
Polycystic Ovary Syndrome (PCOS)
Polycystic Ovary Syndrome (PCOS)
Last Section Update: 12/2024
Contributor(s): Maureen Williams, ND; Carrie Decker, ND, MS
Table of Contents
- Overview
- What is Polycystic Ovary Syndrome?
- Signs & Symptoms of Polycystic Ovary Syndrome
- Health Conditions Associated with Polycystic Ovary Syndrome
- Polycystic Ovary Syndrome Causes & Risk Factors
- Nutrients
- Dietary & Lifestyle Changes for Polycystic Ovary Syndrome
- Diagnosis of Polycystic Ovary Syndrome
- Conventional Polycystic Ovary Syndrome Treatment
- Update History
- References
1 Overview
What is Polycystic Ovary Syndrome?
Polycystic ovary syndrome (PCOS) involves hormone and metabolic imbalances and affects multiple body systems. Individuals with polycystic ovary syndrome often experience absent or irregular periods, ovarian cysts, infertility, facial hair growth, acne, weight gain, problems with blood sugar metabolism, and may have increased cardiovascular disease risk.
Healthy eating habits and losing weight, along with nutrients such as inositol and N-acetylcysteine (NAC), may help reduce polycystic ovary syndrome symptoms when combined with appropriate medical care.
Nutrients for Polycystic Ovary Syndrome
- Myo-inositol and D-chiro-inositol (DCI): These two inositol compounds have been found to help improve insulin sensitivity and triglyceride levels, and may improve menstrual regularity and reproductive health in women with polycystic ovary syndrome.
- Vitamin D: Many women with polycystic ovary syndrome have low levels of vitamin D, and supplementation has been found to improve hormone balance and metabolic health.
- Omega- 3 fatty acids: Clinical trials have found omega-3 fats from fish oil can reduce inflammation and improve markers of glucose and lipid metabolism.
- N-acetylcysteine (NAC): NAC has been shown to improve insulin sensitivity and support improved fertility when combined with ovulation-inducing medications.
- Chromium: Chromium picolinate has been shown to improve glucose tolerance in women with polycystic ovary syndrome.
- Lipoic acid: Women with polycystic ovary syndrome who supplemented with lipoic acid showed an improvement in insulin sensitivity and a reduction in triglycerides, while improvements in menstrual regularity were also seen with a combination of lipoic acid and inositol.
- L-carnitine: Clinical trials have found that L-carnitine and acetyl-L-carnitine may improve glucose metabolism and insulin signaling, BMI, unwanted hair growth (hirsutism), and blood lipid levels in women with polycystic ovary syndrome.
- Melatonin: The sleep-wake cycle may be disrupted in women with polycystic ovary syndrome. Some evidence suggest supplemental melatonin, a sleep-wake (circadian) hormone, may improve unwanted hair growth, insulin levels, levels of male hormones, blood lipid levels, and menstrual regularity among women with polycystic ovary syndrome.
Diet & Lifestyle Changes for Polycystic Ovary Syndrome
- Losing weight can help restore ovulatory cycles and improve metabolic health. Weight loss is generally the first-line treatment consideration for women with polycystic ovary syndrome who are overweight.
- Dietary changes that have been shown to be of benefit are reducing intake of carbohydrates and saturated fat and increasing intake of monounsaturated fats (such as those found in olive oil) and fiber.
- Engage in daily physical activity and regular exercise to improve insulin sensitivity and hormone balance.
Conventional Polycystic Ovary Syndrome Treatment
- Weight loss along with dietary and lifestyle changes
- Oral contraceptives
- Antiandrogens
- Metformin and statins
- Ovulation inducers
2 What is Polycystic Ovary Syndrome?
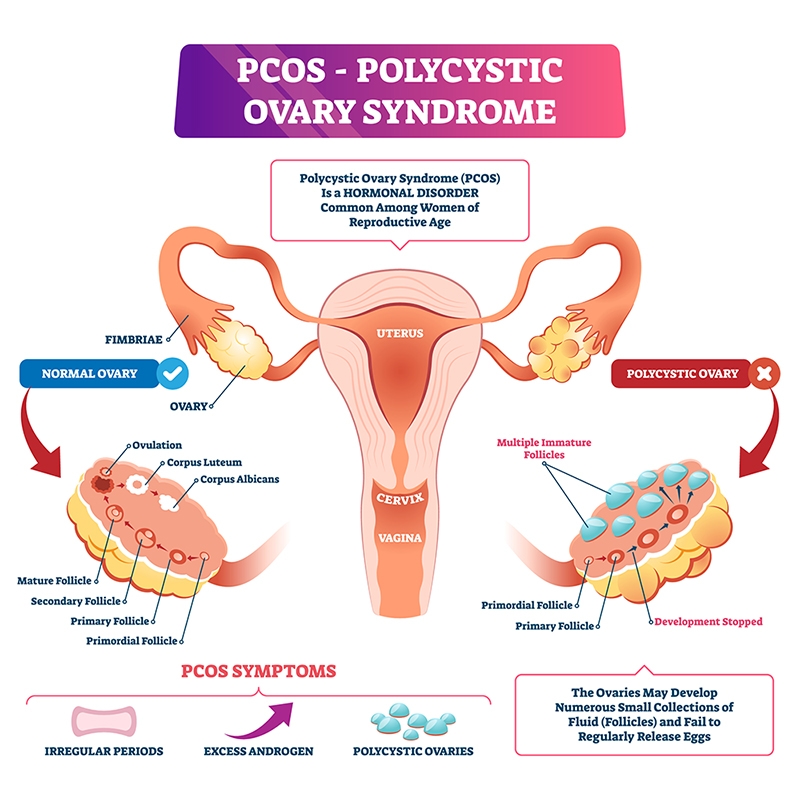
Polycystic ovary syndrome (PCOS) involves hormone and metabolic imbalances and affects multiple body systems. It is characterized by high levels of androgens (testosterone) with ovarian changes that result in menstrual cycle disturbances. polycystic ovary syndrome can affect adult and adolescent females.1,2
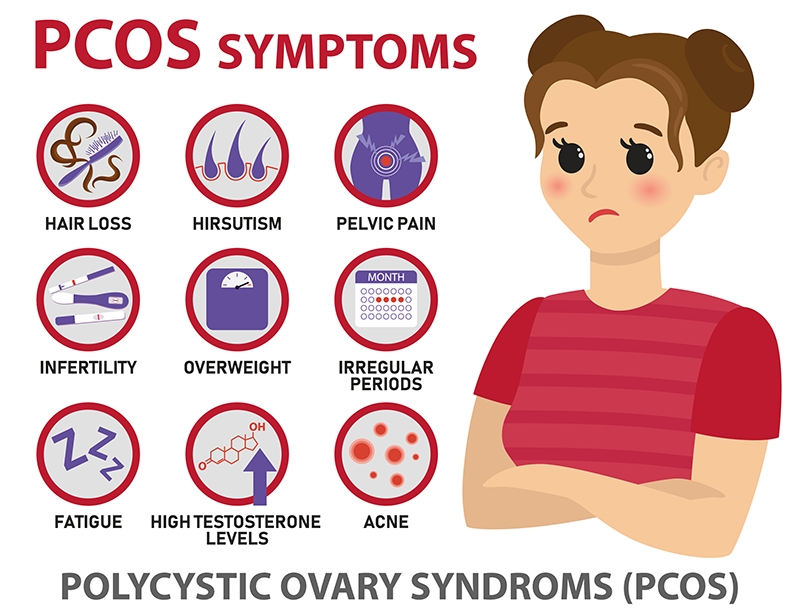
Most adult women with polycystic ovary syndrome have multiple ovarian cysts and enlarged ovaries due to overexposure to androgens. Women with polycystic ovary syndrome typically experience symptoms related to hormonal imbalance, such as menstrual irregularity and infertility.3 Others develop additional distressing signs and symptoms, such as excess facial and body hair, adult acne, hair loss, and increasing body weight.2 Psychological issues, possibly related to body image distress, are significantly higher in women with polycystic ovary syndrome.4 Many women with polycystic ovary syndrome experience mild and ambiguous symptoms.5
Polycystic ovary syndrome is the most common hormonal disorder in women of reproductive age and usually develops during the early stages of puberty.1,7 The true prevalence is unknown because many women with polycystic ovary syndrome are undiagnosed, but it is believed that about 5‒20% of women of reproductive age are affected.8,9
Women with polycystic ovary syndrome are at increased risk of high blood sugar, type 2 diabetes, and other changes that may increase cardiovascular disease risk.10-12 Therefore, lifestyle modification is critical to polycystic ovary syndrome management. Physical exercise and dietary changes may improve metabolic status and reduce levels of male hormones.
Oral contraceptives are generally the first-line pharmacologic treatment for the menstrual irregularities of polycystic ovary syndrome, as well as signs of hyperandrogenism (eg, unwanted hair growth and acne). Other antiandrogenic medications may be considered if the response to birth control is not satisfactory, and medications that promote ovulation can be used to treat infertility related to polycystic ovary syndrome.1,13,14 Metformin, an insulin-sensitizing agent, is a second-line treatment option for metabolic concerns when oral contraceptives and lifestyle changes do not provide satisfactory results.1,13,15,16
In addition, clinical trials show the use of targeted nutrients such as inositol, vitamin D, omega-3 fatty acids, and N-acetylcysteine (NAC) may help relieve polycystic ovary syndrome symptoms and reduce the risk of related chronic health problems.
3 Signs & Symptoms of Polycystic Ovary Syndrome
Common symptoms of polycystic ovary syndrome include:
- Menstrual irregularity. An unpredictable menstrual cycle is a common presenting symptom of polycystic ovary syndrome. This may manifest as cycles longer than 35 days or shorter than 21 days. Despite this, regular menses can occur in polycystic ovary syndrome.8
- Infertility. The menstrual irregularities and absence of ovulation caused by polycystic ovary syndrome can contribute to difficulty becoming pregnant.
