sources include citrus fruits, red and green peppers, kiwifruit, and other fruits and vegetables like broccoli, strawberries, cantaloupe, baked potatoes, and tomatoes.81
Vitamin C can be administered in a solution intravenously. An advantage of this route is that concentrations attained with parenteral administration are significantly higher than when ingested.82 Oral intake of liposomal formats of vitamin C may be another way to achieve supraphysiological levels.83,84 An anti-cancer role for intravenous vitamin C (IVC) has been hotly debated, with data from animal models supporting an anti-tumor role85; however, only preliminary human clinical evidence is available as of early 2021.
In a preliminary phase 1 trial, IVC administered during daily radiotherapy treatments to 14 patients with pancreatic cancer improved overall and progression-free survival relative to institutional and historical controls. Importantly, this study was not statistically powered to detect differences in survival outcomes, but the preliminary results were nonetheless intriguing. Subjects received 50–100 grams IVC according to tolerability, five days weekly during radiation treatments. The subjects were also concomitantly treated with gemcitabine once weekly. The study began enrolling participants in early 2014, and at the time the peer-reviewed paper reporting the results was submitted for publication, in June 2018, five of the 14 patients that received IVC remained alive, three of whom were without evidence of disease recurrence. On the basis of these results, the investigators recommended a phase 2 trial of 75 grams IVC in pancreatic cancer patients.86
A smaller, earlier phase 1 trial published in 2013 found similar results. In this study, nine subjects with stage 4 pancreatic cancer received 15–125 grams IVC twice weekly during concurrent gemcitabine treatment. The IVC was generally well-tolerated, and the trial showed suggestions of survival improvements. Again, however, this trial was too small and preliminary to properly detect meaningful differences in survival outcomes. It nevertheless lends additional support to the notion that further clinical study of IVC in advanced pancreatic cancer is warranted.87
A case report was published on the use of intravenous pharmacologic ascorbic acid (PAA) in a patient with stage 4 pancreatic cancer. The vitamin was given through a port-a-cath in doses of 75‒125 grams per infusion, administered 2–3 times per week. Despite the patient declining systemic chemotherapy and PAA being used alone, the patient had regression of his disease and survived for nearly four years after diagnosis.82
The mechanism of action for the anti-tumor benefit of vitamin C is still unclear but is believed to be due to production of hydrogen peroxide by high-dose IVC, which generates oxidative stress that targets cancer cells.88,89 A preliminary trial published in 2017 by researchers at the University of Kansas suggested that IVC preferentially depletes NAD+ levels in cancer cells. This study also showed IVC did not interfere with the pharmacokinetics of gemcitabine. In this research, 12 pancreatic cancer patients received 75–100 grams IVC three times weekly during concomitant gemcitabine treatment.90
There is sufficient evidence to support continued investigation of the role of IVC in pancreatic cancer treatment, and clinical trials are underway to identify which cancer patients may benefit from vitamin C administration.
Repurposed Drugs
Besides standard chemotherapeutic drugs and the innovative technologies that have been described, some drugs are available that, while not indicated for cancer treatment, potentially have therapeutic properties for cancer patients. For instance, statins are used to treat hypercholesterolemia but also inhibit some pathways that influence cancer cell growth, protein synthesis, and cell cycle progression. Simvastatin (Zocor) has been studied for its potential association with pancreatic cancer, with several retrospective studies indicating it may be associated with reduced mortality, particularly for patients with localized disease.88 A case-control study of 408 pancreatic cancer patients and 816 matched controls found that people who had ever used statins for at least three months had 39% lower odds of pancreatic cancer compared with people who had never used statins.91 A meta-analysis that assessed data from 26 studies including over three million participants with 170,000 cases of pancreatic cancer found that statin use was associated with a 16% reduced relative risk of pancreatic cancer.92
One study showed simvastatin use was associated with improved survival in patients with resectable pancreatic cancer.88,93 Another retrospective study in patients with unresectable pancreatic cancer suggested simvastatin combined with erlotinib (Tarceva) and gemcitabine was linked to improved survival of about eight months compared with about four months in the control group.88,94 In one large study of over 12,000 older patients with pancreatic cancer, those who started statins after their diagnosis had a 31% improved overall survival, and median survival was 10.93 months for postdiagnosis statin initiation versus 4.07 months for pre-diagnosis statin initiation.95 In another study that evaluated the medical records of almost a half million veterans, statin use of six months or longer was associated with a 67% lower risk of developing pancreatic cancer. And statin use for more than four years correlated with a reduction in risk up to 80%.96
Unfortunately, a phase 2, randomized, placebo-controlled trial with simvastatin and gemcitabine in patients with metastatic pancreatic cancer did not show any significant difference in the amount of time the cancer took to progress compared to control treatment with gemcitabine alone.88 Further randomized controlled trials are required to determine the potential role of statins in pancreatic cancer treatment. Several such trials are planned or underway.
Another drug, metformin, also appears to have an association with improved survival for pancreatic cancer in cohort groups studied but has limited trial data available.97 In a study of records of patients with pancreatic cancer and diabetes treated at the University of Texas MD Anderson Cancer Center, the two-year survival rate in those taking metformin was nearly twice that of patients not taking metformin.98 A large 2018 meta-analysis including nearly 4,300 diabetic pancreatic cancer patients, over 2,000 of whom had received metformin, found metformin use in diabetics with pancreatic cancer was associated with a 19% reduced overall mortality risk compared with those who did not use metformin.99
However, some researchers have raised concerns with the methodology of some of the studies reporting links between metformin use and survival in pancreatic cancer, suggesting some of the apparent benefit may have been due to immortal time bias. Thus, more studies are needed.100
Another group of non-cancer drugs that have been considered for use in pancreatic cancer is antihypertensives. Among these, angiotensin receptor blockers (ARBs) have recently been associated with improved survival for patients with pancreatic cancer who are having surgical resection, and their chronic use is independently associated with improved survival, independent of chemotherapy, for those with non-metastatic cancer.101,102
Non-selective beta-blockers have been found to reduce progression of pancreatic cancer that has not metastasized. This finding was seen in two independent studies and confirmed in animal models.9 Another study that analyzed data from the Surveillance, Epidemiology, and End Results (SEER) registry-Medicare linked database found that continuous beta-blocker use before and after pancreatic cancer diagnosis improved survival while use only before diagnosis did not.103
A meta-analysis of studies through 2019 confirmed an inverse association between regular aspirin use and risk of colorectal cancer and other gastrointestinal tract cancers. For pancreatic cancer, regular aspirin use was associated with a reduced risk. For all cancers, aspirin had an improved benefit the longer it was used.104 Aspirin’s role in reducing risk or preventing cancer might be due to its involvement in the breakdown of clots and blood clotting as an anti-thrombotic agent. Although some studies have been mixed, studies that have shown a protective effect for pancreatic cancer risk indicate that long-term aspirin use was associated with reduced risk, and higher doses might be associated with reduced risk, especially for Americans.105
More Information about repurposing drugs for use in the context of cancer in general is available at Life Extension’s Drug Repurposing in Cancer protocol.
Mistletoe
Mistletoe (Viscum album, Viscum coloratum) is a plant with a history of use for various medicinal purposes, and mistletoe extract is available as a subcutaneous injection. The extract is used by some cancer patients to improve quality of life and potentially offer a survival benefit, although its use is controversial and a 2019 review of studies of mistletoe in the context of cancer concluded that evidence generally did not support a survival benefit or quality of life improvement.106,107
Nevertheless, the efficacy of mistletoe as a complement to standard treatment for advanced pancreatic cancer is being studied in a phase 3, randomized, placebo-controlled trial; the trial is estimated to be completed in mid-2022.108 A case report was published in 2019 of a patient with advanced pancreatic cancer who received Viscum album extract (Iscador Qu 10 mg, three injections per week) as adjunctive treatment. The patient’s cancer regressed and he was able to remain recurrence-free for at least 39 months.109
8 Prevention
Several dietary and lifestyle factors may influence pancreatic cancer risk and represent modifiable variables with potential to reduce risk. The effects of diet on cancer in general as well as for pancreatic cancer, specifically, have been extensively studied.
