Life Extension Magazine®
Better Long-Term Cardiac HealthAdult stem cell therapies have also shown clinical benefit in severe chronic heart disease, such as congestive heart failure, of which almost half a million new cases are diagnosed each year. In one study by Brehm and Strauer, bone marrow-derived stem cells were transplanted directly into the heart tissue of 18 male patients who had suffered a heart attack between five months and 8.5 years earlier.18 These patients had progressive chronic heart failure with reduced left ventricular function. A group of patients who did not receive any cell therapy served as controls. After three months, the researchers found that the area of heart tissue damaged by disease was reduced, while oxygen uptake, energy metabolism and left ventricular function all increased compared with the control group, who showed no significant changes in these parameters. In another study, Patel and colleagues studied 20 patients with severe chronic heart disease and very poor left ventricular function classified as chronic heart failure.19 All 20 patients received bypass surgery to improve blood flow. In addition, half of the patients also received an infusion of adult stem cells during surgery, which were injected into the most severely compromised regions of the heart. Six months after surgery, the left ventricular function of the stem cell-treated group increased substantially compared with the control group. The improvement was so great that the stem cell recipients were no longer defined as having chronic heart failure.
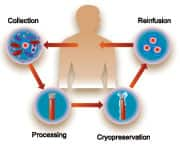
Banking Stem Cells for Heart HealthIt has been suggested that an alternative to stem cell infusion is to administer growth factors that are produced naturally in the body. The use of these chemicals, such as granulocyte colony-stimulating growth factor, alone stimulates the endogenous production of stem cells, which might obviate the need for stem cell infusion. However, a defined benefit from this therapy has not yet been established20 and some evidence suggests that the use of stem cells immediately after a heart attack may even be detrimental.21 Furthermore, there is mounting evidence that those factors that precipitate the onset of heart disease—such as hypertension, diabetes, smoking, and others—also impact the effectiveness of stem cells in terms of their ability to migrate, transdifferentiate, and proliferate. The benefits of banking stem cells before the onset of disease will undoubtedly prove to be clinically important as the use of these therapies becomes more widespread. Despite the uncertainties about their mechanisms of action, scientists broadly agree on the potential of regenerating damaged heart tissue using a patient’s own stem cells to improve cardiac function and performance.22 Autoimmune and Neurological ConditionsAdult stem cells could also offer hope for patients with autoimmune and neurodegenerative diseases.
In autoimmune disorders, the body begins to produce a type of white blood cells called T lymphocytes and protective proteins called antibodies, which, instead of protecting the body against invasive microbes and cancers, attack its own cells and organs. There are more than 70 different types of autoimmune disorders, for example, multiple sclerosis, rheumatoid arthritis, systemic sclerosis (scleroderma), systemic lupus erythematosus, and juvenile idiopathic arthritis. As a class, autoimmune diseases affect approximately 5% of the US population, with common conditions such as systemic lupus erythematosus affecting 1.5 million people, mostly young women. The standard treatment for autoimmune diseases generally consists of immunosuppression, anti-inflammatory medication, or anti-malarial medication, in addition to supportive care. In cases that do not respond to standard treatment or are considered life- or organ-threatening, high doses of immunosuppressive medication have been proposed as a treatment option to eliminate the T cells causing the autoimmune response. However, such high doses also suppress the bone marrow’s production of blood cells (known as “myelosuppression”), necessitating rescue therapy with transfused hematopoietic (blood cell-forming) stem cells.
It has been theorized that regenerating bone marrow with transplanted stem cells normalizes the immune system.23,24 The concept of stem cell therapy following immunosuppressive therapy for autoimmune diseases has led to the publication of consensus guidelines and the initiation of a number of well-controlled clinical trials.25,26 To date, more than 700 patients have received transplants using their own stem cells as treatment for severe autoimmune diseases,27 including 183 patients with multiple sclerosis,28 76 patients with severe rheumatoid arthritis,29 102 patients with systemic sclerosis (scleroderma),30,31 103 patients with systemic lupus erythematosus,32-34 and, most recently, 15 individuals with new onset type I diabetes.35 Numerous studies using adult stem cells to treat other autoimmune diseases such as Crohn’s disease, Behcet’s disease, and relapsing polychondritis have also been published.36,37 Early studies in patients with neurodegenerative diseases—some of which may represent autoimmune processes—have shown promising results, suggesting that stem cells might offer hope for people with neurological disorders, perhaps even for prevalent conditions such as Parkinson’s disease.38-41 Although the clinical outcomes of stem cell treatments have been variable, most of the studies in this field have shown significant amelioration of disease activity, improvement in serological (blood) markers, and either stabilization or reversal of organ dysfunction. The preliminary conclusions of these studies are sufficiently encouraging to proceed to randomized prospective trials of stem cell transplantation for autoimmune diseases as a group, and particularly for those that are most severe and debilitating. Similarly, scientists believe that stem cells therapies offer compelling hope for neurological conditions, and are further exploring their applications for these debilitating disorders.