- Skin and hair changes. Increased male hormone levels are a key feature of polycystic ovary syndrome and may result in excess facial and body hair, adult acne, and hair loss that resembles male-pattern baldness.18 Still, a substantial fraction of women with PCOS have polycystic ovaries and abnormal ovarian function without hyperandrogenism and the related skin and hair changes.9
- Overweight and obesity. It has been estimated that 50‒80% of women with PCOS are obese.8
- Psychological issues. Women with polycystic ovary syndrome have a greater risk of experiencing psychological issues including low self-esteem, depression, anxiety, eating disorders, and of attempting suicide.19,20 Studies suggest these psychological challenges are often related to body image issues.4
4 Health Conditions Associated with Polycystic Ovary Syndrome
Polycystic ovary syndrome is associated with a range of reproductive, metabolic, and psychological disorders.3 It is associated with a high prevalence of cardiovascular risk factors including hyperlipidemia and vascular disease, as well as metabolic abnormalities including type 2 diabetes.8
Reproductive Disorders
Ovarian dysfunction and hyperandrogenism (high levels of male sex hormones) are the main reproductive system-related functional disturbances in polycystic ovary syndrome. Polycystic ovary syndrome is also characterized by a large number of ovarian cysts, which can be viewed with ultrasound; however, evidence of this feature is not required for diagnosis. These reflect a failure of normal follicle or oocyte (ovarian cells that may become eggs) development.9 Many women with PCOS have trouble becoming pregnant due to infrequent ovulation or lack of ovulation altogether, although there still may be menstrual bleeding (known as “anovulatory cycling”). In fact, polycystic ovary syndrome is the most common cause of female infertility.21
PCOS is also associated with complications of both pregnancy and delivery, including miscarriage, gestational diabetes, gestational high blood pressure, preeclampsia, preterm delivery, and perinatal death, as well as increased need for labor induction and Cesarean-section.2,22
Metabolic Disorders
Insulin resistance affects 50–70% of women with PCOS.23 PCOS increases the odds of having impaired glucose tolerance by an estimated three-fold or more.24 These metabolic derangements put women with PCOS at higher risk of type 2 diabetes, obesity, and non-alcoholic fatty liver disease.3,25 In fact, as many as one in 10 women with PCOS develop type 2 diabetes by age 40.23 In addition, high insulin levels resulting from insulin resistance can further increase hyperandrogenism and worsen hormone imbalance.13
Obesity frequently co-occurs with PCOS, and difficulty losing weight is reported to be one of the most distressing aspects of this condition.24 The nature of the relationship between obesity and PCOS appears to be bi-directional, with each contributing to the onset and progression of the other.26
Some evidence suggests obstructive sleep apnea may be more common in women with PCOS. Obstructive sleep apnea is a sleep disorder characterized by partial or complete obstruction of the airways during sleep, leading to intermittent pauses in breathing, low oxygen levels, and fragmented sleep. It is linked to obesity and cardiometabolic diseases.24
Psychological Disorders
Depression, anxiety, eating disorders, reduced quality of life, negative body image, and psychosexual dysfunction all can occur in women with PCOS.3 Studies indicate women with PCOS are more than three times as likely to experience depressive symptoms and over six times more likely to experience anxiety symptoms than those without PCOS.24 A strong association between these psychological issues and body image distress has been shown.3,27 Biochemical features of the disease, such as insulin resistance, systemic inflammation, and high circulating male hormone levels, as well as PCOS manifestations (menstrual problems and infertility), may be contributing factors in the elevated risk of psychological complications.3,27 A heavy emphasis on weight loss as a treatment strategy combined with difficulty losing weight may also contribute to the higher prevalence of eating disorders seen particularly in adolescents with PCOS, thus, a weight neutral approach may be better for addressing women with PCOS who experience psychological issues.23,28 Other psychiatric ailments including personality disorders, schizoaffective disorder, obsessive-compulsive disorder, and panic disorder may also be more common in PCOS patients.27
5 Polycystic Ovary Syndrome Causes & Risk Factors
While the underlying cause of PCOS is unknown, abnormal insulin and hormone secretion and signaling appear to be key factors. Genetic, epigenetic, and environmental factors likely contribute as well.37-39
Abnormal Gonadotropin and Androgen Secretion and Signaling
Excessive secretion of luteinizing hormone (LH), one of the pituitary hormones that stimulates maturation of ovarian follicles, appears to play a role in PCOS.9 A complex interaction of LH, FSH (follicle-stimulating hormone), and GnRH (gonadotropin-releasing hormone) appears to cause increased androgen production and disrupt normal development of follicles.8 At the same time, excessive male hormone levels provoke excessive LH secretion.13 Complicating this further, excessive insulin levels—another prominent feature of a high percentage of cases of PCOS—also contribute to hyperandrogenism.8
Abnormal Insulin Secretion and Signaling
Women with PCOS are prone to defects in insulin signaling, which increases the risk of gaining weight and becoming obese, aggravates the overproduction of androgens in the ovaries and adrenal gland.40,41 Meanwhile, excess androgens encourage insulin resistance, leading to dysfunctional signaling by fat tissue and further elevated insulin levels, which in turn stimulate weight gain and androgen synthesis. The result is a vicious cycle of worsening hormonal and metabolic symptoms and progression of the syndrome.42,43
Premature Sexual Maturation (Adrenarche)
Girls who develop premature signs of puberty (before the age of eight) often have signs and symptoms similar to PCOS. In fact, premature signs of puberty have been found to correlate with high androgen production and irregular menstrual periods, suggesting premature puberty may be an early manifestation of PCOS.44-46
Chronic Inflammation
Chronic systemic inflammation is thought to play a role in PCOS as well. Women with PCOS have been found to have higher levels of markers of inflammation independent of their weight status.47,48 Dysregulated inflammatory signaling by fat tissue and release of growth factors, cytokines, and free radicals from the ovaries, liver, and other tissues contribute to the inflammatory state and promote further metabolic imbalance and insulin resistance.49,50 Inflammation and oxidative stress can also impair ovarian function and follicular maturation.51
Developmental and Early-Life Exposures
Some evidence suggests that certain exposures can affect the development of fetal and immature ovaries. These include in utero exposure to high levels of androgens, as well as fetal or childhood exposure to hormone-disrupting chemicals or drugs. This may even extend to certain foods.1,7 Other maternal factors such as gestational hypertension, gestational diabetes, or smoking have been suggested as contributors to PCOS risk, and the risk may be compounded by environmental contributors in childhood such as poor diet and lack of exercise.7
6 Nutrients
Inositol
Inositol is a sugar alcohol found in plants and made in the body that occurs in multiple structural forms, or isomers. Myo-inositol is the most widely distributed isomer in nature and is abundant in the ovaries. The D-chiro-inositol (DCI) isomer is found in smaller amounts in the body. Some myo-inositol is converted to DCI in the body.52,53
Inositol isomers are present in cells in free form and as components of membrane phospholipids, and are active in cell-to-cell signaling.52 In addition, inositol compounds called inositol phosphates act as second messengers to several hormones, including insulin and FSH, by transmitting external signals to the working interior of cells.53-55 Dysregulation of the inositol phosphate system is associated with insulin resistance and related disorders, including PCOS.55,56 Both myo-inositol and DCI have been found to have positive effects on glucose regulation in clinical trials; however, myo-inositol and DCI appear to have different effects on ovarian and pituitary hormone production, and the optimal ratio of myo-inositol to DCI for treatment of PCOS remains unresolved.54
Myo-inositol. A number of clinical trials have demonstrated that myo-inositol can improve insulin sensitivity and metabolic markers, also influencing ovarian function in women with PCOS.53,57,58 A meta-analysis of six randomized controlled trials with a total of 355 participants found myo-inositol was comparable to metformin with regard to its effects on fasting insulin and insulin sensitivity, testosterone, sex hormone binding globulin (SHBG), and body mass index (BMI) (a measure of body weight status) in women with PCOS, while metformin treatment carried a higher risk of adverse effects.59
Myo-inositol supplementation has also been shown to improve fertility. A randomized controlled trial compared metformin plus 1,800 mg myo-inositol per day to metformin alone for three months in 120 women with PCOS-related infertility undergoing ovulation induction. Women who received metformin plus myo-inositol had more than double the successful childbirth rate compared with those who received metformin alone (55% vs. 27%, respectively).60 Another randomized controlled trial in 116 women with PCOS-related infertility found that those who received 4 grams myo-inositol per day for six months had comparable improvements in metabolic markers and hormone levels to those who received both myo-inositol and metformin. There was no significant advantage for conception with the addition of metformin compared to myo-inositol alone. There were more side effects, mostly gastrointestinal, in the metformin group.61 Other research indicates myo-inositol supplementation reduces the risk of ovarian hyperstimulation syndrome during infertility treatment in patients with PCOS.58,62
D-chiro-inositol (DCI). D-chiro-inositol is important for mediating the insulin response and has been shown to improve insulin sensitivity and improve metabolic conditions related to insulin resistance in PCOS patients.63 By restoring insulin activity, DCI supplements may support improved ovarian function. However, DCI has also been found to inhibit aromatase (the enzyme that promotes conversion of androgens to estrogens in the ovaries), and higher doses have been found to increase circulating testosterone levels in women with PCOS.54,64 In addition, high doses of DCI could interfere with absorption of myo-inositol and thereby decrease the likelihood of a therapeutic benefit, though conclusive data on this relationship is not yet available.54
Myo-inositol plus D-chiro-inositol. Because of differences in their effects, it is possible that myo-inositol and DCI may have complementary actions. A 40:1 ratio of myo-inositol to DCI has been recommended as it is believed to reflect the physiological balance of these compounds in plasma.54,65 One randomized controlled trial in 56 PCOS patients compared the effects of 4 grams of inositol per day for three months using seven different formulations to DCI alone, or myo-inositol to DCI in the following ratios—1:3.5, 2.5:1, 5:1, 20:1, 40:1, or 80:1. The 40:1 ratio of myo-inositol to DCI was most effective for restoring ovulation and normal levels of a range of reproductive hormones, while all formulations improved metabolic markers.65
A randomized controlled trial in 46 participants with PCOS and obesity compared the effects of six months of combined daily treatment with myo-inositol and DCI in a 40:1 ratio (containing 1,100 mg myo-inositol) plus 400 mcg folic acid to 400 mcg folic acid per day alone. Those receiving myo-inositol plus DCI had greater reductions in LH, free testosterone, and fasting insulin levels, increased estrogen levels, and improved insulin sensitivity.66 In another controlled trial in 44 overweight or obese PCOS patients, 10 were treated with diet, 4 grams myo-inositol, and 400 mcg folic acid; 13 with myo-inositol and DCI in a 40:1 ratio containing 1,100 mg myo-inositol; and 21 with diet alone. Weight, BMI, and hip and waist circumference decreased significantly in all groups, while only the 40:1 ratio group experienced a significant return of menstrual regularity.67
In a controlled trial, the quality of oocytes retrieved from 11 women with PCOS-related infertility undergoing a type of in vitro fertilization (IVF) was assessed after 12 weeks of treatment with 550 mg myo-inositol plus either 300 mg or 27.6 mg DCI per day. The higher dose of D-chiro-inositol was found to have a greater positive effect on oocyte quality.68
Vitamin D
Vitamin D is increasingly recognized for its important role in reproductive and metabolic health, and vitamin D deficiency may contribute to hormonal, metabolic, and mental health disorders in women with PCOS.69-71 One observational study found vitamin D deficiency was associated with higher male hormone levels and BMI in women with PCOS, while another found that vitamin D deficiency was more common in women with than without PCOS.72,73
Multiple randomized controlled trials and meta-analyses have demonstrated the beneficial effects of vitamin D therapy on metabolic and hormonal health in women with PCOS. A meta-analysis that included data from 11 randomized controlled trials with a combined total of 483 women with PCOS found that vitamin D, at doses ranging from 4,000–7,143 IUs (100–179 mcg) per day, resulted in lower total testosterone, insulin resistance, total cholesterol, and low-density lipoprotein (LDL)-cholesterol.74 Other meta-analyses of many of these same trials have reached similar conclusions, finding that vitamin D can have broad positive impacts on lipid profile and glucose metabolism in women with PCOS, particularly those with vitamin D deficiency. In a 12-week, randomized, placebo-controlled trial in which 50,000 IUs (1,250 mcg) vitamin D was given weekly to 30 overweight women (age 18‒49) with PCOS and vitamin D deficiency, supplementation led to reduced unwanted hair growth and male hormone levels.75,76 In another randomized controlled trial that included 180 women with PCOS and 150 without, all of whom had serum 25-OH vitamin D levels below 30 ng/mL, those with PCOS who received 20,000 IU (500 mcg) vitamin D per week experienced an improved LH:FSH77 ratio versus placebo.78
Omega-3 Fatty Acids
The anti-inflammatory activity of omega-3 fatty acids may reduce cardiovascular, metabolic, and hormonal complications of PCOS.79 A case-control study in 325 women with PCOS and 325 healthy controls found that omega-3 fats in serum phospholipids were lower in women with PCOS. Women in the highest third of these omega-3 levels had 30‒40% lower odds of having PCOS compared with those in the lowest third.78
A statistical analysis that included nine randomized controlled trials, involving a total of 591 women with PCOS, also reported finding omega-3 fatty acid supplementation improved insulin sensitivity, lowered total cholesterol and triglyceride levels, and raised levels of adiponectin (a signaling peptide primarily produced by fat tissue that improves insulin sensitivity and has anti-inflammatory properties).80,81 Omega-3 fatty acid supplements were also found to lower high-sensitivity C-reactive protein (hs-CRP) levels and increase adiponectin levels in women with PCOS in another statistical analysis.82
In one randomized controlled trial in 88 women with PCOS, supplementing with 2,000 mg omega-3 fatty acids from fish oil (including 360 mg EPA and 240 mg DHA) per day for six months resulted in reductions in waist circumference and triglyceride and cholesterol levels.83
N-acetylcysteine (NAC)
N-acetylcysteine (NAC) is a form of the sulfur-containing amino acid cysteine and a precursor to glutathione, one of the body's most important antioxidants and detoxifiers.
One randomized controlled trial in 100 subjects with PCOS compared treatment with NAC (1,800 mg/day) to metformin (1,500 mg/day) for 24 weeks. NAC treatment significantly improved BMI, waist circumference and waist-to-hip ratio, while metformin did not. Additionally, markers of insulin sensitivity such as fasting insulin, fasting glucose, and fasting glucose/insulin ratio improved significantly with NAC but not with metformin. A greater reduction in total testosterone levels and fewer side effects were seen in NAC-treated participants.84
Multiple controlled trials have shown NAC can improve the effectiveness of ovulation-inducing medications in women with infertility due to PCOS. A randomized controlled trial in 162 women with PCOS-related infertility being treated unsuccessfully with the fertility drug clomiphene citrate (Serophene, Clomid) compared the effects of adding NAC or L-carnitine to treatment for three months. The NAC group experienced greater improvement in free testosterone levels and insulin resistance markers, while both groups experienced similar improvements in menstrual regularity and LH and FSH levels; however, only the L-carnitine group had improvements in lipid levels.85 In another controlled trial, 1,800 mg NAC per day was compared to 1,500 mg metformin as an addition to clomiphene citrate therapy in 108 women with clomiphene-resistant PCOS-related infertility. NAC led to greater reductions in unwanted hair growth and fasting glucose levels; metformin led to greater reductions in BMI and insulin levels. Also, NAC and metformin had similar positive effects on ovulation and fertility rates after 12 weeks.86
NAC may also be helpful in women undergoing infertility treatment with letrozole (Femara). A double-blind, randomized, placebo-controlled trial in 130 PCOS patients showed the combination of letrozole plus 1,200 mg NAC per day resulted in more follicles greater than 18 mm in diameter (a size associated with greater likelihood of ovulation) after five days than letrozole alone or placebo. In addition, those who received NAC as an adjunct to letrozole had higher rates of ovulation and pregnancy.87 However, another randomized controlled trial that included 97 women with PCOS found 1,200 mg NAC per day did not improve the effects of combination therapy with clomiphene citrate plus letrozole for inducing ovulation.88
NAC may also have value as a supportive therapy prior to IVF. In a placebo-controlled trial in 80 women with PCOS preparing for IVF, 1,800 mg NAC per day for six weeks appeared to improve the quality of oocytes.89
Berberine
Berberine is a bioactive alkaloid found in several medicinal plants including goldenseal and phellodendron.90 These plants have been widely used in traditional Asian medicine for infections, metabolic disorders, and other indications. Its efficacy in improving insulin sensitivity, reducing serum androgens, and modulating chronic inflammation have made berberine a subject of interest in PCOS research.91-94
A trial in 89 women with PCOS and insulin resistance found that berberine, in combination with hormone treatment, improved lipid profiles and SHBG levels, as well as glucose metabolism and waist circumference compared with combinations of either metformin or placebo and the same hormone treatment.95 In a small clinical trial, 12 women with newly diagnosed PCOS were given 550 mg of a berberine-phospholipid complex twice daily for 60 days. After treatment, there were significant improvements in insulin sensitivity, testosterone levels, and markers of inflammation.91 Berberine may also improve IVF outcomes: 150 women with PCOS undergoing IVF treatment were randomized to receive either metformin, berberine, or placebo for three months before ovarian stimulation. The women given metformin or berberine had reductions in testosterone and insulin resistance, as well as increased pregnancy rates compared with those given placebo. The women who took berberine also had improved rates of live births and fewer adverse effects than metformin.96
Berberine has been proposed to affect hormone levels and insulin metabolism by inhibiting the PI3K/AKT and MAPK pathways, activating AMPK, and interacting with multiple other targets.94,97 Additionally, berberine may ameliorate certain aspects of PCOS pathology by reducing levels of several pro-inflammatory markers such as nuclear factor-kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), and interleukins 1 (IL-1) and 6 (IL-6).98
Although berberine has been studied in human clinical trials and shown to have several metabolic benefits, concerns about long-term use of berberine have been raised on the basis of certain preclinical studies.99-101 Some evidence suggests long-term berberine use, especially at high doses, may impair some aspects of cellular metabolism in specific types of cells. The implications of this preclinical research are yet to be determined by long-term human clinical trials; therefore, Life Extension currently recommends short-term use of berberine.
