
Irritable Bowel Syndrome (IBS)
Irritable Bowel Syndrome (IBS)
Last Section Update: 10/2024
Contributor(s): Shayna Sandhaus, PhD; Carrie Decker, ND, MS
Table of Contents
- Overview
- Introduction
- Nutrients
- Dietary Considerations for Irritable Bowel Syndrome (IBS)
- Lifestyle Considerations for Irritable Bowel Syndrome (IBS)
- Irritable Bowel Syndrome (IBS) Causes & Risk Factors
- Symptoms & Diagnosis of Irritable Bowel Syndrome (IBS)
- Treatment of Irritable Bowel Syndrome (IBS)
- Novel & Emerging Therapies
- Update History
- References
1 Overview
Summary and Quick Facts for Irritable Bowel Syndrome (IBS)
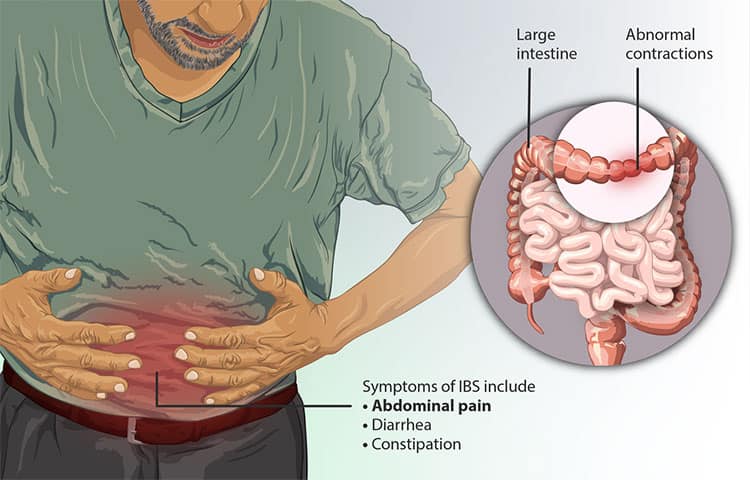
- Irritable bowel syndrome (IBS) is characterized by chronic abdominal pain, cramps, bloating, and altered stool patterns, which can include diarrhea and/or constipation. IBS should not be confused with inflammatory bowel disease.
- This protocol will review several potential causes of IBS and how it is diagnosed. A comprehensive approach to IBS management that combines conventional treatments with dietary, lifestyle, and nutritional interventions will be presented.
- Following a recommended diet, managing stress, and combining conventional medications with nutrients may help alleviate symptoms of IBS and improve one’s overall quality of life.
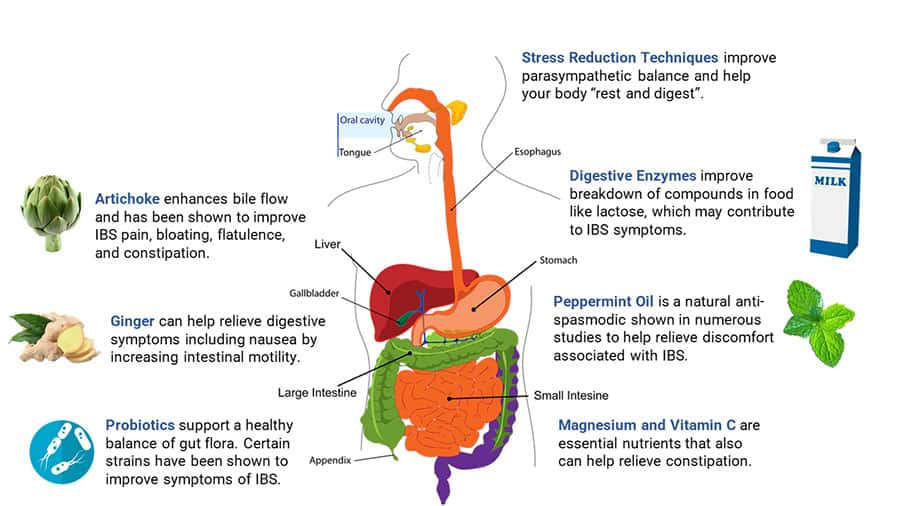
- Probiotics, ginger, artichoke, and other natural agents have been shown to support digestive health and may ease symptoms of IBS.
What is Irritable Bowel Syndrome (IBS)?
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by chronic abdominal pain, cramps, bloating, and altered bowel habits that may include diarrhea and/or constipation. As a functional disorder, it is not linked to tissue damage and is diagnosed by criteria related to key symptoms. Importantly, IBS should not be confused with inflammatory bowel disease (IBD), a broad term describing conditions characterized by chronic inflammation of the gastrointestinal tract.
IBS is very common and often contributes to reduced quality of life. IBS symptoms often can be improved with dietary and lifestyle changes along with nutrient supplementation. However, more serious cases may require drug treatment.
Nutrients & Irritable Bowel Syndrome
- Probiotics. As alterations in the gut microbiome can cause or exacerbate IBS symptoms, supplementation with probiotic bacteria or yeast may help rebalance it. Certain probiotic strains have clinical evidence for improving bowel frequency, abdominal pain, and other symptoms related to IBS.
- Ginger. Ginger may improve constipation by stimulating activity of a nerve-muscle complex that facilitates intestinal motility.
- Digestive enzymes. A comprehensive blend of digestive enzymes taken with meals can improve mealtime symptoms related to foods that commonly trigger IBS-like symptoms, such as lactose and beans.
- Artichoke. Artichoke supports healthy digestive function by promoting bile production. In one study, artichoke leaf extract almost eliminated abdominal pain, cramps, bloating, flatulence, and constipation in patients with IBS. A specific combination of artichoke and ginger extracts has also been shown to relieve bloating, pain, and other functional gastrointestinal symptoms.