Dietary Considerations
Carbohydrates. Diets low in simple and low-fiber carbohydrates are used for weight reduction and can help improve blood sugar regulation, but until recently their effect on the risk of pancreatic cancer was unknown. The effect of carbohydrate consumption on cancer risk was investigated in a study involving nearly 100,000 individuals over about nine years. Scores were obtained to evaluate individuals’ compliance with the low-carbohydrate dietary restrictions. At the end of the study period, a total of 351 cases of pancreatic cancer were identified. The quartile of study patients with the lowest carbohydrate diet had the lowest risk. This finding was more pronounced in individuals 65 years or older. These data support that a low-carbohydrate diet may be associated with a reduced risk of pancreatic cancer in the US population.110
An investigation of cancer risk depending on type of carbohydrates consumed was done in a systematic review of meta-analyses. Eating whole grains was consistently associated with a lower risk of pancreatic cancer, as well as other cancers. The specific effect of refined grains on cancer risk was less clear due to limitations of the studies, although high intake was associated with an increased risk of colon and gastric cancer.111 Sufficient data exists to support an increase in dietary consumption of whole grains for cancer prevention.
Related to carbohydrate consumption is the glycemic index (GI) of foods (ie, their capacity to stimulate the body to release insulin). A term related to GI is “glycemic load" (GL), which takes into account the serving size (dosage) of the food. It was hypothesized that, due to their link with diabetes and obesity, diets composed of high-GI foods might increase the risk of pancreatic cancer. Meta-analyses of dietary GI and risk of cancer so far have produced mixed but promising results.112
Red meat and processed meats. Another dietary factor studied in cancer prevention is red meat and processed meats. In a meta-analysis of 11 case-control studies, greater red meat consumption was associated with a 48% increased risk of pancreatic cancer.113 The effect of meat consumption on acidity has also been explored. The human body has a stable pH around 7.4, while tumor microenvironments are known to be acidic. Along with high fruit and vegetable consumption, low meat and dairy intake along with increased consumption of fruits and vegetables may significantly alkalinize the urine pH.112 A retrospective study in Japanese patients investigated an alkalinizing diet (high fruit and vegetable intake with no meat or dairy) with chemotherapy (including gemcitabine) on advanced or recurrent pancreatic cancer. The study participants also received intravenous vitamin C and were given oral bicarbonate by request or if urinary pH did not increase above 7.0 when following the diet. Urinary pH was significantly increased by following the diet, and the median survival from the time of starting the diet was significantly longer for patients with a urine pH of 7.0 or higher (about 16 months) compared to those with a pH less than 7.0 (4.7 months).112,114
Healthy fats. Oily fish are excellent sources of omega-3 fatty acids, which are anti-inflammatory, anti-proliferative, and anti-metastatic. Consuming omega-3 fish oils prolonged the survival of pancreatic cancer patients with unresectable tumors, by 37% in some, in an analysis of clinical data. Ingestion of omega-3 fatty acids can also improve quality of life of pancreatic cancer patients by stabilizing weight, reducing cachexia, and improving performance following surgery.112
Nuts. A study of over 75,000 women found that frequent nut consumption, specifically at least a 28 grams (1 oz) serving ≥2 times per week, was associated with a 35% reduced risk of pancreatic cancer when compared with women who generally abstained from eating nuts.115 A systematic review and meta-analysis investigating nut consumption and cancer in general concluded that nuts may reduce cancer risk, but more studies are needed.116
Cruciferous vegetables. Cruciferous vegetables, such as broccoli, Brussels sprouts, and cabbage, contain the beneficial phytonutrients sulforaphane and indole-3-carbinol, which converts to 3,3’-diindolymethane. Reviews and meta-analyses of observational and epidemiological evidence found that greater intake of cruciferous vegetables was associated with lower pancreatic cancer risk. Moreover, there is some limited evidence that greater sulforaphane intake (as freeze-dried broccoli sprouts) may benefit pancreatic cancer patients undergoing palliative chemotherapy.117-119
Fasting and ketogenic diets. Several studies have evaluated the effects of fasting and ketogenic diets during chemotherapy. Although cancer can cause cachexia, plus one of the side effects of chemotherapy is nausea and vomiting, both of these interventions are well-tolerated and feasible during chemotherapy. There is some support that fasting and ketogenic diets may lead to improvements in quality of life and fatigue, particularly in the first eight days after starting chemotherapy.120 (See also the sidebar titled “Mitigating Chemotherapy Side Effects with Intermittent Fasting” in Life Extension’s Chemotherapy protocol.) However, ketogenic diets further acidify urine, and the possible implications of this long term (vs. following an alkalinizing diet) should be considered.
Exercise
Exercise is known to improve quality of life and can play a role in post-surgical rehabilitation. Its efficacy and safety for patients with pancreatic cancer were investigated in a review of studies published through July 2020. Exercise was associated with improvements in muscle strength, functional capacity, body composition, fatigue, and quality of life.121
9 Nutrients
Several nutritional agents can play roles in complementary and adjunctive therapy for pancreatic cancer. Because of the poor prognosis of pancreatic cancer, diagnosed patients often turn to nutritional and integrative adjuvant therapies. Although the strength of the evidence varies depending on the agent, and the underlying mechanisms and effects may differ for each, there is some degree of association with pancreatic cancer for all agents described in this section.
In addition, research supported by Life Extension in collaboration with City of Hope, a comprehensive cancer center in California, showed that an integrative treatment regimen consisting of multiple nutrients and natural agents was generally well-tolerated and feasible in patients with pancreatic cancer undergoing chemotherapy with gemcitabine and nab-paclitaxel.122
Omega-3 Fatty Acids
Omega-3 fatty acids, essential components of cell membranes, can affect the function of receptors on cells, and are involved in formation of hormones, contraction of blood vessels, and modulating signaling through inflammatory pathways, including the NF-κB pathway.123 There are three primary omega-3 fatty acids: eicosapentaenoic acid (EPA), alpha-linolenic acid, and docosahexaenoic acid (DHA). As a supplement, omega-3 fatty acids have been extensively studied in cancer, including in pancreatic cancer, with evidence supporting that consumption improves survival in patients with unresectable pancreatic cancer and that omega-3 use can ameliorate some symptoms associated with the cancer.112 One mechanism recently identified by which the omega-3 fatty acid DHA may contribute to improved outcomes in cancer treatment is by triggering tumor cell death when it becomes peroxidized in the acidic tumor microenvironment.124
A meta-analysis that included 11 randomized controlled trials found that use of omega-3 enriched nutritional supplementation was associated with improved overall survival in people with advanced pancreatic cancer. Overall survival ranged from 130–259 days among patients receiving omega-3-enriched nutritional supplementation and from 63–130 days among those who received conventional nutritional support. This analysis also found that omega-3-enriched nutritional regimens were associated with increases in bodyweight and lean body mass compared with standard nutrition support. In the studies included in the analysis, omega-3 supplemental nutrition regimens typically consisted of EPA alone or in combination with DHA. The dosages ranged from 1– 2.2 grams daily in most of the studies, and one study used 6 grams EPA per day.125
The use of high-dose intravenous omega-3 fatty acids in conjunction with chemotherapy (gemcitabine) was studied in a phase 2 trial of patients with unresectable pancreatic cancer. Eighteen subjects received the omega-3 infusions, containing 10 grams of omega-3s (including 4.3–8.6 grams EPA and DHA) while nine served as controls and received standard of care. The trial found that the combination of intravenous omega-3s and gemcitabine resulted in significant changes in certain inflammatory cells, which was postulated to be due to a reduction in pro-inflammatory mediators. Moreover, median progression-free survival was 5.65 months in the omega-3 group compared with 1.8 months in the control group. There was no difference in overall survival; however, the trial was not statistically powered to detect survival differences, so these results should be interpreted cautiously.126
Other small, early-stage clinical studies have found that various omega-3 preparations improved several aspects of nutritional status and quality of life in subjects with pancreatic cancer.127-129 Overall, the available evidence is compelling enough to warrant evaluation of omega-3 preparations, both oral and intravenous, in larger, more rigorous trials in pancreatic cancer patients.