Probiotics
The gut microbiome plays a critical role in modulating immune function and metabolism, and may play a role in the unwanted hair growth that is part of PCOS.29 A statistical analysis of 17 randomized controlled trials that included a total of over 1,000 participants found that intake of probiotics, prebiotics (partially or completely indigestible carbohydrates that enhance growth of beneficial gut bacteria), or synbiotics (combinations of probiotics and prebiotics) was of benefit for metabolic aspects of PCOS. Specifically, improvements occurred in fasting glucose and insulin levels, insulin sensitivity, total and LDL-cholesterol, and triglyceride levels.35 Another statistical analysis of findings from nine randomized controlled trials with a combined total of 587 participants found treatment with probiotics or synbiotics for eight weeks or longer improved unwanted hair growth, fasting blood sugar and insulin, insulin sensitivity, and BMI. Testosterone as well as measures of inflammation and oxidative stress improved as well.34
However, in a placebo-controlled trial in 99 subjects with PCOS, 12 weeks of treatment with a synbiotic supplement providing the prebiotic inulin plus a combination of seven probiotic strains improved LDL- and high-density lipoprotein (HDL)-cholesterol levels, but did not alter triglyceride levels, body weight, or body composition.102
Magnesium
Magnesium plays an important role in regulating glucose metabolism and insulin sensitivity, and low magnesium status may be a contributing factor in the progression of insulin resistance to type 2 diabetes and heart disease.103 A statistical analysis of eight studies in a total of over 2,000 women found that serum magnesium concentrations were lower in overweight or obese women with PCOS than normal-weight women with PCOS.104 Furthermore, a study that examined the magnesium status of more than 1,000 women with PCOS found lower magnesium levels were associated with worse insulin resistance and higher testosterone levels.105
Another placebo-controlled trial in 60 PCOS-affected subjects found 250 mg magnesium oxide per day for eight weeks increased LH levels and reduced BMI but did not affect markers of glucose or lipid metabolism, or multiple indices of hormonal status.106
Curcumin
Curcumin, a polyphenol from turmeric (Curcuma longa), reduces oxidative stress and inflammation and has been shown to improve glucose and lipid metabolism as well as insulin sensitivity.107 A statistical analysis of three randomized controlled trials with a combined total of 168 participants found curcumin supplementation, at doses of 500–1,500 mg per day, led to improvements in fasting glucose, insulin levels, measures of insulin sensitivity, and HDL- and total cholesterol in women with PCOS.108
In one trial included in the meta-analysis, 60 women with PCOS received 500 mg curcumin or placebo twice daily for six weeks. The curcumin-treated women experienced a significant reduction in serum insulin and an improvement in insulin sensitivity compared with placebo.109 In another trial in the statistical analysis, 67 women with PCOS received 500 mg curcumin three times daily or placebo for 12 weeks. At the end of the trial, levels of fasting plasma glucose and androgens decreased significantly in the curcumin group compared with controls.110 Treatment with 1,500 mg curcumin per day for 12 weeks was also shown to upregulate expression of a PPAR-γ activator molecule and improve markers of oxidative stress more than placebo in a controlled trial in 72 women with PCOS.111
Cinnamon
A review of multiple clinical trials concluded that cinnamon (Cinnamomum zeylanicum) bark powder, in doses of 1‒6 grams per day, has glucose-lowering effects in people with type 2 diabetes.112 Cinnamon has demonstrated anti-hypertensive, anti-obesity, and lipid-lowering properties in both clinical and preclinical settings, making it a likely candidate for metabolic syndrome (a cluster of co-occurring risk factors for cardiovascular disease and type 2 diabetes) and PCOS treatment.113,114
A statistical analysis of five randomized controlled trials found that cinnamon improves a measure of insulin resistance in women with PCOS.115 In one of the trials 80 women with PCOS were randomly assigned to receive either a total of 1.5 grams cinnamon powder daily, taken in three divided doses, or placebo for 12 weeks. The cinnamon group experienced significantly greater reductions in fasting insulin, insulin resistance, and LDL-cholesterol than the placebo group.116 In a placebo-controlled trial in 84 subjects (age 20‒38 years) with PCOS and overweight or obesity, 1.5 grams of cinnamon daily for eight weeks increased antioxidant status, decreased a measure of oxidative stress, and improved total, LDL-, and HDL-cholesterol levels.117 In an rodent model of PCOS, the addition of cinnamon to androgen treatment blunted the changes in ovarian function and structure that occurred with androgen treatment alone. The addition of cinnamon also resulted in more normal levels of multiple sex hormones.118
Lipoic Acid
Lipoic acid (alpha-lipoic acid, ALA; R-lipoic acid, R-LA [a highly bioavailable form]) is an important antioxidant nutrient that has been shown to support healthy glucose metabolism.119 A large statistical analysis found that ALA lowers serum insulin and improves insulin sensitivity.120 In an uncontrolled trial in 32 women with PCOS and obesity, 12 weeks of daily treatment with 400 mg ALA resulted in reduced insulin and glucose levels, BMI, and a measure of insulin resistance.121 In another small uncontrolled trial, 600 mg twice daily of controlled-release lipoic acid was administered to six normal-weight women with PCOS for 16 weeks. Insulin resistance, triglyceride levels, and LDL particle size improved. In addition, menstrual irregularities improved in the two subjects not taking oral contraception.122
Several clinical trials have noted positive effects of a combination of lipoic acid and inositol on biomarkers and symptoms of PCOS. In a case-control study in 23 adolescents with PCOS and 21 healthy controls, myo-inositol plus lipoic acid treatment for six months was studied. Treatment significantly reduced insulin resistance and serum insulin levels.123 In an uncontrolled pilot study in 40 women with PCOS, the combination of myo-inositol and lipoic acid decreased male hormone levels, improved menstrual regularity, and reduced unwanted hair growth and BMI without affecting glucose or insulin parameters.124 Long-term treatment with daily lipoic acid at 800 mg and myo-inositol at 2,000 mg for 24 months resulted in significant reductions in menstrual irregularities in an uncontrolled trial in 44 PCOS patients with a history of infrequent menstrual periods. Insulin response to a glucose challenge improved as well.125 In a six-month uncontrolled trial in 71 women with PCOS, 800 mg ALA plus either 1,000 or 2,000 mg of inositol daily improved menstrual regularity. However, substantially fewer women who received 1,000 mg derived this benefit.126
Chromium
The mineral chromium may benefit women with PCOS due to its ability to support glucose regulation and metabolic health.127 A case-control study in 42 women with PCOS (14 with metabolic syndrome and 28 without) found lower intake of chromium, as well as of several antioxidant micronutrients, in participants with metabolic syndrome and PCOS.128
In a double-blind, randomized, controlled trial in 85 women with PCOS who received either 1,000 mcg chromium picolinate or placebo daily for six months, chromium picolinate supplementation led to significant improvements in BMI and fasting serum insulin levels. Chromium supplementation also increased the chances of ovulation and regular menstruation nearly two-fold after five months.129 In a small pilot trial that enrolled 10 women with PCOS and randomized them to receive 200 mcg chromium picolinate or placebo daily for four months, chromium supplementation improved glucose tolerance but did not improve ovulatory frequency.130 Another small study also reported improvements in glucose metabolism in response to 1,000 mcg chromium picolinate supplementation in obese women with PCOS.131
A statistical analysis of data from seven randomized controlled trials found chromium supplementation significantly reduced BMI and free testosterone levels.132 However, another meta-analysis that included six randomized controlled trials with a total of 351 participants found that although chromium supplementation decreased insulin resistance it also markedly increased total and free testosterone levels.133 On the basis of these divergent conclusions from meta-analyses regarding free testosterone levels, it would be prudent for women with PCOS who choose to take chromium to regularly monitor their free testosterone levels.
L-carnitine
L-carnitine is an amino acid with a critical role in lipid metabolism. Clinical evidence shows it may be useful for treating metabolic disorders such as type 2 diabetes and non-alcoholic fatty liver disease (NAFLD).134,135 An observational study found women with PCOS, especially those with obesity, had lower L-carnitine levels than those without PCOS.136
In an uncontrolled trial of 74 women with PCOS, daily treatment with 3 grams L-carnitine over a period of three months resulted in significant improvements in insulin sensitivity, BMI, unwanted hair growth, lipid levels, and menstrual cycle regularity.137 A randomized controlled trial enrolled 170 women with PCOS-related infertility who had proven to be resistant to the effects of clomiphene. The participants were randomized to receive 150 mg clomiphene in addition to placebo or 3 grams of L-carnitine on days 3‒7 of their menstrual cycles. Compared with the placebo, L-carnitine improved the ovulation rate (64.4% vs. 17.4%) and pregnancy rate (51.5% vs. 5.8%).138
In a randomized placebo-controlled trial in 147 women with PCOS, L-carnitine in the form of acetyl-L-carnitine (ALC), at a dosage of 500 mg twice daily, was found to add to the benefits of the anti-diabetes medications metformin and pioglitazone (Actos). One group received only the medications, another received the medications plus ALC, and the third group received placebo. Both active treatments resulted in decreased serum insulin levels, though the effect was more pronounced in the group that received ALC. Luteinizing hormone (LH) decreased, and psychological scores improved, in a similar manner. Both treatments increased serum adiponectin and insulin sensitivity, and improved testosterone and FSH levels. Menstrual regularity and waist circumference improved only in those who received ALC.139
Resveratrol
Resveratrol, a polyphenol found in grape skins, red wine, peanuts, Japanese knotweed, and berries, is a phytoestrogen that has demonstrated estrogenic effects in both animal studies and clinical trials.140,141 Findings from animal and human studies suggest resveratrol may improve metabolic parameters, male hormone levels, ovarian function, and fertility in PCOS.142
In a randomized placebo-controlled trial in 78 PCOS patients, 1,000 mg resveratrol daily for three months resulted in increased menstrual regularity and reduced hair loss, though other reproductive and metabolic indices were unchanged.143 A randomized controlled trial in 30 women with PCOS found 1,500 mg resveratrol daily for three months reduced total testosterone, dehydroepiandrosterone-sulfate (DHEA-S), and fasting insulin levels; resveratrol treatment also increased insulin sensitivity.144
In a randomized controlled trial, 61 women with PCOS-related infertility were given either 800 mg resveratrol or placebo daily for 40 days prior to ovulation induction and oocyte retrieval for IVF. Those who received resveratrol had a greater number of high-quality oocytes and post-fertilization embryos. They were also found to have reduced total testosterone and LH levels and increased FSH levels compared with placebo.145 An open trial in 40 subjects with PCOS found 40 days of 800 mg resveratrol per day reduced inflammatory cytokine and CRP levels as well as levels of markers of a type of cellular stress called endoplasmic reticulum stress.146 Abnormal proteins resulting from endoplasmic reticulum stress are thought to play a role in PCOS-related ovarian dysfunction.147
Melatonin
Melatonin is a hormone secreted by the pineal gland that regulates sleep-wake (circadian) cycles in the body and has free radical-scavenging effects. In healthy individuals, melatonin levels exhibit a daily pattern, peaking at night and dipping in the morning. Genetic variants of melatonin receptors have been linked to increased risk of PCOS, and sleep disturbances including sleep apnea are common in women with PCOS.148
Circadian control systems appear to be dysregulated in women with PCOS. In a study that included 321 female volunteers with and without signs of PCOS, higher morning melatonin levels were associated with expression of a higher number of PCOS-related features (hyperandrogenism, few or no periods, and cystic ovaries), suggesting PCOS may be associated with disrupted circadian signaling. The study also found a relationship between excessively high morning cortisol levels and number of PCOS features. Since cortisol secretion also follows a regular circadian pattern roughly opposite to that of melatonin, this finding provides additional confirmation of possible day-night cycle disturbance in women with PCOS.149 A cross-sectional study in adolescent girls noted a relationship between morning circadian misalignment, based on irregular melatonin secretion, and PCOS-related metabolic disturbance.150
A randomized, double-blind, placebo-controlled trial in 84 women with PCOS found that 6 mg of melatonin, along with 250 mg magnesium oxide, daily for eight weeks reduced unwanted hair growth, serum insulin, total testosterone, total and LDL-cholesterol, and raised HDL-cholesterol and total antioxidant capacity compared with baseline.151,152 A prospective cohort study in 40 women with PCOS found that melatonin supplementation reduced male hormone levels, normalized other reproductive hormones, and improved menstrual regularity.153
Flaxseeds and Flaxseed Oil
Flaxseeds are a source of lignans (plant compounds that modulate estrogenic activity) and the omega-3 fatty acid alpha-linolenic acid. In animal models, flaxseed oil has been found to modulate hormone signaling, improve lipid metabolism, and reduce inflammation related to PCOS partly through altering gut microbiome composition.155-157 Flaxseed powder is also a source of dietary fiber, which also may be of benefit in PCOS.