Current and Future Stem Cell TherapiesImportantly, all of the studies that have been mentioned so far were carried out using stem cells that were collected after the onset of disease. It is intriguing to speculate on the improvement in outcome that might be achieved if a patient’s own stem cells were available before the onset of disease. The table on page 46 summarizes the current status of regenerative therapy, divided into those diseases being treated with adult stem cells today and those in which experimental evidence from animal studies strongly indicates potential benefits in the future.
ConclusionAdult stem cells may one day yield cures for the most dreaded diseases that plague adults. A plentiful supply of adult stem cells for personal use collected while healthy and available may offer all adults powerful insurance against the consequences of a range of diseases, both chronic and acute, that is growing daily. Only by having a readily accessible source of stem cells can the full benefits of regenerative medicine be realized. While it remains to be seen whether adult stem cells can prevent or reverse aging or extend life span, ongoing research promises to propel the field of regenerative medicine forward. Regardless of these unanswered questions, it is clear that banking stem cells for long-term storage may truly represent a “bio-insurance policy” that can help provide for your optimal health in the future. Authors’ AffiliationsDenis Rodgerson, PhD: NeoStem California Laboratory, 637 South Lucas Avenue, Suite 508, Los Angeles, CA 90017. Ron Rothenberg, MD, FACEP: California HealthSpan Institute, 320 Santa Fe Drive, Encinitas, CA 92024. Wayne Marasco, MD, PhD: Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney Street, Boston, MA 02115. Disclosures: All three authors have a financial interest in NeoStem, Inc. (www.neostem.com), a company that specializes in the banking and long-term storage of adult stem cells. If you have any questions about the scientific content of this article, please call one of our Health Advisors at 1-800-226-2370. | ||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||
| 1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145-7. 2. Rossant J. Stem cells: the magic brew. Nature. 2007 Jul 19;448(7151):260-2. 3. Anon. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005 Aug 1;23(22):5074-87. 4. Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007 Apr 1;109(7):3108-14. 5. Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001 Apr;7(4):430-6. 6. Fuchs S, Baffour R, Zhou YF, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001 May;37(6):1726-32. 7. Schuster MD, Kocher AA, Seki T, et al. Myocardial neovascularization by bone marrow angioblasts results in cardiomyocyte regeneration. Am J Physiol Heart Circ Physiol. 2004 Aug;287(2):H525-32. 8. Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002 Oct 8;106(15):1913-8. 9. Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002 Dec 10;106(24):3009-17. 10. Britten MB, Abolmaali ND, Assmus B, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003 Nov 4;108(18):2212-8. 11. Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004 Oct 19;44(8):1690-9. 12. Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004 Jul 10;364(9429):141-8. 13. Drexler H, Meyer GP, Wollert KC. Bone-marrow-derived cell transfer after ST-elevation myocardial infarction: lessons from the BOOST trial. Nat Clin Pract Cardiovasc Med. 2006 Mar;3 Suppl 1S65-8. 14. Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1210-21. 15. Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1222-32. 16. Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1199-209. 17. Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006 Jan 14;367(9505):113-21. 18. Brehm M, Strauer BE. Stem cell therapy in postinfarction chronic coronary heart disease. Nat Clin Pract Cardiovasc Med. 2006 Mar;3 Suppl 1S101-4. 19. Patel AN, Geffner L, Vina RF, et al. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg. 2005 Dec;130(6):1631-8. 20. Ince H, Petzsch M, Kleine HD, et al. Prevention of left ventricular remodeling with granulocyte colony-stimulating factor after acute myocardial infarction: final 1-year results of the Front-Integrated Revascularization and Stem Cell Liberation in Evolving Acute Myocardial Infarction by Granulocyte Colony-Stimulating Factor (FIRSTLINE-AMI) Trial. Circulation. 2005 Aug 30;112(9 Suppl):I73-80. 21. Kang HJ, Kim HS, Zhang SY, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004 Mar 6;363(9411):751-6. 22. Bartunek J, Dimmeler S, Drexler H, et al. The consensus of the task force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for repair of the heart. Eur Heart J. 2006 Jun;27(11):1338-40. 23. Burt RK, Traynor AE. Hematopoietic stem cell transplantation: a new therapy for autoimmune disease. Stem Cells. 1999;17(6):366-72. 