Vitamin D
A major function of vitamin D is to aid in absorption of calcium, and it also has roles in regulation of cell proliferation and differentiation and immune function.130 Vitamin D can be made by the body from ultraviolet light exposure, but the amount produced is limited and dependent upon both individual and environmental factors. Plasma 25-hydroxyvitamin D [25(OH)D] is the precursor of the active form of vitamin D and is used as a biomarker to assess levels of vitamin D.131 Most dietary vitamin D comes from consumption of fortified foods, particularly milk and cereals, since the vitamin occurs in very few non-processed foods. Natural sources of vitamin D include fatty fish (including fish liver oils), beef liver, cheese, egg yolks, and mushrooms.130
Several observational and epidemiological studies have shown that higher vitamin D intake is linked with lower pancreatic cancer risk. Moreover, vitamin D deficiency appears to occur frequently in people with pancreatic cancer, and higher blood levels of 25(OH)D have been associated with longer survival. However, not all studies have corroborated these findings. Overall, data regarding the role of vitamin D levels and intake in pancreatic risk and treatment is mixed.132
An analysis of data from prospective US cohort studies evaluated prediagnostic 25(OH)D levels and survival time in 493 pancreatic cancer patients. The researchers categorized patients according their prediagnostic 25(OH)D level as insufficient (<20 ng/mL), relative insufficiency (20 to < 30 ng/mL), and sufficient (≥30 ng/mL). Survival was greater among patients with sufficient 25(OH)D levels compared to those with insufficient levels. The trend for lower risk of death with increasing 25(OH)D was statistically significant. However, a major limitation of this study was that the prediagnostic 25(OH)D levels were assessed a median of 6.7 years before diagnosis.133
One study reported that in patients with stage 1 or 2 pancreatic cancer who underwent surgical resection, those who had 25(OH)D levels >20 ng/mL had improved survival compared with similarly-staged patients who had vitamin D deficiency. This association was not seen for patients with advanced pancreatic cancer. Furthermore, the patients with vitamin D deficiency also had elevated inflammatory biomarkers.134
A meta-analysis evaluating 12 studies aimed at investigating the potential role of vitamin D in pancreatic cancer risk and mortality concluded that higher plasma 25(OH)D levels were associated with improved survival.131 The meta-analysis did not find an association between vitamin D and pancreatic cancer risk. Another meta-analysis specifically examined this relationship, finding that intake of vitamins, particularly vitamins D and B12, were associated with reduced risk of pancreatic cancer. This analysis included three prospective studies that assessed low-dose vitamin D intake (10 mcg [400 IU] daily). When these three studies were analyzed together, a statistically significant 25% reduction in pancreatic cancer risk was found for vitamin D intake.135
Lipoic Acid
Alpha-lipoic acid (ALA) is a cofactor for mitochondrial enzymes and is involved with cellular glucose uptake. The compound is produced by the body and therefore not required from the diet in healthy individuals. A limited number of studies have investigated ALA’s role in cancer. One notable case report detailed how a patient with advanced pancreatic cancer with spread to the liver was treated with ALA plus low-dose naltrexone.136 The doses and routes of administration were intravenous ALA 300‒600 mg twice weekly and low-dose naltrexone 4.5 mg at bedtime, plus oral ALA 600 mg daily, selenium 200 mcg twice daily, and silymarin 300 mg four times daily. Despite a grim prognosis with little hope for survival, the patient was still alive without progression of his cancer 6.5 years later. The authors of the original case report described three additional cases with similar positive outcomes in response to the ALA/low-dose naltrexone protocol.137 Randomized controlled trials are needed to formally evaluate this approach in the context of pancreatic cancer.
Vitamin E
Vitamin E functions primarily as an antioxidant to protect the body against free radicals. It consists of eight isomers, four tocotrienols and four tocopherols, and can be obtained naturally in the diet from nuts, seeds, sunflower oil, soybeans, avocado, and green leafy vegetables.112 A meta-analysis of 10 studies, including over 250,000 participants and 2,976 pancreatic cancer patients, investigated the role of vitamin E intake and risk of pancreatic cancer. It concluded that a high level of vitamin E intake could be protective for populations at risk of pancreatic cancer.138 Another meta-analysis that included 18 studies found that higher dietary intake of vitamin E, among other nutrients, may be associated with reduced pancreatic cancer risk.139
The role of vitamin E in cancer prevention is unclear, but it has been postulated that it could be due in part to increasing expression of the tumor suppressor p27.140 In vitro and in vivo (mouse) studies have investigated the combination of gamma-tocotrienols with gemcitabine in pancreatic cancer and found an anti-tumor benefit, while a phase 1 trial on pancreatic cancer patients showed that administering vitamin E supplementation (as 200–1,600 mg delta-tocotrienol daily) for two weeks prior to surgery resulted in apoptotic cell death of cancer cells.112
Vitamin A and Carotenoids
Vitamin A is needed to protect fatty tissues and lipid molecules from oxidative damage. It is derived in the diet from ingested carotenoids that can, in turn, be used to generate the active form of vitamin A (retinol).141 Dietary sources are diverse and include carrots, eggs, leafy green vegetables, dried apricots, cantaloupe, bell peppers, fish, liver, and tropical fruits.112
Carotenoids, in addition to serving as vitamin A precursors, have demonstrated anti-cancer properties. A diet high in carotenoids, including α-carotene, β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene, has been correlated with lower risks of several types of cancers, including pancreatic cancer.22,112,142-146 In one study, men with the highest intake of lycopene were 31% less likely than men with the lowest intake to develop pancreatic cancer.145 Similarly, β-carotene and zeaxanthin intakes (highest vs. lowest) have been associated with a reduced risk of 48% and 47%, respectively.144
A meta-analysis of 11 studies that included a total of 2,705 patients was performed to assess the association of dietary vitamin A with risk of pancreatic cancer. The conclusion of the meta-analysis was that there is convincing evidence of risk reduction, although the authors did not state what doses of vitamin A are needed for this effect.147 The potential mechanism of action and cellular effects of vitamin A and carotenoids in pancreatic cancer are multiple and include its involvement in metabolic processes and regulation of growth and differentiation of epithelial, bone, and immune cells.112,148,149
B Vitamins
Several B vitamins play roles in the body as antioxidants, coenzymes in cellular metabolism, and production of energy. They are widely available in foods including fortified cereals. Vitamin B6 (pyridoxine) is found in beans, some meats, bananas, and nuts, and vitamin B12 (cobalamin) is found in animal products including dairy.
A meta-analysis was performed to investigate the roles of vitamin B6 and vitamin B12 levels or dietary intake in the risk of pancreatic cancer. Although no association was found with vitamin B12, there was a significant inverse association of vitamin B6 intake and blood concentration (measuring the active form pyridoxal 5’-phosphate [PLP]) with risk of cancer. For blood concentration, the risk of developing pancreatic cancer decreased by 9% for every 10 nmol/L increase in blood PLP.150 Another meta-analysis found that consumption of 10 mcg per day of vitamin B12 reduced the incidence of pancreatic cancer by 27%. However, the risk reduction observed in this analysis was derived from a single prospective study.135
A case-control study based in San Francisco examined dietary B-vitamin intake in 532 individuals with pancreatic cancer and 1,701 controls. This study found that total folate (vitamin B9) intake was inversely associated with pancreatic cancer risk. However, it also observed a slight increased risk of pancreatic cancer associated with increasing B12 intake from food, which is inconsistent with other findings.151 Other observational and epidemiological studies have observed inverse associations between folate intake and pancreatic cancer risk.117
A study that evaluated several mouse pancreatic tumor models found that alternating treatment with oral nicotinamide (a form of vitamin B3) and intravenous gemcitabine led to improved tumor burden and survival compared with controls.152
Curcumin
Curcumin is a major active constituent in turmeric, which is a spice used throughout Asia that is derived from turmeric root (Curcuma longa). Many studies have investigated a possible anti-cancer role of curcumin. In vitro studies provide evidence that curcumin can increase the sensitivity of pancreatic cancer cells to radiotherapy,153 and inhibit pancreatic cancer cell metastasis and migration.154 The compound has also been shown to boost the sensitivity of pancreatic cancer cells to certain chemotherapy drugs, including gemcitabine,155,156 and inhibit the growth of new blood vessels (angiogenesis), which is involved in tumor growth and spread.157,158
In a prospective phase 2 trial, a proprietary curcumin phospholipid preparation was well tolerated in 44 consecutive patients with locally advanced or metastatic pancreatic cancer. Although preliminary, this trial also provided some evidence of improvements in response rates.159 A 2010 trial in 17 pancreatic cancer patients showed that 8,000 mg of curcumin daily, in conjunction with gemcitabine, was often not well tolerated and led to curcumin discontinuation due to abdominal complaints in 29% of subjects.160 However, two earlier preliminary trials found that 8,000 mg curcumin daily was sufficiently tolerable to warrant further studies. These trials also revealed some evidence that this high-dose curcumin regimen generated biological activity in pancreatic cancer patients as assessed by biomarker analyses. However, there was considerable interpatient variability in plasma curcumin levels among study participants, suggesting that variable curcumin absorption and tissue distribution may be a challenge in this context.161,162
L-carnitine
L-carnitine, which is derived from the amino acid lysine or obtained via nutritional supplementation, has a critical role in transportation of fatty acids into cells to be used for energy, especially in skeletal muscles.163 Because of its role in energy production, a possible role in cachexia and fatigue has been investigated in cancer patients. One study found that supplementation with 1,500 mg L-carnitine daily reduced fatigue in cancer patients undergoing chemotherapy.164 A randomized controlled trial of patients with advanced pancreatic cancer found that oral supplementation with L-carnitine decreased malnutrition, increased body weight, and improved overall body composition. Participants in this study took 4,000 mg L-carnitine or placebo daily for 12 weeks. Among subjects taking L-carnitine, BMI increased 3.4% during the study, while BMI dropped by 1.5% in the placebo group. Quality of life improved in the L-carnitine group as well.165
Magnesium
Magnesium, like zinc, is an essential element that must be obtained either from the diet or in supplemental form. Magnesium functions as a coenzyme for numerous reactions in the body and is needed for energy production. Studies have shown that low magnesium intake may be associated with diabetes risk,166-168 and diabetes is a risk factor for pancreatic cancer.