In a randomized, open-label, controlled trial completed by 41 participants with PCOS, those who undertook lifestyle modification and received 30 grams flaxseed powder (about 2 tablespoons) per day for 12 weeks exhibited several improvements compared with lifestyle modifications alone. These changes included improvements in body weight, insulin resistance, triglycerides, hs-CRP, leptin (an appetite-regulating hormone, high levels of which are often associated with obesity), HDL-cholesterol, and adiponectin.158 In a case report, 30 grams flaxseed powder daily for four months was associated with reductions in androgen levels and unwanted hair growth in a 31-year-old PCOS patient.157
7 Dietary & Lifestyle Changes for Polycystic Ovary Syndrome
Weight Loss
Losing as little as 5‒10% of bodyweight can reduce insulin resistance, cardiometabolic risk, and male hormone levels while improving menstrual function and possibly fertility in women with PCOS.8,14 The primary method for accomplishing this is diet, exercise, and lifestyle measures, although in indicated cases bariatric surgery has proven remarkably successful when a more conservative approach does not suffice.
Exercise
For women with PCOS, participation in a regular exercise regimen has many potential benefits, including improving menstrual problems, ovulation, cardiovascular and metabolic health, and mental health.159 However, two studies that compared the response of women with PCOS to that of healthy controls found that, while exercise did deliver many of the expected benefits to women with PCOS, it failed to alter the glucose-insulin dynamic in the body as a whole and in skeletal muscle, and it did not have the expected effect on fat burning. Exercise improved these parameters in the control group; thus, metabolic response to physical activity appears to be blunted in individuals with PCOS.160,161 A meta-analysis of 19 trials that included 777 women found that exercise intensity is more important than duration to derive benefits to insulin sensitivity and waist circumference. The authors concluded that a minimum of 120 minutes per week of vigorous-intensity exercise is needed for women with PCOS.162 A systematic literature review concluded that vigorous aerobic exercise and resistance training can lower male hormone levels and improve insulin sensitivity, while a randomized controlled trial in 61 women with PCOS found that a program of yoga exercise also may be of benefit as it reduced unwanted hair growth, abdominal circumference, and hip circumference compared with controls.163,164
Diet
Eating fewer calories and reducing dietary glycemic value (a measure of a food’s potential to raise blood glucose levels) may be beneficial in women with PCOS, especially those who are overweight or obese. In a 24-week trial in 62 overweight or obese women, 28 with PCOS and 34 without, a reduced-calorie, low-glycemic index diet significantly improved acne, menstrual regularity, and testosterone and SHBG levels.165
Reduced-carbohydrate diets may be beneficial in PCOS patients. A meta-analysis that included data from eight randomized controlled trials with a total of 327 participants with PCOS found reduced-carbohydrate diets, most lowering carbohydrate intake to 40–45% of daily calories versus 55‒60% of calories in control diets, reduced BMI, insulin resistance, and total and LDL-cholesterol levels. Maintaining carbohydrate restriction for more than four weeks raised SHBG and FSH levels and reduced testosterone levels. Diets with reduced amounts of both carbohydrate and fat had greater effects on FSH and SHBG.166
A ketogenic diet is a high-fat (approximately 55‒60% of calories) diet that severely restricts carbohydrates (approximately 5–10% of daily calories). Although the medium-term effects and safety of ketogenic diets, followed for as long as two years, have been established in the medical literature, long-term health consequences have not been sufficiently studied.167
Very-low carbohydrate diets such as “keto” cause a steep decline in insulin secretion. At the same time, the body shifts from burning glucose, a readily available fuel given normal carbohydrate consumption, to eventually beginning to produce and burn ketones from fat as a primary energy source. Benefits such as weight loss, increased insulin sensitivity, and improved glucose metabolism have been attributed to keto diets, based on a long history of clinical use as well as clinical studies of up to two years in duration.167
A study in 14 women with PCOS found that eating a hybrid ketogenic-Mediterranean diet, along with food supplements and some phytoextracts, for 12 weeks led to reductions in body weight, BMI, and abdominal fat. Importantly, the diet was designed to emphasize monounsaturated fats such as those found in olive oil and nuts, and limit saturated and polyunsaturated fat intake by restricting the intake of eggs, meat, and fish. Significant reductions were also observed in glucose levels, insulin levels, and an index of insulin resistance.168
Eating a diet high in animal products and low in plant-based foods is associated with insulin resistance, heart disease, and type 2 diabetes.169 Moreover, the quality of carbohydrates in the diet appears to be a critical factor for long-term weight management and cardio-metabolic health. While sugar-sweetened beverages and refined grains are low-quality carbohydrates that raise the risk of obesity, heart disease, and type 2 diabetes, complex and high-fiber carbohydrate foods, such as beans and lentils, vegetables and fruits, and whole grains, have been associated with weight loss and decreased risk of heart disease and type 2 diabetes.170,171
In a study of 87 women with PCOS and 50 women without, those with PCOS and insulin resistance were found to consume less dietary fiber and magnesium and a higher glycemic load diet. Glycemic load, like glycemic index, measures the effect of diet and food on blood sugar. Lower fiber consumption in women with PCOS was associated with insulin resistance, higher fasting insulin and worse glycemic control, and higher levels of androgens.172 An eight-week low-starch/low-dairy dietary intervention study, without calorie restriction, in 10 women with PCOS also reduced body weight and fasting insulin levels.173
The Dietary Approaches to Stop Hypertension (DASH) diet is a mainly plant-based diet that emphasizes vegetables, fruits, whole grains, beans, nuts and seeds, lean meats, low-fat dairy products, and restricts saturated fat, cholesterol, red meat, refined grains, and sweets and salt.174,175 In a randomized controlled three-month trial in 60 women with PCOS, participants were assigned to one of two energy-restricted diets, either DASH or a control. The DASH diet group experienced greater body weight and fat loss compared with controls. Improvements in androstenedione (an androgen) and SHBG levels, as well as antioxidant status, were also observed in the DASH diet group.176
8 Diagnosis of Polycystic Ovary Syndrome
The Rotterdam Criteria were established in 2003 and are the basis of current diagnostic guidelines for PCOS. Diagnosis using these criteria requires the presence of two of the following three features177:
- Oligomenorrhea (absent or irregular periods) or oligo-ovulation (low frequency of ovulation)
- High male hormone levels, based on symptoms or lab tests
- Polycystic ovaries on ultrasound
A complete medical history and physical exam are critical for the diagnosis of PCOS.13 Laboratory testing can also establish the presence of hyperandrogenism, and assessment of cardiometabolic risk, through both blood tests and medical history, is recommended. The third main feature of PCOS is polycystic ovaries, sometimes referred to as polycystic ovarian morphology (PCOM), which is diagnosed by ultrasound.9 Psychological screening is also important given the high prevalence of mood and other related disorders in patients with PCOS.9
Importantly, PCOS is a diagnosis of exclusion, meaning that other causes of ovarian dysfunction and hormone imbalance must be excluded to confirm a diagnosis.177 Other conditions with similar clinical features include thyroid disease, hyperprolactinemia (high levels of the hormone prolactin), an androgen-producing tumor of the ovary or adrenal gland, and non-classical congenital adrenal hyperplasia (NC-CAH), which causes adrenal steroid 21-hydroxylase deficiency.13,178 NC-CAH is an autosomal recessive disorder that is often confused with PCOS due to many overlapping symptoms; it is differentially diagnosed by hormonal profiles, adrenal function tests, and genetic tests.187,218
Although the onset of PCOS frequently occurs near the onset of puberty, diagnosing PCOS in girls and younger women is complicated by the fact that high levels of androgens and menstrual irregularities are common during puberty.1,7 Another consideration is that large, polycystic ovaries can be a normal finding in adolescents.13
Ultrasound
Transvaginal ultrasound is used to visualize the ovaries in suspected cases of PCOS in which hyperandrogenism and ovulatory dysfunction have not been confirmed.8 Most women with PCOS are found to have polycystic ovaries with a high number of ovarian follicles and greater-than-normal ovarian volume visible on ultrasound. However, there is still disagreement regarding the exact number of follicles and volume of ovaries considered to be diagnostic.13,177 Ultrasound is not a useful diagnostic tool in adolescents who have been menstruating for less than eight years.3
Lab Tests
Blood work is used to measure levels of hormones, assess metabolic disturbance, and exclude other possible causes of menstrual abnormalities or androgen excess that mimic PCOS.
Androgens:
- Testosterone. Levels of total testosterone and free testosterone (the fraction that is biologically active) are generally used to diagnose women suspected of having PCOS.7 Free testosterone is currently recognized as a more accurate test for identifying androgen excess, in part because insulin resistance can interfere with measurement of total testosterone.13,179 Free testosterone can be measured directly, or indirectly by means of a calculation from measurements of total testosterone and SHBG.8
- Androstenedione. Androstenedione is an androgen hormone produced from progesterone by the adrenal gland and is a precursor hormone to testosterone. While some PCOS patients have high androstenedione levels, very high levels may indicate an adrenal disorder as the cause of hyperandrogenism.177
- Dehydroepiandrosterone. DHEA is another adrenal androgen and is mostly found in circulation as DHEA-S. Elevated DHEA-sulfate (DHEA-S) levels may be an indicator of PCOS. In fact, in approximately 5% of PCOS patients, the only laboratory sign of androgen excess is an increased DHEA-S level.13
Regulatory Proteins:
- Sex hormone-binding globulin (SHBG). SHBG, a protein involved in regulating sex hormone bioavailability, has emerged as an important biomarker of PCOS, informing both diagnosis and treatment. There is some evidence that it could be useful for early diagnosis as well.180,181 Because SHBG binds to sex hormones and reduces their bioavailability, decreased SHBG levels lead to increased androgen signaling and is closely associated with both ovarian dysfunction and metabolic disturbance. In addition, insulin resistance can reduce production of SHBG.181,182
- Anti-Müllerian hormone (AMH). AMH is a protein produced by growing ovarian follicles that helps regulate follicular growth. Blood levels of AMH are correlated with number of ovarian follicles and have been found to be two to four times higher in women with PCOS than without.183 Some evidence suggests AMH may not only be an indicator of polycystic ovaries but may also be related to PCOS-associated ovarian dysfunction, hyperandrogenism, and infertility, as well as insufficient response to treatment.183-185 Its use in diagnosing and guiding treatment for PCOS is being refined.186
Other Hormones:
- Luteinizing hormone (LH) and follicular stimulating hormone (FSH). LH and FSH are pituitary hormones that regulate ovarian function. Hormonal imbalance in women with PCOS disrupts feedback mechanisms that regulate LH and FSH release, resulting in increased LH production, decreased FSH production, and a rise in the ratio of LH to FSH.1,7
- 17-hydroxyprogesterone. 17-hydroxyprogesterone levels may be necessary to rule out an adrenal cause of androgen excess.187 High 17-hydroxyprogesterone levels are strongly suggestive of NC-CAH, rather than PCOS, particularly after adrenocorticotropic hormone (ACTH) stimulation test, which measures the adrenal glands’ stress response.218
- Prolactin. Prolactin is a hormone related to growth hormone and secreted from the pituitary. It has many functions but is best known for its role in lactation and breast development.188 Excessive secretion of prolactin, known as hyperprolactinemia, can lead to symptoms that mimic PCOS, so prolactin level are sometimes obtained to rule this out.9,13
- Thyroid stimulating hormone (TSH). TSH levels are measured to rule out a thyroid disorder as the cause of PCOS-like symptoms.13 Additional information about thyroid dysfunction is available in Life Extension’s Hypothyroidism protocol.
Other Blood Tests:
- Metabolic tests. Recommended testing for metabolic dysfunction has been insufficiently utilized by many clinicians, despite the high prevalence of insulin resistance, impaired glucose tolerance, metabolic syndrome, type 2 diabetes, and dyslipidemia in PCOS patients compared with their healthy counterparts. Guidelines from the American College of Obstetricians and Gynecologists and the Endocrine Society recommend that all PCOS patients be tested for impaired glucose tolerance and dyslipidemia by means of a two-hour oral glucose tolerance test and fasting lipid profile at diagnosis, and at regular intervals thereafter. Many gynecologists either do not order any metabolic lab tests or order only fasting glucose and hemoglobin A1C tests.189 Lab tests are ultimately ordered at the discretion of the clinician, and may include any or all of these tests.
- Vitamin D. The prevalence of vitamin D deficiency is far higher in women with PCOS than in the general population.190-192 The assessment of vitamin D status has important clinical implications in PCOS, as vitamin D deficiency has been shown to be associated with higher male hormone levels, insulin resistance, ovulatory dysfunction and infertility, and mental health problems.193
- Homocysteine. Several studies have noted levels of homocysteine, an independent cardiovascular risk factor, are elevated in PCOS patients. Higher homocysteine levels are frequently seen in those with insulin resistance and other cardiovascular risk factors; however, high homocysteine levels have been found to be correlated with PCOS diagnosis independently of other indicators of cardio-metabolic risk.194,195 In addition, some studies suggest elevated homocysteine levels may be correlated with high androgen levels and fertility problems in women with PCOS.194
- Inflammatory markers. Chronic low-grade inflammation is a prominent though subclinical feature of PCOS and is implicated in PCOS sequela and co-occurring conditions. These include obesity, insulin resistance, metabolic syndrome, type 2 diabetes, and cardiovascular disease. The inflammatory markers C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), white blood cell count (WBC), and others have all been found to be higher in PCOS patients than in matched healthy individuals.47 These tests can be used to assess baseline status, risk of complications, and response to treatment.
9 Conventional Polycystic Ovary Syndrome Treatment
Polycystic ovary syndrome treatment is individualized to address the patient’s main concerns, which may include menstrual problems, infertility, unwanted hair growth, acne, weight gain, or metabolic disturbance. Dietary and lifestyle changes aimed at improving insulin sensitivity and body composition are a central pillar of an effective treatment plan. Drug therapies are commonly used to bring clinical symptoms and biochemical abnormalities under control, either while the patient attempts diet and lifestyle changes or in the event a patient does not improve sufficiently with nutrition and lifestyle strategies.