24. Gratwohl A. Passweg J. Gerber I. et al. Stem cell transplantation for autoimmune diseases. Best Pract Res Clin Haematology. 2001;14:755. 25. Tyndall A, Gratwohl A. Blood and marrow stem cell transplants in auto-immune disease: a consensus report written on behalf of the European League against Rheumatism (EULAR) and the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 1997 Apr;19(7):643-5. 26. Marmont A. Tyndall A. Gratwold A. Vischer T. Haemapoietic precursor-cell transplants for autoimmune disease. Lancet. 1995;345:978. 27. Tyndall A, Saccardi R. Haematopoietic stem cell transplantation in the treatment of severe autoimmune disease: results from phase I/II studies, prospective randomized trials and future directions. Clin Exp Immunol. 2005 Jul;141(1):1-9. 28. Saccardi R, Kozak T, Bocelli-Tyndall C, et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006 Dec;12(6):814-23. 29. Snowden JA, Passweg J, Moore JJ, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004 Mar;31(3):482-8. 30. Burt RK, Marmont A, Oyama Y, et al. Randomized controlled trials of autologous hematopoietic stem cell transplantation for autoimmune diseases: the evolution from myeloablative to lymphoablative transplant regimens. Arthritis Rheum. 2006 Dec;54(12):3750-60. 31. Loh Y, Oyama Y, Statkute L, et al. Non-myeloablative allogeneic hematopoietic stem cell transplantation for severe systemic sclerosis: graft-versus-autoimmunity without graft-versus-host disease? Bone Marrow Transplant. 2007 Apr;39(7):435-7. 32. Jayne D, Tyndall A. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004;13(5):359-65. 33. Jayne D, Passweg J, Marmont A, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004;13(3):168-76. 34. Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006 Feb 1;295(5):527-35. 35. Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007 Apr 11;297(14):1568-76. 36. Hensel M, Breitbart A, Ho AD. Autologous hematopoietic stem-cell transplantation for Behcet’s disease with pulmonary involvement. N Engl J Med. 2001 Jan 4;344(1):69. 37. Hawkey CJ, Snowden JA, Lobo A, Beglinger C, Tyndall A. Stem cell transplantation for inflammatory bowel disease: practical and ethical issues. Gut. 2000 Jun;46(6):869-72. 38. Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004 Jul;10: SupplS42-S50. 39. Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004 Jun;113(12):1701-10. 40. Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005 Jan;115(1):102-9. 41. Behrstock S, Ebert A, McHugh J, et al. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006 Mar;13(5):379-88. 42. Ballen K, Broxmeyer HE, McCullough J et al. Current status of cord blood banking and transplantation in the United States and Europe. Biol Blood Marrow Transplant. 2001;7(12):635-45. 43. Bacigalupo A, Frassoni F, Van Lint MT. Bone marrow or peripheral blood as a source of stem cells for allogeneic transplants. Curr Opin Hematol. 2000 Nov;7(6):343-7. 44. Available at: www.marrow.org/ABOUT/index.html. Accessed July 11, 2007. 45. Rawley S. Cryopreservation of hematopoietic cells. In Thomas’ Hematopoietic Cell Transplantation. Eds.Blume K. Foreman S. Appelbaum F. Third Edition, P 599. Blackwell Publishing, Malden, MA 2004. 46. Bickford PC, Tan J, Shytle RD, et al. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006 Feb;15(1):118-23. 47. Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007 Sep 1;12:4839-54. 48. Ingram DK, Zhu M, Mamczarz J, et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006 Apr;5(2):97-108. 49. Chen JF, Huang L, Jin J, et al. Relationship between aging and the number and function of bone marrow-derived endothelial progenitor cells in rats. Zhonghua Xin Xue Guan Bing Za Zhi. 2006 Nov;34(11):1026-8. 50. J G, Cq W, Hh F, et al. Effects of resveratrol on endothelial progenitor cells and their contributions to reendothelialization in intima-injured rats. J Cardiovasc Pharmacol. 2006 May;47(5):711-21. 51. Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139(3):991-7. 52. Kidd PM. Neurodegeneration from mitochondrial insufficiency: nutrients, stem cells, growth factors, and prospects for brain rebuilding using integrative management. Altern Med Rev. 2005 Dec;10(4):268-93. 53. Thum T, Hoeber S, Froese S, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007 Feb 16;100(3):434-43. 54. Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens. 2005 Sep;23(9):1699-706. 55. Liu KQ, Qi X, Du JP, et al. Treatment of acute myocardial infarction with autologous bone marrow stem cells mobilization combined with recombinant growth factor in rat. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006 Aug;18(8):494-7. 56. Iwakura A, Shastry S, Luedemann C, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006 Mar 28;113(12):1605-14. 57. Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006 Aug;91(8):3024-33. |