The role of magnesium intake and incidence of pancreatic cancer was investigated in the longitudinal VITamins and Lifestyle (VITAL) study. This cohort included nearly 67,000 men and women aged 50–76 years at baseline. Participants were followed-up from 2000 to 2008. A dose-dependent relationship was found between magnesium intake and pancreatic cancer risk, with a 24% elevation in incidence of cancer for every 100 mg per day decrease below the recommended daily allowance (RDA) of magnesium intake.
Compared with those who met or exceeded the magnesium RDA in the VITAL study, those whose intake was between 75% and 99% of the RDA had a 42% increased risk of pancreatic cancer, and those with magnesium intakes below 75% of the RDA had a 76% increased risk (see Figure 3). The RDA for magnesium is 420 mg for adult men and 320 mg for adult women.
Importantly, the association between pancreatic cancer risk and magnesium intake in the VITAL study appeared to be limited to study participants taking magnesium supplements. The authors concluded that magnesium may be beneficial for prevention of pancreatic cancer.169
Genistein
Genistein is an isoflavone found in soybeans and soy products as well as red clover. Genistein has several potential mechanisms of action in cancer, including modulating growth factor signaling, promoting apoptosis, and inhibiting survival and proliferation of cancer cells and angiogenesis. Investigations of a role for genistein in pancreatic cancer included a cohort study that measured expression of G protein-coupled estrogen receptor 1 (GPER1) mRNA.112 GPER1 is a gene involved in the increased sensitivity of pancreatic cancer cells to genistein, and higher expression of its mRNA was found to be associated with increased survival in pancreatic cancer patients. However, a 2011 phase 2 study in 20 patients with advanced pancreatic cancer found that the addition of 531 mg of soy isoflavones twice daily, including genistein, to treatment with gemcitabine and erlotinib did not improve survival outcomes.170 A 2016 pharmacology study of a chemically modified form of genistein in pancreatic cancer patients found that this formulation, called AXP107-11, led to a favorable pharmacokinetic profile and did not cause hematological or non-hematological toxicities. The investigators concluded that further studies to assess the efficacy of this modified form of genistein in pancreatic cancer are warranted.171
Melatonin
Melatonin is a hormone produced endogenously, primarily by the pineal gland, and is also available in supplemental form. Melatonin is secreted at night in response to darkness as part of the sleep-wake cycle and also has a role in many other bodily functions, including immune regulation. Limited clinical studies have been done on melatonin and pancreatic cancer (typically considering melatonin in combination with other interventions and various advanced solid tumors considered to be untreatable), but both in vitro and in vivo studies have been reported.172,173 An in vivo study in mice with melatonin alone or in combination with gemcitabine found inhibition of growth of pancreatic tumor cells, which was suggested to be due to its promotion of cellular turnover and death by decreasing Bcl-2 and increasing BAX expression (key regulators of apoptotic cell death).174 Another preclinical study with both in vitro and in vivo components found that melatonin synergized with sorafenib (Nexavar) to suppress the growth of pancreatic cancer.175
Zinc
Zinc, an essential element required by the body for DNA and protein synthesis as well as for immune function, must be acquired from the diet and is also available in supplemental form. A meta-analysis including seven studies and a total of 1,659 patients with pancreatic cancer was performed to investigate a possible association of zinc with pancreatic cancer risk. It found that the highest intake of zinc was significantly associated with lower risk of pancreatic cancer; this was especially the case for Americans.176 Unfortunately, the amount of zinc taken by patients in this highest category was not reported by the study. An earlier review article reported that the copper/zinc ratio tends to be increased in the context of pancreatic cancer, and monitoring this ratio may be helpful for monitoring malignancies and efficacy of treatments.177
Green Tea
Green tea is one of the most unprocessed forms of tea and therefore its leaves maintain high amounts of antioxidants and other beneficial phytochemicals. Because of green tea’s many purported health benefits, its use has been studied in various cancers. Preclinical evidence suggests epigallocatechin gallate (EGCG), the most abundant catechin in green tea, can induce pancreatic cancer cell apoptosis and inhibit tumor progression.178 However, available observational evidence assessing the associations between green tea consumption and pancreatic cancer risk is mixed, and rigorous interventional studies in humans are lacking.
A meta-analysis of eight studies involving 2,317 patients investigated green tea consumption and risk of pancreatic cancer.179 The analyzed data showed no evidence of an association between amount of green tea consumed and pancreatic cancer. However, the authors noted that the results were primarily obtained from case-control studies among the Chinese population, and further studies are warranted. Other meta-analyses have suggested that high consumption levels of green tea may be associated with a lower risk of pancreatic cancer by nearly 25%, but this has been observed mostly in Chinese populaitons.117,180
Additional Nutrients
A variety of additional natural agents have been studied in the context of pancreatic cancer preclinical models. These include milk thistle, berberine, emodin, thymoquinone (from black cumin seed), and garcinol. Although preclinical evidence supports reasonable biological plausibility to suggest that these agents could be beneficial in pancreatic cancer, as of mid-2021, human clinical evidence is insufficient to support a strong recommendation for these agents. More studies are needed.117
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- American Cancer Society. Key Statistics for Pancreatic Cancer. Accessed 03/18/2021, https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html
- Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. Jan 2018;68(1):7-30. doi:10.3322/caac.21442
- McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. Nov 21 2018;24(43):4846-4861. doi:10.3748/wjg.v24.i43.4846
- Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. Jun 25 2018;18(1):688. doi:10.1186/s12885-018-4610-4
- Fernandez-del Castillo C, Jimenez RE. Epidemiology and non-familial risk factors for exocrine pancreatic cancer. UpToDate. Last updated 11/20/20. Accessed 01/28/21. 2020;
- Cellini F, Arcelli A, Simoni N, et al. Basics and Frontiers on Pancreatic Cancer for Radiation Oncology: Target Delineation, SBRT, SIB technique, MRgRT, Particle Therapy, Immunotherapy and Clinical Guidelines. Cancers (Basel). Jun 29 2020;12(7)doi:10.3390/cancers12071729
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. Mar 11 2017;389(10073):1011-1024. doi:10.1016/S0140-6736(16)32409-6
- Zanini S, Renzi S, Limongi AR, Bellavite P, Giovinazzo F, Bermano G. A review of lifestyle and environment risk factors for pancreatic cancer. Eur J Cancer. Mar 2021;145:53-70. doi:10.1016/j.ejca.2020.11.040
- Ilmer M, Westphalen CB, Niess H, et al. Repurposed Drugs in Pancreatic Ductal Adenocarcinoma: An Update. Cancer J. Mar/Apr 2019;25(2):134-138. doi:10.1097/PPO.0000000000000372
- Rouanet M, Lebrin M, Gross F, Bournet B, Cordelier P, Buscail L. Gene Therapy for Pancreatic Cancer: Specificity, Issues and Hopes. Int J Mol Sci. Jun 8 2017;18(6)doi:10.3390/ijms18061231
- Longnecker DS. Pathology of exocrine pancreatic neoplasms. UpToDate. Last updated 01/21/21. Accessed 01/28/21. 2021;
- Fernandez-del Castillo C. Clinical manifestations, diagnosis and staging of exocrine pancreatic cancer. UpToDate. Last updated 02/27/20. Accessed 01/28/21. 2020;
- Mohammed S, Van Buren G, 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. Jul 28 2014;20(28):9354-60. doi:10.3748/wjg.v20.i28.9354
- Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. Apr 2008;247(4):571-9. doi:10.1097/SLA.0b013e31811f4449
- Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. Feb 2007;20 Suppl 1:S94-112. doi:10.1038/modpathol.3800686
- National Cancer Institute, The Genetics of Cancer. https://www.cancer.gov/about-cancer/causes-prevention/genetics
- Johns Hopkins University. What causes pancreatic cancer?
- Ciernikova S, Novisedlakova M, Cholujova D, Stevurkova V, Mego M. The Emerging Role of Microbiota and Microbiome in Pancreatic Ductal Adenocarcinoma. Biomedicines. Dec 3 2020;8(12)doi:10.3390/biomedicines8120565
- Canto MI. Familial risk factors for pancreatic cancer and screening of high-risk patients. UpToDate. Last updated 01/22/21. Accessed 01/28/21. 2021;
- American Society of Clinical Oncology. Familial pancreatic cancer.