Oral Contraceptives
Hormonal contraception providing both estrogen and progestins are the first choice medical treatment for managing menstrual irregularities, unwanted hair growth, acne, and for providing contraception.8,13 Estrogens and progestins lower LH levels, inhibiting androgen production in the ovaries, and increase levels of SHBG, reducing androgen activity.1 Oral contraception is preferred for its beneficial effects on SHBG concentrations.9 In addition, combined oral contraceptives appear to limit conversion of testosterone into the more potent androgen 5-α-dihydrotestosterone by inhibiting the enzyme 5-α-reductase.13
Antiandrogens
Spironolactone (Aldactone) is a drug with an antiandrogenic effect due to its ability to block androgen receptors.196 The use of spironolactone and oral contraceptives has been shown to be more effective than either alone for PCOS-related unwanted hair growth, and is often recommended for treating moderate-to-severe unwanted hair growth, or mild unwanted hair growth that is not sufficiently resolved after six months of treatment with oral contraceptives.1,13
Spironolactone might also be helpful in treating hair loss and acne related to androgen excess.1 For more information about therapies for these conditions, see the Hair Loss and Acne protocols.
Sexually active women should always use contraceptives with spironolactone since it is a strong teratogen (an agent that can cause birth defects). For the same reason, spironolactone should not be used during pregnancy.13,196 Spironolactone can also cause electrolyte imbalance and dehydration.1,196
Finasteride (Proscar) is an antiandrogen that works by inhibiting the conversion of testosterone into its more potent metabolite, dihydrotestosterone. It has been shown to improve hirsutism in women with PCOS. Some evidence indicates finasteride may also improve insulin resistance and glucose metabolism.197
Metformin
Metformin (Glucophage) is an anti-diabetes medication that inhibits liver glucose production, decreases intestinal glucose uptake, and increases insulin sensitivity in peripheral tissues.198 After hormonal contraception metformin is considered second-line therapy in PCOS, although its effects can be variable. Metformin has been shown to improve ovulation and fertility in PCOS patients.199,200 It generally reduces androgen levels but is not a reliable anti-hirsutism agent. Metformin may also support weight loss, but this effect is likely to be modest.8,9,13 Metformin’s effects may be greatest in those at high risk of type 2 diabetes, especially in the presence of obesity.15,16 One setting in which metformin is sometimes considered first-line treatment is in adolescents with PCOS, although oral contraceptives are important as well.1,13
Metformin often causes gastrointestinal side effects (eg, gas and diarrhea). Beginning with a lower dose and working up to a higher dose may help some individuals avoid these side effects.201 Although current evidence suggests metformin may be safe in pregnancy, its use during pregnancy is still controversial.200,202,203
Importantly, metformin can increase homocysteine levels. A statistical analysis of 11 studies found metformin at doses of ≥1,700 mg per day increased serum homocysteine and decreased folic acid levels in non-pregnant women with PCOS.204 Another clinical study using metformin at 500 mg twice daily also reported increased homocysteine levels in overweight and obese women with PCOS.205 Therefore, using the lowest effective dose of metformin and supplementing with folate may be advisable for maintaining healthy homocysteine levels.204,206
Statins
Statins are a family of cholesterol-lowering drugs that includes atorvastatin (Lipitor), lovastatin (Mevacor), simvastatin (Zocor), and others. In addition to improving lipid levels, some research suggests statins, particularly atorvastatin, reduce low-grade inflammation and high testosterone levels in women with polycystic ovary syndrome.197 The potential benefits of statin therapy in PCOS patients are still under investigation.
Weight Loss Surgery
Bariatric surgery improves multiple aspects of PCOS sequela.14 Bariatric surgery should be reserved for women with extreme obesity or with moderate obesity plus additional health concerns. Of course, it is also only indicated after diet, exercise, and lifestyle measures have been applied. One statistical analysis of 13 studies of bariatric surgery in PCOS patients found a marked reduction in symptoms accompanied by an over 50% loss of body weight.9 Another statistical analysis of 21 studies found that bariatric surgery resulted in decreases in hirsutism, menstrual irregularity, infertility, type 2 diabetes, high blood pressure, and depression. Total testosterone, fasting insulin, and LH levels decreased while estradiol increased after three months of follow-up. Longer follow-up times of six months or ≥12 months revealed decreases in testosterone and fasting insulin, blood glucose, and triglyceride levels. High-density lipoprotein and SHBG were significantly improved only ≥12 months after bariatric surgery.207
Hair Removal
Hirsutism that does not respond adequately to hormonal contraception or anti-androgen therapy can also be treated by waxing, shaving, electrolysis, or laser hair removal. In addition, eflornithine hydrochloride cream (Vaniqa) is a topical drug that inhibits hair growth, but it must be used indefinitely to prevent regrowth.14
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Witchel SF, Oberfield SE, Pena AS. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls. Journal of the Endocrine Society. Aug 1 2019;3(8):1545-1573. doi:10.1210/js.2019-00078
- Gilbert EW, Tay CT, Hiam DS, Teede HJ, Moran LJ. Comorbidities and complications of polycystic ovary syndrome: An overview of systematic reviews. Clin Endocrinol (Oxf). Dec 2018;89(6):683-699. doi:10.1111/cen.13828
- Witchel SF, Teede HJ, Peña AS. Curtailing PCOS. Pediatric research. Jan 2020;87(2):353-361. doi:10.1038/s41390-019-0615-1
- Alur-Gupta S, Chemerinski A, Liu C, et al. Body-image distress is increased in women with polycystic ovary syndrome and mediates depression and anxiety. Fertility and sterility. Nov 2019;112(5):930-938.e1. doi:10.1016/j.fertnstert.2019.06.018
- Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int J Environ Res Public Health. Nov 20 2018;15(11)doi:10.3390/ijerph15112589
- VectorMine. Labeled internal reproductive disease comparison scheme with healthy and sick female organs. Set of anatomical symptoms due to elevated androgens. Shutterstock: https://www.shutterstock.com/image-vector/polycystic-ovary-syndrome-pcos-vector-illustration-1474393784.
- Nicolaides NC, Matheou A, Vlachou F, Neocleous V, Skordis N. Polycystic ovarian syndrome in adolescents: From diagnostic criteria to therapeutic management. Acta bio-medica : Atenei Parmensis. Sep 7 2020;91(3):e2020085. doi:10.23750/abm.v91i3.10162
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. Jul 7 2016;375(1):54-64. doi:10.1056/NEJMcp1514916
- Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nature reviews Disease primers. 2016;2(1):1-18.
- Cree-Green M, Cai N, Thurston JE, et al. Using simple clinical measures to predict insulin resistance or hyperglycemia in girls with polycystic ovarian syndrome. Pediatr Diabetes. Dec 2018;19(8):1370-1378. doi:10.1111/pedi.12778
- Borzan V, Lerchbaum E, Missbrenner C, et al. Risk of Insulin Resistance and Metabolic Syndrome in Women with Hyperandrogenemia: A Comparison between PCOS Phenotypes and Beyond. J Clin Med. Feb 18 2021;10(4)doi:10.3390/jcm10040829
- Wekker V, van Dammen L, Koning A, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Human reproduction update. Nov 1 2020;26(6):942-960. doi:10.1093/humupd/dmaa029
- Leon LIR, Anastasopoulou C, Mayrin JV. Polycystic Ovarian Disease. StatPearls. StatPearls; 2021.
- Barbieri RL, Ehrmann DA. Treatment of polycystic ovary syndrome in adults. UpToDate. Updated 8/31/2020. Accessed 12/3/2021, https://www.uptodate.com/contents/treatment-of-polycystic-ovary-syndrome-in-adults
- Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human reproduction (Oxford, England). Sep 1 2018;33(9):1602-1618. doi:10.1093/humrep/dey256
- Guan Y, Wang D, Bu H, Zhao T, Wang H. The Effect of Metformin on Polycystic Ovary Syndrome in Overweight Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International journal of endocrinology. 2020/09/16 2020;2020:5150684. doi:10.1155/2020/5150684
- Anana_go. Symptoms of PCOS. Shutterstock: https://wwwshutterstockcom/image-vector/symptoms-pcos-infographics-detailed-vector-womens-1434467078.
- Rodriguez Paris V, Bertoldo MJ. The Mechanism of Androgen Actions in PCOS Etiology. Med Sci (Basel). Aug 28 2019;7(9)doi:10.3390/medsci7090089
- Lee I, Cooney LG, Saini S, et al. Increased risk of disordered eating in polycystic ovary syndrome. Fertility and sterility. Mar 2017;107(3):796-802. doi:10.1016/j.fertnstert.2016.12.014
- Cesta CE, Månsson M, Palm C, Lichtenstein P, Iliadou AN, Landén M. Polycystic ovary syndrome and psychiatric disorders: Co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. Nov 2016;73:196-203. doi:10.1016/j.psyneuen.2016.08.005
- Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics (Sao Paulo, Brazil). Nov 2015;70(11):765-9. doi:10.6061/clinics/2015(11)09
- Bahri Khomami M, Joham AE, Boyle JA, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-A systematic review, meta-analysis, and meta-regression. Obes Rev. May 2019;20(5):659-674. doi:10.1111/obr.12829
- Bellver J, Rodriguez-Tabernero L, Robles A, et al. Polycystic ovary syndrome throughout a woman's life. J Assist Reprod Genet. Jan 2018;35(1):25-39. doi:10.1007/s10815-017-1047-7
- Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertility and sterility. Oct 2018;110(5):794-809. doi:10.1016/j.fertnstert.2018.08.021
- Paschou SA, Polyzos SA, Anagnostis P, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Endocrine. Jan 2020;67(1):1-8. doi:10.1007/s12020-019-02085-7
- Barber TM, Hanson P, Weickert MO, Franks S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin Med Insights Reprod Health. 2019;13:1179558119874042. doi:10.1177/1179558119874042
- Cooney LG, Dokras A. Depression and Anxiety in Polycystic Ovary Syndrome: Etiology and Treatment. Curr Psychiatry Rep. Sep 20 2017;19(11):83. doi:10.1007/s11920-017-0834-2
- Ee C, Pirotta S, Mousa A, Moran L, Lim S. Providing lifestyle advice to women with PCOS: an overview of practical issues affecting success. BMC endocrine disorders. 2021/11/23 2021;21(1):234. doi:10.1186/s12902-021-00890-8
- He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. Journal of ovarian research. Jun 17 2020;13(1):73. doi:10.1186/s13048-020-00670-3
- Giampaolino P, Foreste V, Di Filippo C, et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int J Mol Sci. Feb 19 2021;22(4)doi:10.3390/ijms22042048
- Qi X, Yun C, Sun L, et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019/08/01 2019;25(8):1225-1233. doi:10.1038/s41591-019-0509-0
- Rizk MG, Thackray VG. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. Journal of the Endocrine Society. Feb 1 2021;5(2):bvaa177. doi:10.1210/jendso/bvaa177
- Zeng B, Lai Z, Sun L, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol. Jan-Feb 2019;170(1):43-52. doi:10.1016/j.resmic.2018.09.002
- Cozzolino M, Vitagliano A, Pellegrini L, et al. Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. Eur J Nutr. Oct 2020;59(7):2841-2856. doi:10.1007/s00394-020-02233-0
- Li Y, Tan Y, Xia G, Shuai J. Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. Jul 21 2021:1-17. doi:10.1080/10408398.2021.1951155
- Shirvani-Rad S, Tabatabaei-Malazy O, Mohseni S, et al. Probiotics as a Complementary Therapy for Management of Obesity: A Systematic Review. Evid Based Complement Alternat Med. 2021;2021:6688450. doi:10.1155/2021/6688450
- Dumesic DA, Hoyos LR, Chazenbalk GD, Naik R, Padmanabhan V, Abbott DH. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction. Jan 2020;159(1):R1-R13. doi:10.1530/REP-19-0197
- Khan MJ, Ullah A, Basit S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl Clin Genet. 2019;12:249-260. doi:10.2147/TACG.S200341
- Saddick SY. Identifying genes associated with the development of human polycystic ovary syndrome. Saudi J Biol Sci. May 2020;27(5):1271-1279. doi:10.1016/j.sjbs.2020.01.012
- Wang F, Wang S, Zhang Z, et al. Defective insulin signaling and the protective effects of dimethyldiguanide during follicular development in the ovaries of polycystic ovary syndrome. Mol Med Rep. Dec 2017;16(6):8164-8170. doi:10.3892/mmr.2017.7678
- Romualdi D, Versace V, Lanzone A. What is new in the landscape of insulin-sensitizing agents for polycystic ovary syndrome treatment. Ther Adv Reprod Health. Jan-Dec 2020;14:2633494120908709. doi:10.1177/2633494120908709
- Raperport C, Homburg R. The Source of Polycystic Ovarian Syndrome. Clin Med Insights Reprod Health. 2019;13:1179558119871467. doi:10.1177/1179558119871467
- Yanes Cardozo LL, Romero DG, Reckelhoff JF. Cardiometabolic Features of Polycystic Ovary Syndrome: Role of Androgens. Physiology (Bethesda). Sep 2017;32(5):357-366. doi:10.1152/physiol.00030.2016
- Guarnotta V, Lucchese S, Mineo MI, et al. Predictive factors of polycystic ovary syndrome in girls with precocious pubarche. Endocr Connect. Jul 21 2021;10(7):796-804. doi:10.1530/ec-21-0118
- Witchel SF, Rosenfield RL. Premature adrenarche. UpToDate. Updated 6/16/2021. Accessed 12/3/2021, https://www.uptodate.com/contents/premature-adrenarche
- Livadas S, Bothou C, Kanaka-Gantenbein C, et al. Young lean women with evidence of both premature adrenarche and pubarche display a metabolic, hormonal and psychologic profile that is similar to that of their peers with polycystic ovary syndrome. Endocrine Abstracts. 05/08 2018;doi:10.1530/endoabs.56.P918
- Rudnicka E, Suchta K, Grymowicz M, et al. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int J Mol Sci. Apr 6 2021;22(7)doi:10.3390/ijms22073789
- Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HE, Amer S. The Role of Chronic Inflammation in Polycystic Ovarian Syndrome-A Systematic Review and Meta-Analysis. Int J Mol Sci. Mar 8 2021;22(5)doi:10.3390/ijms22052734
- Rocha AL, Oliveira FR, Azevedo RC, et al. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Res. 2019;8doi:10.12688/f1000research.15318.1