- Collaborators GBDPC. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. Dec 2019;4(12):934-947. doi:10.1016/S2468-1253(19)30347-4
- International Agency for Research on Cancer. Pancreas fact sheet.
- Johns Hopkins University. Individuals of Ashkenazi Jewish ancestry.
- Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. Mar 18 2009;101(6):424-31. doi:10.1093/jnci/djp020
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. Feb 2019;10(1):10-27. doi:10.14740/wjon1166
- American Cancer Society. Pancreatic cancer risk factors.
- Zhang AMY, Magrill J, de Winter TJJ, et al. Endogenous Hyperinsulinemia Contributes to Pancreatic Cancer Development. Cell Metab. Sep 3 2019;30(3):403-404. doi:10.1016/j.cmet.2019.07.003
- Shadhu K, Xi C. Inflammation and pancreatic cancer: An updated review. Saudi J Gastroenterol. Jan-Feb 2019;25(1):3-13. doi:10.4103/sjg.SJG_390_18
- Knab LM, Grippo PJ, Bentrem DJ. Involvement of eicosanoids in the pathogenesis of pancreatic cancer: the roles of cyclooxygenase-2 and 5-lipoxygenase. World J Gastroenterol. Aug 21 2014;20(31):10729-39. doi:10.3748/wjg.v20.i31.10729
- Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients. Apr 1 2019;11(4)doi:10.3390/nu11040766
- Wan G, Sun X, Li F, et al. Survival Benefit of Metformin Adjuvant Treatment For Pancreatic Cancer Patients: a Systematic Review and Meta-Analysis. Cell Physiol Biochem. 2018;49(3):837-847. doi:10.1159/000493214
- Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. Jun 20 2001;93(12):937-41. doi:10.1093/jnci/93.12.937
- Huang J, Magnusson M, Torner A, Ye W, Duberg AS. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cancer. Nov 26 2013;109(11):2917-23. doi:10.1038/bjc.2013.689
- Chang JS, Tsai CR, Chen LT, Shan YS. Investigating the Association Between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas. Jan 2016;45(1):134-41. doi:10.1097/MPA.0000000000000419
- Casari I, Falasca M. Diet and Pancreatic Cancer Prevention. Cancers (Basel). Nov 23 2015;7(4):2309-17. doi:10.3390/cancers7040892
- American Cancer Society. Signs and symptoms of pancreatic cancer.
- Sadr-Azodi O, Gudbjornsdottir S, Ljung R. Pattern of increasing HbA1c levels in patients with diabetes mellitus before clinical detection of pancreatic cancer - a population-based nationwide case-control study. Acta oncologica (Stockholm, Sweden). Jul 2015;54(7):986-92. doi:10.3109/0284186X.2015.1006402
- Wang S, Qiu Y, Bai B. The Expression, Regulation, and Biomarker Potential of Glypican-1 in Cancer. Mini Review. Frontiers in oncology. 2019-July-12 2019;9(614):614. doi:10.3389/fonc.2019.00614
- Sarah L. Cartwright MPK. Evaluation of acute abdominal pain in adults. American Family Physician. 2008;77(7):971-978.
- Kim S, Park BK, Seo JH, et al. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci Rep. Jun 1 2020;10(1):8820. doi:10.1038/s41598-020-65720-8
- Sangha Brar JS, Gupta S, Haja Mohideen SM, Liauw L, Lath N. The pancreatic and extrapancreatic manifestations of IgG4-related disease. Diagnostic and interventional radiology (Ankara, Turkey). Mar-Apr 2018;24(2):83-88. doi:10.5152/dir.2018.17319
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. Jun 27 2020;395(10242):2008-2020. doi:10.1016/S0140-6736(20)30974-0
- Baenas DF, Miretti VS, Caeiro F, Paira S. Differential diagnosis between pancreatic involvement in IgG4-related disease and pancreatic cancer. Gastroenterol Hepatol. Feb 2021;44(2):144-155. Diagnostico diferencial entre compromiso pancreatico en enfermedad relacionada con IgG4 y cancer de pancreas. doi:10.1016/j.gastrohep.2020.05.019
- ACA. American Cancer Society. Pancreatic Cancer Stages. Available at https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/staging.html . Last updated 12/18/17. Accessed 01/30/21. 2017;
- Edge SB, Edge SB, American Joint Committee on C. AJCC cancer staging manual 8th ed. Springer.; 2017.
- Michael D Kluger JC. UpToDate. Total pancreatectomy. https://www.uptodate.com/contents/total-pancreatectomy
- Columbia Surgery. Pancreatectomy surgery (removal of the pancreas).
- Sodergren MH, Mangal N, Wasan H, et al. Immunological combination treatment holds the key to improving survival in pancreatic cancer. J Cancer Res Clin Oncol. Nov 2020;146(11):2897-2911. doi:10.1007/s00432-020-03332-5
- Ryan DP, Mamon H. Initial chemotherapy and radiation for non-metastatic, locally advanced, unresectable and borderline resectable, exocrine pancreatic cancer. Last updated 12/15/20. Accessed 01/28/21. 2020;
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. New England Journal of Medicine. 2018;379(25):2395-2406. doi:10.1056/NEJMoa1809775
- Ryan DP. Initial systemic chemotherapy for metastatic exocrine pancreatic cancer. UpToDate. Last updated 01/21/21. Accessed 01/28/21. 2021;
- Ryan DP. Second-line systemic therapy for advanced exocrine pancreatic cancer. UpToDate. Last updated 09/29/20. Accessed 01/28/21. 2020;
- Singh RR, O'Reilly EM. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs. May 2020;80(7):647-669. doi:10.1007/s40265-020-01304-0
- Dahan L, Phelip JM, Le Malicot K, et al. FOLFIRINOX until progression, FOLFIRINOX with maintenance treatment, or sequential treatment with gemcitabine and FOLFIRI.3 for first-line treatment of metastatic pancreatic cancer: A randomized phase II trial (PRODIGE 35-PANOPTIMOX). Journal of Clinical Oncology. 2018;36(15_suppl):4000-4000. doi:10.1200/JCO.2018.36.15_suppl.4000
- van Manen L, Stegehuis PL, Farina-Sarasqueta A, et al. Validation of full-field optical coherence tomography in distinguishing malignant and benign tissue in resected pancreatic cancer specimens. PLoS One. 2017;12(4):e0175862. doi:10.1371/journal.pone.0175862
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. Jul 1 2019;5(7):1020-1027. doi:10.1001/jamaoncol.2019.0892
- Angelova A, Ferreira T, Bretscher C, Rommelaere J, Marchini A. Parvovirus-Based Combinatorial Immunotherapy: A Reinforced Therapeutic Strategy against Poor-Prognosis Solid Cancers. Cancers (Basel). Jan 19 2021;13(2)doi:10.3390/cancers13020342
- Looi C-K, Chung FF-L, Leong C-O, Wong S-F, Rosli R, Mai C-W. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. Journal of Experimental & Clinical Cancer Research. 2019/04/15 2019;38(1):162. doi:10.1186/s13046-019-1153-8
- Ware MB, El-Rayes BF, Lesinski GB. Mirage or long-awaited oasis: reinvigorating T-cell responses in pancreatic cancer. J Immunother Cancer. Aug 2020;8(2)doi:10.1136/jitc-2020-001100
- Nagai K, Adachi T, Harada H, Eguchi S, Sugiyama H, Miyazaki Y. Dendritic Cell-based Immunotherapy Pulsed With Wilms Tumor 1 Peptide and Mucin 1 as an Adjuvant Therapy for Pancreatic Ductal Adenocarcinoma After Curative Resection: A Phase I/IIa Clinical Trial. Anticancer Res. Oct 2020;40(10):5765-5776. doi:10.21873/anticanres.14593
- Balachandran VP, Beatty GL, Dougan SK. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology. May 2019;156(7):2056-2072. doi:10.1053/j.gastro.2018.12.038
- Choudhry H, Helmi N, Abdulaal WH, et al. Prospects of IL-2 in Cancer Immunotherapy. Biomed Res Int. 2018;2018:9056173. doi:10.1155/2018/9056173
- Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. Jun 2016;5(6):e1163462. doi:10.1080/2162402x.2016.1163462
- Ayars M, O'Sullivan E, Macgregor-Das A, et al. IL2RG, identified as overexpressed by RNA-seq profiling of pancreatic intraepithelial neoplasia, mediates pancreatic cancer growth. Oncotarget. Oct 13 2017;8(48):83370-83383. doi:10.18632/oncotarget.19848
- Angelini C, Bovo G, Muselli P, et al. Preoperative interleukin-2 immunotherapy in pancreatic cancer: preliminary results. Hepato-gastroenterology. Jan-Feb 2006;53(67):141-4.