- Delitala AP, Capobianco G, Delitala G, Cherchi PL, Dessole S. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Archives of gynecology and obstetrics. Sep 2017;296(3):405-419. doi:10.1007/s00404-017-4429-2
- Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. Sep 2019;158(3):R79-r90. doi:10.1530/rep-18-0583
- Sortino MA, Salomone S, Carruba MO, Drago F. Polycystic Ovary Syndrome: Insights into the Therapeutic Approach with Inositols. Front Pharmacol. 2017;8:341. doi:10.3389/fphar.2017.00341
- Kamenov Z, Gateva A. Inositols in PCOS. Molecules. Nov 27 2020;25(23)doi:10.3390/molecules25235566
- Monastra G, Vucenik I, Harrath AH, et al. PCOS and Inositols: Controversial Results and Necessary Clarifications. Basic Differences Between D-Chiro and Myo-Inositol. Front Endocrinol (Lausanne). 2021;12:660381. doi:10.3389/fendo.2021.660381
- Caputo M, Bona E, Leone I, et al. Inositols and metabolic disorders: From farm to bedside. Journal of traditional and complementary medicine. May 2020;10(3):252-259. doi:10.1016/j.jtcme.2020.03.005
- Watkins OC, Yong HEJ, Sharma N, Chan SY. A review of the role of inositols in conditions of insulin dysregulation and in uncomplicated and pathological pregnancy. Crit Rev Food Sci Nutr. Dec 7 2020:1-49. doi:10.1080/10408398.2020.1845604
- Unfer V, Facchinetti F, Orru B, Giordani B, Nestler J. Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr Connect. Nov 2017;6(8):647-658. doi:10.1530/EC-17-0243
- Regidor PA, Schindler AE, Lesoine B, Druckman R. Management of women with PCOS using myo-inositol and folic acid. New clinical data and review of the literature. Hormone molecular biology and clinical investigation. Mar 2 2018;34(2)doi:10.1515/hmbci-2017-0067
- Facchinetti F, Orru B, Grandi G, Unfer V. Short-term effects of metformin and myo-inositol in women with polycystic ovarian syndrome (PCOS): a meta-analysis of randomized clinical trials. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Mar 2019;35(3):198-206. doi:10.1080/09513590.2018.1540578
- Agrawal A, Mahey R, Kachhawa G, Khadgawat R, Vanamail P, Kriplani A. Comparison of metformin plus myoinositol vs metformin alone in PCOS women undergoing ovulation induction cycles: randomized controlled trial. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Jun 2019;35(6):511-514. doi:10.1080/09513590.2018.1549656
- Prabhakar P, Mahey R, Gupta M, et al. Impact of myoinositol with metformin and myoinositol alone in infertile PCOS women undergoing ovulation induction cycles - randomized controlled trial. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Apr 2021;37(4):332-336. doi:10.1080/09513590.2020.1810657
- Lagana AS, Vitagliano A, Noventa M, Ambrosini G, D'Anna R. Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: a systematic review and meta-analysis of randomized controlled trials. Archives of gynecology and obstetrics. Oct 2018;298(4):675-684. doi:10.1007/s00404-018-4861-y
- Davinelli S, Nicolosi D, Di Cesare C, Scapagnini G, Di Marco R. Targeting Metabolic Consequences of Insulin Resistance in Polycystic Ovary Syndrome by D-chiro-inositol and Emerging Nutraceuticals: A Focused Review. J Clin Med. Apr 2 2020;9(4)doi:10.3390/jcm9040987
- Gambioli R, Forte G, Aragona C, Bevilacqua A, Bizzarri M, Unfer V. The use of D-chiro-Inositol in clinical practice. Eur Rev Med Pharmacol Sci. Jan 2021;25(1):438-446. doi:10.26355/eurrev_202101_24412
- Nordio M, Basciani S, Camajani E. The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: comparison with other ratios. Eur Rev Med Pharmacol Sci. Jun 2019;23(12):5512-5521. doi:10.26355/eurrev_201906_18223
- Benelli E, Del Ghianda S, Di Cosmo C, Tonacchera M. A Combined Therapy with Myo-Inositol and D-Chiro-Inositol Improves Endocrine Parameters and Insulin Resistance in PCOS Young Overweight Women. International journal of endocrinology. 2016;2016:3204083. doi:10.1155/2016/3204083
- Le Donne M, Metro D, Alibrandi A, Papa M, Benvenga S. Effects of three treatment modalities (diet, myoinositol or myoinositol associated with D-chiro-inositol) on clinical and body composition outcomes in women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. Mar 2019;23(5):2293-2301. doi:10.26355/eurrev_201903_17278
- Mendoza N, Galan MI, Molina C, et al. High dose of d-chiro-inositol improves oocyte quality in women with polycystic ovary syndrome undergoing ICSI: a randomized controlled trial. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. May 2020;36(5):398-401. doi:10.1080/09513590.2019.1681959
- Muscogiuri G, Altieri B, de Angelis C, et al. Shedding new light on female fertility: The role of vitamin D. Rev Endocr Metab Disord. Sep 2017;18(3):273-283. doi:10.1007/s11154-017-9407-2
- Mu Y, Cheng D, Yin TL, Yang J. Vitamin D and Polycystic Ovary Syndrome: a Narrative Review. Reprod Sci. Aug 2021;28(8):2110-2117. doi:10.1007/s43032-020-00369-2
- Kalyanaraman R, Pal L. A Narrative Review of Current Understanding of the Pathophysiology of Polycystic Ovary Syndrome: Focus on Plausible Relevance of Vitamin D. Int J Mol Sci. May 5 2021;22(9)doi:10.3390/ijms22094905
- Davis EM, Peck JD, Hansen KR, Neas BR, Craig LB. Associations between vitamin D levels and polycystic ovary syndrome phenotypes. Minerva endocrinologica. Jun 2019;44(2):176-184. doi:10.23736/S0391-1977.18.02824-9
- Kumar A, Barki S, Raghav V, Chaturvedi A, Kumar K. Correlation of Vitamin D with metabolic parameters in polycystic ovarian syndrome. J Family Med Prim Care. Jan-Mar 2017;6(1):115-119. doi:10.4103/2249-4863.214985
- Miao CY, Fang XJ, Chen Y, Zhang Q. Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp Ther Med. Apr 2020;19(4):2641-2649. doi:10.3892/etm.2020.8525
- Jin B, Qian L, Fu X, Zhu J, Shu J. Influence of vitamin D supplementation on lipid levels in polycystic ovary syndrome patients: a meta-analysis of randomized controlled trials. J Int Med Res. Aug 2020;48(8):300060520935313. doi:10.1177/0300060520935313
- Guo S, Tal R, Jiang H, Yuan T, Liu Y. Vitamin D Supplementation Ameliorates Metabolic Dysfunction in Patients with PCOS: A SystematicReview of RCTs and Insight into the Underlying Mechanism. International journal of endocrinology. 2020;2020:7850816. doi:10.1155/2020/7850816
- Al-Bayyari N, Al-Domi H, Zayed F, Hailat R, Eaton A. Androgens and hirsutism score of overweight women with polycystic ovary syndrome improved after vitamin D treatment: A randomized placebo controlled clinical trial. Clin Nutr. Mar 2021;40(3):870-878. doi:10.1016/j.clnu.2020.09.024
- Lu L, Li X, Lv L, Xu Y, Wu B, Huang C. Dietary and serum omega-3 polyunsaturated fatty acids and PCOS: A matched case-control study. Br J Nutr. Aug 10 2021:1-31. doi:10.1017/s0007114521003007
- Salek M, Clark CCT, Taghizadeh M, Jafarnejad S. N-3 fatty acids as preventive and therapeutic agents in attenuating PCOS complications. Excli j. 2019;18:558-575. doi:10.17179/excli2019-1534
- Yang K, Zeng L, Bao T, Ge J. Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reproductive biology and endocrinology : RB&E. Mar 27 2018;16(1):27. doi:10.1186/s12958-018-0346-x
- ScienceDirect. Adiponectin. 12/3/2021. https://www.sciencedirect.com/topics/medicine-and-dentistry/adiponectin
- Tosatti JAG, Alves MT, Cândido AL, Reis FM, Araújo VE, Gomes KB. Influence of n-3 fatty acid supplementation on inflammatory and oxidative stress markers in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Br J Nutr. Mar 28 2021;125(6):657-668. doi:10.1017/s0007114520003207
- Khani B, Mardanian F, Fesharaki SJ. Omega-3 supplementation effects on polycystic ovary syndrome symptoms and metabolic syndrome. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2017;22:64. doi:10.4103/jrms.JRMS_644_16
- Chandil N, Pande S, Sen SS, Gupta D. Comparison of Metformin and N Acetylcysteine on Clinical, Metabolic Parameter and Hormonal Profile in Women with Polycystic Ovarian Syndrome. J Obstet Gynaecol India. Feb 2019;69(1):77-81. doi:10.1007/s13224-018-1135-3
- El Sharkwy IA, Abd El Aziz WM. Randomized controlled trial of N-acetylcysteine versus l-carnitine among women with clomiphene-citrate-resistant polycystic ovary syndrome. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. Oct 2019;147(1):59-64. doi:10.1002/ijgo.12902
- Nemati M, Nemati S, Taheri AM, Heidari B. Comparison of metformin and N-acetyl cysteine, as an adjuvant to clomiphene citrate, in clomiphene-resistant women with polycystic ovary syndrome. J Gynecol Obstet Hum Reprod. Sep 2017;46(7):579-585. doi:10.1016/j.jogoh.2017.07.004
- Mostajeran F, Tehrani HG, Rahbary B. N-Acetylcysteine as an Adjuvant to Letrozole for Induction of Ovulation in Infertile Patients with Polycystic Ovary Syndrome. Advanced biomedical research. 2018;7:100. doi:10.4103/abr.abr_157_17
- Behrouzi Lak T, Hajshafiha M, Nanbakhsh F, Oshnouei S. N-acetyl cysteine in ovulation induction of PCOS women underwent intrauterine insemination: An RCT. Int J Reprod Biomed. Apr 2017;15(4):203-208.
- Cheraghi E, Soleimani Mehranjani M, Shariatzadeh SMA, Nasr Esfahani MH, Alani B. N-Acetylcysteine Compared to Metformin, Improves The Expression Profile of Growth Differentiation Factor-9 and Receptor Tyrosine Kinase c-Kit in The Oocytes of Patients with Polycystic Ovarian Syndrome. Int J Fertil Steril. Jan 2018;11(4):270-278. doi:10.22074/ijfs.2018.5142
- Tillhon M, Guaman Ortiz LM, Lombardi P, Scovassi AI. Berberine: new perspectives for old remedies. Biochem Pharmacol. Nov 15 2012;84(10):1260-7. doi:10.1016/j.bcp.2012.07.018
- Rondanelli M, Riva A, Petrangolini G, et al. Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest-Post-Test Explanatory Study. Nutrients. 2021;13(10):3665. doi:10.3390/nu13103665
- Li MF, Zhou XM, Li XL. The Effect of Berberine on Polycystic Ovary Syndrome Patients with Insulin Resistance (PCOS-IR): A Meta-Analysis and Systematic Review. Evid Based Complement Alternat Med. 2018;2018:2532935. doi:10.1155/2018/2532935
- Wang Z, Nie K, Su H, et al. Berberine improves ovulation and endometrial receptivity in polycystic ovary syndrome. Phytomedicine. Oct 2021;91:153654. doi:10.1016/j.phymed.2021.153654
- Zhang SW, Zhou J, Gober HJ, Leung WT, Wang L. Effect and mechanism of berberine against polycystic ovary syndrome. Biomed Pharmacother. Jun 2021;138:111468. doi:10.1016/j.biopha.2021.111468
- Wei W, Zhao H, Wang A, et al. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur J Endocrinol. Jan 2012;166(1):99-105. doi:10.1530/eje-11-0616
- An Y, Sun Z, Zhang Y, Liu B, Guan Y, Lu M. The use of berberine for women with polycystic ovary syndrome undergoing IVF treatment. Clin Endocrinol (Oxf). Mar 2014;80(3):425-31. doi:10.1111/cen.12294
- Yu J, Ding C, Hua Z, Jiang X, Wang C. Protective effects of berberine in a rat model of polycystic ovary syndrome mediated via the PI3K/AKT pathway. J Obstet Gynaecol Res. May 2021;47(5):1789-1803. doi:10.1111/jog.14730
- Shen HR, Xu X, Li XL. Berberine exerts a protective effect on rats with polycystic ovary syndrome by inhibiting the inflammatory response and cell apoptosis. Reproductive biology and endocrinology : RB&E. Jan 7 2021;19(1):3. doi:10.1186/s12958-020-00684-y
- Kysenius K, Brunello CA, Huttunen HJ. Mitochondria and NMDA receptor-dependent toxicity of berberine sensitizes neurons to glutamate and rotenone injury. PLoS One. 2014;9(9):e107129. doi:10.1371/journal.pone.0107129
- Mikes V, Dadák V. Berberine derivatives as cationic fluorescent probes for the investigation of the energized state of mitochondria. Biochim Biophys Acta. May 27 1983;723(2):231-9. doi:10.1016/0005-2728(83)90122-6
- Mikes V, Yaguzhinskij LS. Interaction of fluorescent berberine alkyl derivatives with respiratory chain of rat liver mitochondria. J Bioenerg Biomembr. Feb 1985;17(1):23-32. doi:10.1007/BF00744986
- Karimi E, Heshmati J, Shirzad N, et al. The effect of synbiotics supplementation on anthropometric indicators and lipid profiles in women with polycystic ovary syndrome: a randomized controlled trial. Lipids in health and disease. Apr 6 2020;19(1):60. doi:10.1186/s12944-020-01244-4
- Kostov K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int J Mol Sci. Mar 18 2019;20(6)doi:10.3390/ijms20061351
- Babapour M, Mohammadi H, Kazemi M, Hadi A, Rezazadegan M, Askari G. Associations Between Serum Magnesium Concentrations and Polycystic Ovary Syndrome Status: a Systematic Review and Meta-analysis. Biol Trace Elem Res. Apr 2021;199(4):1297-1305. doi:10.1007/s12011-020-02275-9
- Luo X, Cai WY, Ma HL, et al. Associations of Serum Magnesium With Insulin Resistance and Testosterone in Women With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2021;12:683040. doi:10.3389/fendo.2021.683040
- Farsinejad-Marj M, Azadbakht L, Mardanian F, Saneei P, Esmaillzadeh A. Clinical and Metabolic Responses to Magnesium Supplementation in Women with Polycystic Ovary Syndrome. Biol Trace Elem Res. Aug 2020;196(2):349-358. doi:10.1007/s12011-019-01923-z
- Den Hartogh DJ, Gabriel A, Tsiani E. Antidiabetic Properties of Curcumin II: Evidence from In Vivo Studies. Nutrients. Dec 25 2019;12(1)doi:10.3390/nu12010058
- Chien YJ, Chang CY, Wu MY, Chen CH, Horng YS, Wu HC. Effects of Curcumin on Glycemic Control and Lipid Profile in Polycystic Ovary Syndrome: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Nutrients. Feb 21 2021;13(2)doi:10.3390/nu13020684
- Sohaei S, Amani R, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. Dec 2019;47:102201. doi:10.1016/j.ctim.2019.102201
- Heshmati J, Moini A, Sepidarkish M, et al. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine. Jan 2021;80:153395. doi:10.1016/j.phymed.2020.153395
- Heshmati J, Golab F, Morvaridzadeh M, et al. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor gamma coactivator 1alpha gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab Syndr. Mar - Apr 2020;14(2):77-82. doi:10.1016/j.dsx.2020.01.002
- Santos HO, da Silva GAR. To what extent does cinnamon administration improve the glycemic and lipid profiles? Clin Nutr ESPEN. Oct 2018;27:1-9. doi:10.1016/j.clnesp.2018.07.011
- Mollazadeh H, Hosseinzadeh H. Cinnamon effects on metabolic syndrome: a review based on its mechanisms. Iranian journal of basic medical sciences. Dec 2016;19(12):1258-1270. doi:10.22038/ijbms.2016.7906
- Shirzad F, Morovatdar N, Rezaee R, Tsarouhas K, Abdollahi Moghadam A. Cinnamon effects on blood pressure and metabolic profile: A double-blind, randomized, placebo-controlled trial in patients with stage 1 hypertension. Avicenna journal of phytomedicine. Jan-Feb 2021;11(1):91-100.
- Heshmati J, Sepidarkish M, Morvaridzadeh M, et al. The effect of cinnamon supplementation on glycemic control in women with polycystic ovary syndrome: A systematic review and meta-analysis. J Food Biochem. Jan 2021;45(1):e13543. doi:10.1111/jfbc.13543
- Hajimonfarednejad M, Nimrouzi M, Heydari M, Zarshenas MM, Raee MJ, Jahromi BN. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: A randomized double-blind placebo controlled clinical trial. Phytother Res. Feb 2018;32(2):276-283. doi:10.1002/ptr.5970
- Borzoei A, Rafraf M, Niromanesh S, Farzadi L, Narimani F, Doostan F. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. Journal of traditional and complementary medicine. Jan 2018;8(1):128-133. doi:10.1016/j.jtcme.2017.04.008
- Dou L, Zheng Y, Li L, et al. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reproductive biology and endocrinology : RB&E. Oct 19 2018;16(1):99. doi:10.1186/s12958-018-0418-y
- Higdon J. Lipoic Acid. Linus Pauling Institute. Data on file.
- Mahmoudi-Nezhad M, Vajdi M, Farhangi MA. An updated systematic review and dose-response meta-analysis of the effects of α-lipoic acid supplementation on glycemic markers in adults. Nutrition. Feb 2021;82:111041. doi:10.1016/j.nut.2020.111041
- Genazzani AD, Shefer K, Della Casa D, et al. Modulatory effects of alpha-lipoic acid (ALA) administration on insulin sensitivity in obese PCOS patients. J Endocrinol Invest. May 2018;41(5):583-590. doi:10.1007/s40618-017-0782-z
- Masharani U, Gjerde C, Evans JL, Youngren JF, Goldfine ID. Effects of controlled-release alpha lipoic acid in lean, nondiabetic patients with polycystic ovary syndrome. J Diabetes Sci Technol. Mar 1 2010;4(2):359-64. doi:10.1177/193229681000400218
- Cirillo F, Catellani C, Lazzeroni P, et al. HMGB1 is increased in adolescents with polycystic ovary syndrome (PCOS) and decreases after treatment with myo-inositol (MYO) in combination with alpha-lipoic acid (ALA). Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Jul 2020;36(7):588-593. doi:10.1080/09513590.2020.1725967
- De Cicco S, Immediata V, Romualdi D, et al. Myoinositol combined with alpha-lipoic acid may improve the clinical and endocrine features of polycystic ovary syndrome through an insulin-independent action. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Sep 2017;33(9):698-701. doi:10.1080/09513590.2017.1313972
- Fruzzetti F, Fidecicchi T, Palla G, Gambacciani M. Long-term treatment with alpha-lipoic acid and myo-inositol positively affects clinical and metabolic features of polycystic ovary syndrome. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Feb 2020;36(2):152-155. doi:10.1080/09513590.2019.1640673
- Fruzzetti F, Benelli E, Fidecicchi T, Tonacchera M. Clinical and Metabolic Effects of Alpha-Lipoic Acid Associated with Two Different Doses of Myo-Inositol in Women with Polycystic Ovary Syndrome. International journal of endocrinology. 2020;2020:2901393. doi:10.1155/2020/2901393
- Piotrowska A, Pilch W, Czerwińska-Ledwig O, et al. The Possibilities of Using Chromium Salts as an Agent Supporting Treatment of Polycystic Ovary Syndrome. Biol Trace Elem Res. Dec 2019;192(2):91-97. doi:10.1007/s12011-019-1654-5
- Zaeemzadeh N, Jahanian Sadatmahalleh S, Ziaei S, et al. Comparison of dietary micronutrient intake in PCOS patients with and without metabolic syndrome. Journal of ovarian research. Jan 9 2021;14(1):10. doi:10.1186/s13048-020-00746-0
- Ashoush S, Abou-Gamrah A, Bayoumy H, Othman N. Chromium picolinate reduces insulin resistance in polycystic ovary syndrome: Randomized controlled trial. J Obstet Gynaecol Res. Mar 2016;42(3):279-85. doi:10.1111/jog.12907
- Lucidi RS, Thyer AC, Easton CA, Holden AE, Schenken RS, Brzyski RG. Effect of chromium supplementation on insulin resistance and ovarian and menstrual cyclicity in women with polycystic ovary syndrome. Fertility and sterility. Dec 2005;84(6):1755-7. doi:10.1016/j.fertnstert.2005.06.028
- Lydic ML, McNurlan M, Bembo S, Mitchell L, Komaroff E, Gelato M. Chromium picolinate improves insulin sensitivity in obese subjects with polycystic ovary syndrome. Fertility and sterility. Jul 2006;86(1):243-6. doi:10.1016/j.fertnstert.2005.11.069
- Fazelian S, Rouhani MH, Bank SS, Amani R. Chromium supplementation and polycystic ovary syndrome: A systematic review and meta-analysis. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS). Jul 2017;42:92-96. doi:10.1016/j.jtemb.2017.04.008
- Tang XL, Sun Z, Gong L. Chromium supplementation in women with polycystic ovary syndrome: Systematic review and meta-analysis. J Obstet Gynaecol Res. Jan 2018;44(1):134-143. doi:10.1111/jog.13462
- Bene J, Hadzsiev K, Melegh B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr Diabetes. Mar 7 2018;8(1):8. doi:10.1038/s41387-018-0017-1
- Savic D, Hodson L, Neubauer S, Pavlides M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. Jul 22 2020;12(8)doi:10.3390/nu12082178
- Celik F, Kose M, Yilmazer M, Köken GN, Arioz DT, Kanat Pektas M. Plasma L-carnitine levels of obese and non-obese polycystic ovary syndrome patients. J Obstet Gynaecol. May 2017;37(4):476-479. doi:10.1080/01443615.2016.1264375
- Salehpour S, Nazari L, Hoseini S, Moghaddam PB, Gachkar L. Effects of L-carnitine on Polycystic Ovary Syndrome. JBRA Assist Reprod. Oct 14 2019;23(4):392-395. doi:10.5935/1518-0557.20190033
- Ismail AM, Hamed AH, Saso S, Thabet HH. Adding L-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. European journal of obstetrics, gynecology, and reproductive biology. Sep 2014;180:148-52. doi:10.1016/j.ejogrb.2014.06.008
- Tauqir S, Israr M, Rauf B, et al. Acetyl-L-Carnitine Ameliorates Metabolic and Endocrine Alterations in Women with PCOS: A Double-Blind Randomized Clinical Trial. Adv Ther. Jul 2021;38(7):3842-3856. doi:10.1007/s12325-021-01789-5
- Cai X, Liu M, Zhang B, Zhao SJ, Jiang SW. Phytoestrogens for the Management of Endometriosis: Findings and Issues. Pharmaceuticals (Basel). Jun 14 2021;14(6)doi:10.3390/ph14060569
- Pasquariello R, Verdile N, Brevini TAL, et al. The Role of Resveratrol in Mammalian Reproduction. Molecules. Oct 5 2020;25(19)doi:10.3390/molecules25194554
- Shojaei-Zarghani S, Rafraf M. Resveratrol and Markers of Polycystic Ovary Syndrome: a Systematic Review of Animal and Clinical Studies. Reprod Sci. Jul 26 2021;doi:10.1007/s43032-021-00653-9
- Mansour A, Samadi M, Sanginabadi M, et al. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with PCOS. Clin Nutr. Jun 2021;40(6):4106-4112. doi:10.1016/j.clnu.2021.02.004
- Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of Resveratrol on Polycystic Ovary Syndrome: A Double-blind, Randomized, Placebo-controlled Trial. J Clin Endocrinol Metab. Nov 2016;101(11):4322-4328. doi:10.1210/jc.2016-1858
- Bahramrezaie M, Amidi F, Aleyasin A, et al. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: a triple-blind randomized clinical trial. J Assist Reprod Genet. Aug 2019;36(8):1701-1712. doi:10.1007/s10815-019-01461-6
- Brenjian S, Moini A, Yamini N, et al. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am J Reprod Immunol. Jan 2020;83(1):e13186. doi:10.1111/aji.13186
- Kunitomi C, Harada M, Kusamoto A, et al. Induction of aryl hydrocarbon receptor in granulosa cells by endoplasmic reticulum stress contributes to pathology of polycystic ovary syndrome. Mol Hum Reprod. Feb 27 2021;27(3)doi:10.1093/molehr/gaab003
- Spinedi E, Cardinali DP. The Polycystic Ovary Syndrome and the Metabolic Syndrome: A Possible Chronobiotic-Cytoprotective Adjuvant Therapy. International journal of endocrinology. 2018;2018:1349868. doi:10.1155/2018/1349868
- Lim AJR, Indran IR, Kramer MS, Yong EL. Phenotypic spectrum of polycystic ovary syndrome and their relationship to the circadian biomarkers, melatonin and cortisol. Endocrinol Diabetes Metab. Jul 2019;2(3):e00047. doi:10.1002/edm2.47
- Simon SL, McWhirter L, Diniz Behn C, et al. Morning Circadian Misalignment Is Associated With Insulin Resistance in Girls With Obesity and Polycystic Ovarian Syndrome. J Clin Endocrinol Metab. Aug 1 2019;104(8):3525-3534. doi:10.1210/jc.2018-02385
- Alizadeh M, Karandish M, Asghari Jafarabadi M, et al. Metabolic and hormonal effects of melatonin and/or magnesium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Nutr Metab (Lond). Jun 6 2021;18(1):57. doi:10.1186/s12986-021-00586-9
- Mousavi R, Alizadeh M, Asghari Jafarabadi M, et al. Effects of Melatonin and/or Magnesium Supplementation on Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res. May 19 2021;doi:10.1007/s12011-021-02725-y
- Tagliaferri V, Romualdi D, Scarinci E, et al. Melatonin Treatment May Be Able to Restore Menstrual Cyclicity in Women With PCOS: A Pilot Study. Reprod Sci. Feb 2018;25(2):269-275. doi:10.1177/1933719117711262
- Wang T, Sha L, Li Y, et al. Dietary α-Linolenic Acid-Rich Flaxseed Oil Exerts Beneficial Effects on Polycystic Ovary Syndrome Through Sex Steroid Hormones-Microbiota-Inflammation Axis in Rats. Front Endocrinol (Lausanne). 2020;11:284. doi:10.3389/fendo.2020.00284
- Komal F, Khan MK, Imran M, et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in PCOS rat model. J Transl Med. Sep 14 2020;18(1):349. doi:10.1186/s12967-020-02519-1
- Nowak DA, Snyder DC, Brown AJ, Demark-Wahnefried W. The Effect of Flaxseed Supplementation on Hormonal Levels Associated with Polycystic Ovarian Syndrome: A Case Study. Curr Top Nutraceutical Res. 2007;5(4):177-181.