- Caprotti R, Brivio F, Fumagalli L, et al. Free-from-progression period and overall short preoperative immunotherapy with IL-2 increases the survival of pancreatic cancer patients treated with macroscopically radical surgery. Anticancer Res. May-Jun 2008;28(3B):1951-4.
- Uggeri F, Caprotti R, De Grate L, et al. Short-term preoperative IL-2 immunotherapy in operable pancreatic cancer: a randomized study. Hepato-gastroenterology. May-Jun 2009;56(91-92):861-5.
- Lo Re G, Lo Re F, Doretto P, et al. Cyclophosphamide with or without fluorouracil followed by subcutaneous or intravenous interleukin-2 use in solid tumors: A feasibility off-label experience. Cytokine. Jan 2019;113:50-60. doi:10.1016/j.cyto.2018.06.005
- Sun Z, Ren Z, Yang K, et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8+ T-cell response and effective tumor control. Nature Communications. 2019/08/28 2019;10(1):3874. doi:10.1038/s41467-019-11782-w
- Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B. Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy. Mini Review. Frontiers in Immunology. 2018-December-18 2018;9(2905)doi:10.3389/fimmu.2018.02905
- Hall M, Liu H, Malafa M, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer. 2016;4:61. doi:10.1186/s40425-016-0164-7
- Santos JM, Havunen R, Siurala M, et al. Adenoviral production of interleukin-2 at the tumor site removes the need for systemic postconditioning in adoptive cell therapy. Int J Cancer. Oct 1 2017;141(7):1458-1468. doi:10.1002/ijc.30839
- Santos JM, Cervera-Carrascon V, Havunen R, et al. Adenovirus Coding for Interleukin-2 and Tumor Necrosis Factor Alpha Replaces Lymphodepleting Chemotherapy in Adoptive T Cell Therapy. Mol Ther. Sep 5 2018;26(9):2243-2254. doi:10.1016/j.ymthe.2018.06.001
- Quixabeira DCA, Zafar S, Santos JM, et al. Oncolytic Adenovirus Coding for a Variant Interleukin 2 (vIL-2) Cytokine Re-Programs the Tumor Microenvironment and Confers Enhanced Tumor Control. Front Immunol . 2021;12:674400. doi:10.3389/fimmu.2021.674400
- Yamaguchi Y, Katata Y, Okawaki M, Sawaki A, Yamamura M. A Prospective Observational Study of Adoptive Immunotherapy for Cancer Using Zoledronate-Activated Killer (ZAK) Cells - An Analysis for Patients with Incurable Pancreatic Cancer. Anticancer Res. May 2016;36(5):2307-13.
- LaRocca CJ, Warner SG. A New Role for Vitamin D: The Enhancement of Oncolytic Viral Therapy in Pancreatic Cancer. Biomedicines. Nov 5 2018;6(4)doi:10.3390/biomedicines6040104
- Qian Y, Gong Y, Fan Z, et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol Oncol. Oct 2 2020;13(1):130. doi:10.1186/s13045-020-00958-3
- Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. Aug 26 2014;111(5):817-22. doi:10.1038/bjc.2014.215
- Yang FA, Chen YC, Tiong C. Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. Sep 12 2020;12(9):2798. doi:10.3390/nu12092798
- National Institutes of Health. Vitamin C: Fact Sheet for Consumers.
- Drisko JA, Serrano OK, Spruce LR, Chen Q, Levine M. Treatment of pancreatic cancer with intravenous vitamin C: a case report. Anti-cancer drugs. Apr 2018;29(4):373-379. doi:10.1097/CAD.0000000000000603
- Łukawski M, Dałek P, Borowik T, et al. New oral liposomal vitamin C formulation: properties and bioavailability. J Liposome Res. Sep 2020;30(3):227-234. doi:10.1080/08982104.2019.1630642
- Gopi S, Balakrishnan P. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J Liposome Res. Oct 6 2020:1-9. doi:10.1080/08982104.2020.1820521
- Vissers MCM, Das AB. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Review. Front Physiol. 2018-July-03 2018;9(809):809. doi:10.3389/fphys.2018.00809
- Alexander MS, Wilkes JG, Schroeder SR, et al. Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer. Cancer Research. 2018;78(24):6838-6851. doi:10.1158/0008-5472.Can-18-1680
- Welsh JL, Wagner BA, van't Erve TJ, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. Mar 2013;71(3):765-75. doi:10.1007/s00280-013-2070-8
- Bigelsen S. Evidence-based complementary treatment of pancreatic cancer: a review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin C, statins, metformin, curcumin, and aspirin. Cancer Manag Res. 2018;10:2003-2018. doi:10.2147/CMAR.S161824
- O'Leary BR, Alexander MS, Du J, Moose DL, Henry MD, Cullen JJ. Pharmacological ascorbate inhibits pancreatic cancer metastases via a peroxide-mediated mechanism. Sci Rep. Oct 19 2020;10(1):17649. doi:10.1038/s41598-020-74806-2
- Polireddy K, Dong R, Reed G, et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci Rep. Dec 7 2017;7(1):17188. doi:10.1038/s41598-017-17568-8
- Archibugi L, Piciucchi M, Stigliano S, et al. Exclusive and Combined Use of Statins and Aspirin and the Risk of Pancreatic Cancer: a Case-Control Study. Sci Rep. Oct 12 2017;7(1):13024. doi:10.1038/s41598-017-13430-z
- Zhang Y, Liang M, Sun C, et al. Statin Use and Risk of Pancreatic Cancer: An Updated Meta-analysis of 26 Studies. Pancreas. Feb 2019;48(2):142-150. doi:10.1097/MPA.0000000000001226
- Jeon CY, Pandol SJ, Wu B, et al. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PLoS One. 2015;10(4):e0121783. doi:10.1371/journal.pone.0121783
- Moon do C, Lee HS, Lee YI, et al. Concomitant Statin Use Has a Favorable Effect on Gemcitabine-Erlotinib Combination Chemotherapy for Advanced Pancreatic Cancer. Yonsei medical journal. Sep 2016;57(5):1124-30. doi:10.3349/ymj.2016.57.5.1124
- E JY, Lu SE, Lin Y, et al. Differential and Joint Effects of Metformin and Statins on Overall Survival of Elderly Patients with Pancreatic Adenocarcinoma: A Large Population-Based Study. Cancer Epidemiol Biomarkers Prev. Aug 2017;26(8):1225-1232. doi:10.1158/1055-9965.EPI-17-0227
- Khurana V, Sheth A, Caldito G, Barkin JS. Statins reduce the risk of pancreatic cancer in humans: a case-control study of half a million veterans. Pancreas. Mar 2007;34(2):260-5. doi:10.1097/MPA.0b013e318030e963
- Broadhurst PJ, Hart AR. Metformin as an Adjunctive Therapy for Pancreatic Cancer: A Review of the Literature on Its Potential Therapeutic Use. Dig Dis Sci. Nov 2018;63(11):2840-2852. doi:10.1007/s10620-018-5233-y
- Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. May 15 2012;18(10):2905-12. doi:10.1158/1078-0432.CCR-11-2994
- Xin W, Fang L, Fang Q, Zheng X, Huang P. Effects of metformin on survival outcomes of pancreatic cancer patients with diabetes: A meta-analysis. Molecular and clinical oncology. Mar 2018;8(3):483-488. doi:10.3892/mco.2017.1541
- Wei M, Liu Y, Bi Y, Zhang Z-J. Metformin and pancreatic cancer survival: Real effect or immortal time bias? International Journal of Cancer . 2019;145(7):1822-1828. doi:https://doi.org/10.1002/ijc.32254
- Liu H, Naxerova K, Pinter M, et al. Use of Angiotensin System Inhibitors Is Associated with Immune Activation and Longer Survival in Nonmetastatic Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. Oct 1 2017;23(19):5959-5969. doi:10.1158/1078-0432.CCR-17-0256
- Cerullo M, Gani F, Chen SY, Canner JK, Pawlik TM. Impact of Angiotensin Receptor Blocker Use on Overall Survival Among Patients Undergoing Resection for Pancreatic Cancer. World journal of surgery. Sep 2017;41(9):2361-2370. doi:10.1007/s00268-017-4021-8
- Yang A, Zylberberg HM, Rustgi SD, et al. Beta-blockers have no impact on survival in pancreatic ductal adenocarcinoma prior to cancer diagnosis. Sci Rep. Jan 13 2021;11(1):1038. doi:10.1038/s41598-020-79999-0
- Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. May 2020;31(5):558-568. doi:10.1016/j.annonc.2020.02.012
- Fang L, Xu Q, Qian J, Zhou JY. Aberrant Factors of Fibrinolysis and Coagulation in Pancreatic Cancer. Onco Targets Ther. 2021;14:53-65. doi:10.2147/OTT.S281251
- Freuding M, Keinki C, Micke O, Buentzel J, Huebner J. Mistletoe in oncological treatment: a systematic review : Part 1: survival and safety. J Cancer Res Clin Oncol. Mar 2019;145(3):695-707. doi:10.1007/s00432-018-02837-4
- Freuding M, Keinki C, Kutschan S, Micke O, Buentzel J, Huebner J. Mistletoe in oncological treatment: a systematic review : Part 2: quality of life and toxicity of cancer treatment. J Cancer Res Clin Oncol. Apr 2019;145(4):927-939. doi:10.1007/s00432-018-02838-3
- Wode K, Hok Nordberg J, Kienle GS, et al. Efficacy of mistletoe extract as a complement to standard treatment in advanced pancreatic cancer: study protocol for a multicentre, parallel group, double-blind, randomised, placebo-controlled clinical trial (MISTRAL). Trials. Sep 11 2020;21(1):783. doi:10.1186/s13063-020-04581-y
- Werthmann PG, Kempenich R, Lang-Averous G, Kienle GS. Long-term survival of a patient with advanced pancreatic cancer under adjunct treatment with Viscum album extracts: A case report. World J Gastroenterol. Mar 28 2019;25(12):1524-1530. doi:10.3748/wjg.v25.i12.1524
- Zhong GC, Li QJ, Yang PF, et al. Low-carbohydrate diets and the risk of pancreatic cancer: a large prospective cohort study. Carcinogenesis. May 28 2021;42(5):724-732. doi:10.1093/carcin/bgab006
- Gaesser GA. Whole Grains, Refined Grains, and Cancer Risk: A Systematic Review of Meta-Analyses of Observational Studies. Nutrients. Dec 7 2020;12(12)doi:10.3390/nu12123756
- Jentzsch V, Davis JAA, Djamgoz MBA. Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management. Cancers (Basel). Oct 23 2020;12(11)doi:10.3390/cancers12113096
- Paluszkiewicz P, Smolińska K, Dębińska I, Turski WA. Main dietary compounds and pancreatic cancer risk. The quantitative analysis of case-control and cohort studies. Cancer Epidemiol. Feb 2012;36(1):60-7. doi:10.1016/j.canep.2011.05.004
- HAMAGUCHI R, NARUI R, WADA H. Effects of Alkalization Therapy on Chemotherapy Outcomes in Metastatic or Recurrent Pancreatic Cancer. Anticancer Research. 2020;40(2):873-880. doi:10.21873/anticanres.14020
- Bao Y, Hu FB, Giovannucci EL, et al. Nut consumption and risk of pancreatic cancer in women. Br J Cancer. Nov 26 2013;109(11):2911-6. doi:10.1038/bjc.2013.665
- Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. Jul 2015;73(7):409-25. doi:10.1093/nutrit/nuv006
- Djamgoz MBA, Jentzsch V. Integrative Management of Pancreatic Cancer (PDAC): Emerging Complementary Agents and Modalities. Nutrition and Cancer. Jun 4 2021:1-24. doi:10.1080/01635581.2021.1934043
- Li L-y, Luo Y, Lu M-d, Xu X-w, Lin H-d, Zheng Z-q. Cruciferous vegetable consumption and the risk of pancreatic cancer: a meta-analysis. World Journal of Surgical Oncology. 2015/02/12 2015;13(1):44. doi:10.1186/s12957-015-0454-4
- Lozanovski VJ, Polychronidis G, Gross W, et al. Broccoli sprout supplementation in patients with advanced pancreatic cancer is difficult despite positive effects—results from the POUDER pilot study. Investigational New Drugs. 2020/06/01 2020;38(3):776-784. doi:10.1007/s10637-019-00826-z
- Plotti F, Terranova C, Luvero D, et al. Diet and Chemotherapy: The Effects of Fasting and Ketogenic Diet on Cancer Treatment. Chemotherapy. 2020;65(3-4):77-84. doi:10.1159/000510839
- O'Connor D, Brown M, Eatock M, Turkington RC, Prue G. Exercise efficacy and prescription during treatment for pancreatic ductal adenocarcinoma: a systematic review. BMC Cancer. Jan 9 2021;21(1):43. doi:10.1186/s12885-020-07733-0
- Chung V, Frankel P, Shibata S, et al. Pilot trial of gemcitabine, nab-paclitaxel, metformin and a standardized dietary supplement in patients with unresectable pancreatic cancer. Presented at: American Society of Clinical Oncology: Gastrointestinal Cancers Symposium. 2020:
- Park M, Lim JW, Kim H. Docoxahexaenoic Acid Induces Apoptosis of Pancreatic Cancer Cells by Suppressing Activation of STAT3 and NF-kappaB. Nutrients. Nov 2 2018;10(11)doi:10.3390/nu10111621
- Dierge E, Debock E, Guilbaud C, et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. Jun 8 2021;doi:10.1016/j.cmet.2021.05.016
- Ma YJ, Yu J, Xiao J, Cao BW. The consumption of omega-3 polyunsaturated fatty acids improves clinical outcomes and prognosis in pancreatic cancer patients: a systematic evaluation. Nutr Cancer. 2015/01/02 2015;67(1):112-8. doi:10.1080/01635581.2015.976315
- Isherwood J, Arshad A, Chung WY, et al. Myeloid derived suppressor cells are reduced and T regulatory cells stabilised in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega 3. Ann Transl Med. Mar 2020;8(5):172. doi:10.21037/atm.2020.02.02
- Abe K, Uwagawa T, Haruki K, et al. Effects of ω-3 Fatty Acid Supplementation in Patients with Bile Duct or Pancreatic Cancer Undergoing Chemotherapy. Anticancer Res. Apr 2018;38(4):2369-2375. doi:10.21873/anticanres.12485
- Akita H, Takahashi H, Asukai K, et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin Nutr ESPEN. Oct 2019;33:148-153. doi:10.1016/j.clnesp.2019.06.003
- Arshad A, Isherwood J, Mann C, et al. Intravenous ω-3 Fatty Acids Plus Gemcitabine. JPEN J Parenter Enteral Nutr. Mar 2017;41(3):398-403. doi:10.1177/0148607115595221
- National Institutes of Health. Vitamin D: Fact Sheet for Consumers.
- Zhang X, Huang XZ, Chen WJ, et al. Plasma 25-hydroxyvitamin D levels, vitamin D intake, and pancreatic cancer risk or mortality: a meta-analysis. Oncotarget. Sep 8 2017;8(38):64395-64406. doi:10.18632/oncotarget.18888
- Wei D, Wang L, Zuo X, Bresalier RS. Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer. Cancers (Basel). May 31 2021;13(11)doi:10.3390/cancers13112716
- Yuan C, Qian ZR, Babic A, et al. Prediagnostic Plasma 25-Hydroxyvitamin D and Pancreatic Cancer Survival. J Clin Oncol. Aug 20 2016;34(24):2899-905. doi:10.1200/JCO.2015.66.3005
- Rasmussen LS, Yilmaz MK, Falkmer UG, et al. Pre-treatment serum vitamin D deficiency is associated with increased inflammatory biomarkers and short overall survival in patients with pancreatic cancer. Eur J Cancer. Feb 2021;144:72-80. doi:10.1016/j.ejca.2020.10.038
- Liu Y, Wang X, Sun X, Lu S, Liu S. Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies. Medicine (Baltimore). Mar 2018;97(13):e0114. doi:10.1097/MD.0000000000010114
- Berkson BM, Rubin DM, Berkson AJ. The long-term survival of a patient with pancreatic cancer with metastases to the liver after treatment with the intravenous alpha-lipoic acid/low-dose naltrexone protocol. Integr Cancer Ther. Mar 2006;5(1):83-9. doi:10.1177/1534735405285901
- Berkson BM, Rubin DM, Berkson AJ. Revisiting the ALA/N (alpha-lipoic acid/low-dose naltrexone) protocol for people with metastatic and nonmetastatic pancreatic cancer: a report of 3 new cases. Integr Cancer Ther. Dec 2009;8(4):416-22. doi:10.1177/1534735409352082
- Lujian Peng XL, Qian Lu, Tengqian Tang, Zhanyu Yang. Vitamin E Intake and Pancreatic Cancer Risk: A Meta-Analysis of Observational Studies. Med Sci Monit. 2015 (21):1249-1255.
- Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int J Food Sci Nutr. Nov 2016;67(7):744-53. doi:10.1080/09637486.2016.1197892
- Salem AA, Mackenzie GG. Pancreatic cancer: A critical review of dietary risk. Nutr Res. Apr 2018;52:1-13. doi:10.1016/j.nutres.2017.12.001
- Life Extension. Cancer adjuvant therapy.
- Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. International journal of food sciences and nutrition. 2016/10/02 2016;67(7):744-753. doi:10.1080/09637486.2016.1197892
- Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J Gastrointest Cancer. Jun 2013;44(2):152-61. doi:10.1007/s12029-012-9441-y
- Jeurnink SM, Ros MM, Leenders M, et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: a nested case-control study: plasma micronutrients and pancreatic cancer risk. Int J Cancer. Mar 15 2015;136(6):E665-76. doi:10.1002/ijc.29175
- Nkondjock A, Ghadirian P, Johnson KC, Krewski D, Canadian Cancer Registries Epidemiology Research G. Dietary intake of lycopene is associated with reduced pancreatic cancer risk. J Nutr. Mar 2005;135(3):592-7. doi:10.1093/jn/135.3.592
- Huang X, Gao Y, Zhi X, Ta N, Jiang H, Zheng J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci Rep. Dec 12 2016;6:38936. doi:10.1038/srep38936
- Zhang T, Chen H, Qin S, et al. The association between dietary vitamin A intake and pancreatic cancer risk: a meta-analysis of 11 studies. Bioscience reports. Dec 2016;36(6)doi:10.1042/BSR20160341
- Jeong Y, Lim JW, Kim H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-kappaB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients. Apr 1 2019;11(4)doi:10.3390/nu11040762
- Yan T, Li HY, Wu JS, et al. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol Lett. Nov 2017;14(5):5400-5408. doi:10.3892/ol.2017.6836
- Wei DH, Mao QQ. Vitamin B6, vitamin B12 and methionine and risk of pancreatic cancer: a meta-analysis. Nutr J. Oct 4 2020;19(1):111. doi:10.1186/s12937-020-00628-7
- Gong Z, Holly EA, Bracci PM. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control. Oct 2009;20(8):1317-25. doi:10.1007/s10552-009-9352-9
- Selvanesan BC, Meena K, Beck A, et al. Nicotinamide combined with gemcitabine is an immunomodulatory therapy that restrains pancreatic cancer in mice. J Immunother Cancer. Nov 2020;8(2)doi:10.1136/jitc-2020-001250
- Schwarz K, Dobiasch S, Nguyen L, Schilling D, Combs SE. Modification of radiosensitivity by Curcumin in human pancreatic cancer cell lines. Sci Rep. Mar 2 2020;10(1):3815. doi:10.1038/s41598-020-60765-1
- Wang Q, Qu C, Xie F, et al. Curcumin suppresses epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells by inhibiting cancer-associated fibroblasts. Am J Cancer Res. 2017;7(1):125-133.
- Yoshida K, Toden S, Ravindranathan P, Han H, Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. Oct 1 2017;38(10):1036-1046. doi:10.1093/carcin/bgx065
- Liu P, Ying Q, Liu H, et al. Curcumin enhances anticancer efficacy of either gemcitabine or docetaxel on pancreatic cancer cells. Oncol Rep. Oct 2020;44(4):1393-1402. doi:10.3892/or.2020.7713
- Bimonte S, Barbieri A, Leongito M, et al. Curcumin AntiCancer Studies in Pancreatic Cancer. Nutrients. Jul 16 2016;8(7)doi:10.3390/nu8070433
- Bimonte S, Barbieri A, Palma G, Luciano A, Rea D, Arra C. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. Biomed Res Int. 2013;2013:810423. doi:10.1155/2013/810423
- Pastorelli D, Fabricio ASC, Giovanis P, et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol Res. Jun 2018;132:72-79. doi:10.1016/j.phrs.2018.03.013
- Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62(8):1137-41. doi:10.1080/01635581.2010.513802
- Kanai M, Yoshimura K, Asada M, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. Jul 2011;68(1):157-64. doi:10.1007/s00280-010-1470-2
- Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. Jul 15 2008;14(14):4491-9. doi:10.1158/1078-0432.Ccr-08-0024
- National Institutes of Health. Carnitine: Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Carnitine-HealthProfessional/
- Matsui H, Einama T, Shichi S, et al. L-Carnitine supplementation reduces the general fatigue of cancer patients during chemotherapy. Molecular and clinical oncology. Mar 2018;8(3):413-416. doi:10.3892/mco.2018.1557
- Kraft M, Kraft K, Gartner S, et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)--a randomized multicentre trial. Nutr J . Jul 23 2012;11:52. doi:10.1186/1475-2891-11-52
- Lopez-Ridaura R, Willett WC, Rimm EB, et al. Magnesium Intake and Risk of Type 2 Diabetes in Men and Women. Diabetes Care. 2004;27(1):134-140. doi:10.2337/diacare.27.1.134
- Barbagallo M, Dominguez LJ. Magnesium and type 2 diabetes. World journal of diabetes. 2015;6(10):1152-1157. doi:10.4239/wjd.v6.i10.1152
- Hata A, Doi Y, Ninomiya T, et al. Magnesium intake decreases Type 2 diabetes risk through the improvement of insulin resistance and inflammation: the Hisayama Study. Diabetic Med. 2013;30(12):1487-1494. doi:https://doi.org/10.1111/dme.12250
- Dibaba D, Xun P, Yokota K, White E, He K. Magnesium intake and incidence of pancreatic cancer: the VITamins and Lifestyle study. Br J Cancer. Dec 1 2015;113(11):1615-21. doi:10.1038/bjc.2015.382
- El-Rayes BF, Philip PA, Sarkar FH, et al. A phase II study of isoflavones, erlotinib, and gemcitabine in advanced pancreatic cancer. Invest New Drugs. Aug 2011;29(4):694-9. doi:10.1007/s10637-010-9386-6
- Löhr JM, Karimi M, Omazic B, et al. A phase I dose escalation trial of AXP107-11, a novel multi-component crystalline form of genistein, in combination with gemcitabine in chemotherapy-naive patients with unresectable pancreatic cancer. Pancreatology. Jul-Aug 2016;16(4):640-5. doi:10.1016/j.pan.2016.05.002
- Pourhanifeh MH, Mehrzadi S, Kamali M, Hosseinzadeh A. Melatonin and gastrointestinal cancers: Current evidence based on underlying signaling pathways. Eur J Pharmacol. Nov 5 2020;886:173471. doi:10.1016/j.ejphar.2020.173471
- Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, Asemi Z. Melatonin and pancreatic cancer: Current knowledge and future perspectives. J Cell Physiol. May 2019;234(5):5372-5378. doi:10.1002/jcp.27372
- Xu C, Wu A, Zhu H, et al. Melatonin is involved in the apoptosis and necrosis of pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax balance. Biomed Pharmacother. Mar 2013;67(2):133-9. doi:10.1016/j.biopha.2012.10.005
- Fang Z, Jung KH, Yan HH, et al. Melatonin Synergizes with Sorafenib to Suppress Pancreatic Cancer via Melatonin Receptor and PDGFR-β/STAT3 Pathway. Cellular Physiology and Biochemistry. 2018;47(5):1751-1768. doi:10.1159/000491058
- Li L, Gai X. The association between dietary zinc intake and risk of pancreatic cancer: a meta-analysis. Bioscience reports. Jun 30 2017;37(3)doi:10.1042/BSR20170155
- Ebadi M, Swanson S. The status of zinc, copper, and metallothionein in cancer patients. Progress in clinical and biological research. 1988;259:161-75.
- Bimonte S, Cascella M, Leongito M, et al. An overview of pre-clinical studies on the effects of (-)-epigallocatechin-3-gallate, a catechin found in green tea, in treatment of pancreatic cancer. Recenti progressi in medicina. Jun 2017;108(6):282-287. An overview of pre-clinical studies on the effects of (-)-epigallocatechin-3-gallate, a catechin found in green tea, in treatment of pancreatic cancer. doi:10.1701/2715.27715
- Zeng JL, Li ZH, Wang ZC, Zhang HL. Green tea consumption and risk of pancreatic cancer: a meta-analysis. Nutrients. Oct 28 2014;6(11):4640-50. doi:10.3390/nu6114640
- Chen K, Zhang Q, Peng M, Shen Y, Wan P, Xie G. Relationship between tea consumption and pancreatic cancer risk: a meta-analysis based on prospective cohort studies and case-control studies. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) . Sep 2014;23(5):353-60. doi:10.1097/CEJ.0000000000000033