- Haidari F, Banaei-Jahromi N, Zakerkish M, Ahmadi K. The effects of flaxseed supplementation on metabolic status in women with polycystic ovary syndrome: a randomized open-labeled controlled clinical trial. Nutr J. Jan 24 2020;19(1):8. doi:10.1186/s12937-020-0524-5
- Woodward A, Klonizakis M, Broom D. Exercise and Polycystic Ovary Syndrome. Adv Exp Med Biol. 2020;1228:123-136. doi:10.1007/978-981-15-1792-1_8
- Hansen SL, Bojsen-Møller KN, Lundsgaard AM, et al. Mechanisms Underlying Absent Training-Induced Improvement in Insulin Action in Lean, Hyperandrogenic Women With Polycystic Ovary Syndrome. Diabetes. Nov 2020;69(11):2267-2280. doi:10.2337/db20-0062
- Lionett S, Kiel IA, Røsbjørgen R, Lydersen S, Larsen S, Moholdt T. Absent Exercise-Induced Improvements in Fat Oxidation in Women With Polycystic Ovary Syndrome After High-Intensity Interval Training. Front Physiol. 2021;12:649794. doi:10.3389/fphys.2021.649794
- Patten RK, Boyle RA, Moholdt T, et al. Exercise Interventions in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front Physiol. 2020;11:606. doi:10.3389/fphys.2020.00606
- Shele G, Genkil J, Speelman D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J Funct Morphol Kinesiol. May 31 2020;5(2)doi:10.3390/jfmk5020035
- Mohseni M, Eghbali M, Bahrami H, Dastaran F, Amini L. Yoga Effects on Anthropometric Indices and Polycystic Ovary Syndrome Symptoms in Women Undergoing Infertility Treatment: A Randomized Controlled Clinical Trial. Evid Based Complement Alternat Med. 2021;2021:5564824. doi:10.1155/2021/5564824
- Shishehgar F, Mirmiran P, Rahmati M, Tohidi M, Ramezani Tehrani F. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC endocrine disorders. Sep 2 2019;19(1):93. doi:10.1186/s12902-019-0420-1
- Zhang X, Zheng Y, Guo Y, Lai Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. International journal of endocrinology. 2019;2019:4386401. doi:10.1155/2019/4386401
- Masood W, Annamaraju P, Uppaluri KR. Ketogenic Diet. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. Feb 27 2020;18(1):104. doi:10.1186/s12967-020-02277-0
- Adeva-Andany MM, González-Lucán M, Fernández-Fernández C, Carneiro-Freire N, Seco-Filgueira M, Pedre-Piñeiro AM. Effect of diet composition on insulin sensitivity in humans. Clin Nutr ESPEN. Oct 2019;33:29-38. doi:10.1016/j.clnesp.2019.05.014
- Sievenpiper JL. Low-carbohydrate diets and cardiometabolic health: the importance of carbohydrate quality over quantity. Nutr Rev. Aug 1 2020;78(Suppl 1):69-77. doi:10.1093/nutrit/nuz082
- Martínez-González MA, Fernandez-Lazaro CI, Toledo E, et al. Carbohydrate quality changes and concurrent changes in cardiovascular risk factors: a longitudinal analysis in the PREDIMED-Plus randomized trial. Am J Clin Nutr. Feb 1 2020;111(2):291-306. doi:10.1093/ajcn/nqz298
- Cutler DA, Pride SM, Cheung AP. Low intakes of dietary fiber and magnesium are associated with insulin resistance and hyperandrogenism in polycystic ovary syndrome: A cohort study. Food science & nutrition. Apr 2019;7(4):1426-1437. doi:10.1002/fsn3.977
- Pohlmeier AM, Phy JL, Watkins P, et al. Effect of a low-starch/low-dairy diet on fat oxidation in overweight and obese women with polycystic ovary syndrome. Appl Physiol Nutr Metab. Nov 2014;39(11):1237-44. doi:10.1139/apnm-2014-0073
- MedlinePlus. DASH diet to lower high blood pressure. National Library of Medicine. Updated 10/18/2020. Accessed 12/3/2021, https://medlineplus.gov/ency/patientinstructions/000770.htm
- MedlinePlus. DASH Eating Plan. National Library of Medicine. Updated 10/1/2021. Accessed 12/3/2021, https://medlineplus.gov/dasheatingplan.html
- Azadi-Yazdi M, Karimi-Zarchi M, Salehi-Abargouei A, Fallahzadeh H, Nadjarzadeh A. Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. J Hum Nutr Diet. Jun 2017;30(3):275-283. doi:10.1111/jhn.12433
- Rao P, Bhide P. Controversies in the diagnosis of polycystic ovary syndrome. Ther Adv Reprod Health. Jan-Dec 2020;14:2633494120913032. doi:10.1177/2633494120913032
- MedlinePlus. 21-hydroxylase deficiency. National Library of Medicine. Updated 8/18/2020. Accessed 12/3/2021, https://medlineplus.gov/genetics/condition/21-hydroxylase-deficiency/
- Karakas SE. New biomarkers for diagnosis and management of polycystic ovary syndrome. Clin Chim Acta. Aug 2017;471:248-253. doi:10.1016/j.cca.2017.06.009
- Deswal R, Yadav A, Dang AS. Sex hormone binding globulin - an important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst Biol Reprod Med. Feb 2018;64(1):12-24. doi:10.1080/19396368.2017.1410591
- Qu X, Donnelly R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int J Mol Sci. Nov 1 2020;21(21)doi:10.3390/ijms21218191
- Zhu JL, Chen Z, Feng WJ, Long SL, Mo ZC. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. Dec 2019;499:142-148. doi:10.1016/j.cca.2019.09.010
- Dewailly D, Barbotin AL, Dumont A, Catteau-Jonard S, Robin G. Role of Anti-Mullerian Hormone in the Pathogenesis of Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2020;11:641. doi:10.3389/fendo.2020.00641
- Pasquali R. New perspectives on the role of anti-Mullerian hormone (AMH) in women. Ann Transl Med. Dec 2018;6(Suppl 2):S94. doi:10.21037/atm.2018.11.19
- Vagios S, Sacha CR, Hammer KC, et al. Response to ovulation induction treatments in women with polycystic ovary syndrome as a function of serum anti-Mullerian hormone levels. J Assist Reprod Genet. May 1 2021;doi:10.1007/s10815-021-02217-x
- Ramezani Tehrani F, Rahmati M, Mahboobifard F, Firouzi F, Hashemi N, Azizi F. Age-specific cut-off levels of anti-Müllerian hormone can be used as diagnostic markers for polycystic ovary syndrome. Reproductive Biology and Endocrinology. 2021/05/22 2021;19(1):76. doi:10.1186/s12958-021-00755-8
- Papadakis G, Kandaraki EA, Tseniklidi E, Papalou O, Diamanti-Kandarakis E. Polycystic Ovary Syndrome and NC-CAH: Distinct Characteristics and Common Findings. A Systematic Review. Systematic Review. Frontiers in Endocrinology. 2019-June-19 2019;10(388)doi:10.3389/fendo.2019.00388
- Al-Chalabi M, Bass AN, Alsalman I. Physiology, Prolactin. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Dhesi AS, Murtough KL, Lim JK, et al. Metabolic screening in patients with polycystic ovary syndrome is largely underutilized among obstetrician-gynecologists. Am J Obstet Gynecol. Nov 2016;215(5):579.e1-579.e5. doi:10.1016/j.ajog.2016.07.043
- Lin M-W, Wu M-H. The role of vitamin D in polycystic ovary syndrome. The Indian journal of medical research. 2015;142(3):238-240. doi:10.4103/0971-5916.166527
- Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism. Oct 2011;60(10):1475-81. doi:10.1016/j.metabol.2011.03.002
- Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. Jan 2011;31(1):48-54. doi:10.1016/j.nutres.2010.12.001
- Trummer C, Pilz S, Schwetz V, Obermayer-Pietsch B, Lerchbaum E. Vitamin D, PCOS and androgens in men: a systematic review. Endocr Connect. Mar 2018;7(3):R95-r113. doi:10.1530/ec-18-0009
- Kondapaneni V, Gutlapalli SD, Poudel S, Zeb M, Toulassi IA, Cancarevic I. Significance of Homocysteine Levels in the Management of Polycystic Ovarian Syndrome: A Literature Review. Cureus. Oct 23 2020;12(10):e11110. doi:10.7759/cureus.11110
- Meng Y, Chen X, Peng Z, Liu X, Sun Y, Dai S. Association between High Serum Homocysteine Levels and Biochemical Characteristics in Women with Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(6):e0157389. doi:10.1371/journal.pone.0157389
- Patibandla S, Heaton J, Kyaw H. Spironolactone. StatPearls. 2021.
- Cignarella A, Mioni R, Sabbadin C, et al. Pharmacological Approaches to Controlling Cardiometabolic Risk in Women with PCOS. Int J Mol Sci. Dec 15 2020;21(24)doi:10.3390/ijms21249554
- Dumitrescu R, Mehedintu C, Briceag I, Purcarea VL, Hudita D. Metformin-clinical pharmacology in PCOs. J Med Life. Apr-Jun 2015;8(2):187-92.
- Pasquali R. Contemporary approaches to the management of polycystic ovary syndrome. Therapeutic advances in endocrinology and metabolism. Apr 2018;9(4):123-134. doi:10.1177/2042018818756790
- Practice Committee of the American Society for Reproductive Medicine. Electronic address Aao, Practice Committee of the American Society for Reproductive M. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline. Fertility and sterility. Sep 2017;108(3):426-441. doi:10.1016/j.fertnstert.2017.06.026
- Yang PK, Hsu CY, Chen MJ, et al. The Efficacy of 24-Month Metformin for Improving Menses, Hormones, and Metabolic Profiles in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. Mar 1 2018;103(3):890-899. doi:10.1210/jc.2017-01739
- Panchaud A, Rousson V, Vial T, et al. Pregnancy outcomes in women on metformin for diabetes or other indications among those seeking teratology information services. Br J Clin Pharmacol. Mar 2018;84(3):568-578. doi:10.1111/bcp.13481
- Ouyang H, Al-Mureish A, Wu N. Research progress of metformin in gestational diabetes mellitus: a narrative review. Ann Palliat Med. Mar 2021;10(3):3423-3437. doi:10.21037/apm-21-192
- Li X, Fang Z, Yang X, et al. The effect of metformin on homocysteine levels in patients with polycystic ovary syndrome: A systematic review and meta-analysis. J Obstet Gynaecol Res. May 2021;47(5):1804-1816. doi:10.1111/jog.14725
- Esmaeilzadeh S, Gholinezhad-Chari M, Ghadimi R. The Effect of Metformin Treatment on the Serum Levels of Homocysteine, Folic Acid, and Vitamin B12 in Patients with Polycystic Ovary Syndrome. J Hum Reprod Sci. Apr-Jun 2017;10(2):95-101. doi:10.4103/jhrs.JHRS_74_16
- Vijayakumar A, Kim EK, Kim H, Choi YJ, Huh KB, Chang N. Effects of folic acid supplementation on serum homocysteine levels, lipid profiles, and vascular parameters in post-menopausal Korean women with type 2 diabetes mellitus. Nutrition research and practice. Aug 2017;11(4):327-333. doi:10.4162/nrp.2017.11.4.327
- Tian Z, Zhang YC, Wang Y, Chang XH, Zhu HL, Zhao Y. Effects of bariatric surgery on patients with obesity and polycystic ovary syndrome: a meta-analysis. Surg Obes Relat Dis. Aug 2021;17(8):1399-1408. doi:10.1016/j.soard.2021.04.009
- Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Human reproduction update. Nov 2016;22(6):687-708. doi:10.1093/humupd/dmw025
- Costello M, Garad R, Hart R, et al. A Review of First Line Infertility Treatments and Supporting Evidence in Women with Polycystic Ovary Syndrome. Med Sci (Basel). Sep 10 2019;7(9)doi:10.3390/medsci7090095
- Rushing JS, Santoro N. Fertility Issues in Polycystic Ovarian Disease: A Systematic Approach. Endocrinol Metab Clin North Am. Mar 2021;50(1):43-55. doi:10.1016/j.ecl.2020.10.004
- Kulkarni AD, Jamieson DJ, Jones HW, Jr., et al. Fertility treatments and multiple births in the United States. N Engl J Med. Dec 5 2013;369(23):2218-25. doi:10.1056/NEJMoa1301467
- Hu S, Yu Q, Wang Y, Wang M, Xia W, Zhu C. Letrozole versus clomiphene citrate in polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Archives of gynecology and obstetrics. May 2018;297(5):1081-1088. doi:10.1007/s00404-018-4688-6
- Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. May 24 2018;5(5):Cd010287. doi:10.1002/14651858.CD010287.pub3
- Tsiami AP, Goulis DG, Sotiriadis AI, Kolibianakis EM. Higher ovulation rate with letrozole as compared with clomiphene citrate in infertile women with polycystic ovary syndrome: a systematic review and meta-analysis. Hormones (Athens). May 25 2021;doi:10.1007/s42000-021-00289-z
- Gadalla MA, Norman RJ, Tay CT, et al. Medical and Surgical Treatment of Reproductive Outcomes in Polycystic Ovary Syndrome: An Overview of Systematic Reviews. Int J Fertil Steril. Jan 2020;13(4):257-270. doi:10.22074/ijfs.2020.5608
- Weiss NS, Kostova E, Nahuis M, Mol BWJ, van der Veen F, van Wely M. Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. Cochrane Database Syst Rev. Jan 16 2019;1:CD010290. doi:10.1002/14651858.CD010290.pub3
- Paulson R. In vitro fertilization. UpToDate. Updated 7/9/2021. Accessed 12/7/2021, https://www.uptodate.com/contents/in-vitro-fertilization
- New MI, Ghizzoni L, Meyer-Bahlburg H, et al. Fertility in patients with nonclassical congenital adrenal hyperplasia. Fertility and Sterility. 2019;111(1):13-20. https://pubmed.ncbi.nlm.nih.gov/30611403/