- Melatonin. Melatonin, a multifunctional hormone, can be useful for more than just improving sleep. IBS patients taking melatonin had reduced abdominal pain, bloating, and overall IBS symptoms.
What Dietary & Lifestyle Changes Can Help IBS?
- Consider certain elimination diets (ie, low FODMAP or gluten-free diet); foods to avoid may include legumes, wheat, barley, rye, milk, certain fruits, and others.
- Get tested for and avoid foods that trigger high levels of IgG antibodies (ie, foods that cause sensitivities)
- Stress reduction techniques (eg, yoga, cognitive behavioral therapy, etc.)
- Engage in a regular form of low- to moderate-intensity exercise
- Consider acupuncture or hypnotherapy
What are the Signs & Symptoms of IBS?
- Recurrent abdominal pain or cramps typically related to defecation and associated with change in stool frequency or appearance
- Changes in stool could include constipation (in constipation-predominant IBS [IBS-C]) or diarrhea (in diarrhea-predominant IBS [IBS-D])
- Other symptoms not directly associated with the gastrointestinal tract may include headaches, backaches, lethargy, and anxiety or depression
What are the Risk Factors for IBS?
- Genetic predisposition
- Stress, anxiety, and/or depression
- Food sensitivities and intolerances
- Gastrointestinal infections
- Small intestinal bacteria overgrowth (SIBO)
- Intestinal inflammation
- Female gender and hormonal fluctuations
- Use of certain medications, including proton pump inhibitors and broad-spectrum antibiotics
How is IBS Treated?
- Diet and lifestyle interventions
- Bulking agents like dietary fiber and fiber supplementation
- Laxatives and stool softeners (eg, lubiprostone and polyethylene glycol)
- Antidiarrheals such as loperamide or bile acid sequestrants (eg, colesevelam)
- Antispasmodic medications (eg, pinaverium bromide)
- Serotonergic agents, which can be common classes of antidepressants (eg, tricyclic antidepressants [TCAs] or selective serotonin reuptake inhibitors [SSRIs]) or those that locally interact with serotonin receptors (agonists or antagonists) in the gut (eg, alosetron)
- Antibiotics to treat SIBO (rifaximin)
What are Some Novel & Emerging Therapies for IBS?
- Mesalazine, a drug used to treat inflammatory bowel disease, may also benefit patients with IBS
- Mast cell stabilizers (cromolyn or medications known as cromoglycates)
- Transcutaneous vagal nerve stimulation
2 Introduction
Irritable bowel syndrome (IBS) is a very common functional gastrointestinal disorder. The estimated prevalence of IBS varies widely depending on which criteria are used and differ from country to country; about 10–15% of people in the United States are thought to be affected by IBS. Women are affected more frequently than men, and the condition is less common among people over age 50.1-3 Because not everyone with symptoms possibly attributable to IBS seeks treatment, it is estimated that about 40% of individuals who would meet diagnostic criteria for IBS do not have a formal diagnosis.4
Typical IBS symptoms include recurrent abdominal pain (typically related to defecation), bloating, and varying bouts of diarrhea and constipation. The condition is generally associated with a reduced quality of life.4,5 Importantly, IBS should not be confused with inflammatory bowel disease (IBD). IBD includes Crohn’s disease and ulcerative colitis, which are characterized by inflammatory lesions in the intestines.6
IBS can be diarrhea-predominant (IBS-D), constipation-predominant (IBS-C), mixed (IBS-M), or unclassified. Subtype classification is important because the approach to treatment may vary depending on which IBS subtype an individual has. For instance, some medications are only approved for IBS-C while others only have approval for IBS-D.7
Many factors may cause or exacerbate IBS symptoms. For example, stress, anxiety, depression, food sensitivities, small intestinal bacterial overgrowth (SIBO), excessive exercise, and hormonal fluctuations are all associated with IBS.8-13
Addressing psychological conditions in IBS patients is especially important because irritable bowel symptoms often persist despite drug therapy if these issues are not addressed.14-21 Moreover, various strategies of dietary modification, targeting food intolerances and sensitivities, often lead to symptom improvement without the need for pharmaceutical interventions.22-25
This protocol will discuss the causes of and risk factors for IBS along with its diagnosis and conventional treatment; emerging drug strategies will be examined as well. The important role of dietary and lifestyle modification will be reviewed, and evidence on nutrients that may alleviate IBS symptoms will also be summarized.
3 Nutrients
Probiotics
Probiotics are microorganisms that may provide health benefits to their host when administered at sufficient levels.28 Probiotics, as a whole and when taken consistently, may help rebalance intestinal flora and alleviate IBS symptoms, and are recommended in the 2018 American College of Gastroenterology (ACG) guidelines for the treatment of overall IBS symptoms as well as the treatment of bloating and flatulence.29 However, the 2021 ACG guidelines concluded that although probiotics are an important area of research for IBS treatment, there is still not yet enough evidence to recommend them for global IBS symptoms.30 Certain probiotic strains, and blends of probiotics, have been clinically studied in individuals with IBS and shown positive effects.
A particularly important type of bacteria, Bifidobacteria, is found in reduced quantities in the gastrointestinal (GI) tracts of both constipation-predominant IBS31 and diarrhea-predominant IBS32 sufferers relative to healthy individuals.33 Bifidobacterium longum BB536 is one specific strain of Bifidobacteria that has been well studied clinically and shown in various settings, including antibiotic use, to improve defecation patterns in both diarrhea and constipation. It has also been shown to help eliminate opportunistic GI pathogens and have beneficial effects on immune function.34 Another specific Bifidobacteria strain shown to improve bowel symptoms, particularly in those struggling with constipation, is B. lactis HN019. A randomized controlled trial found that treatment with B. lactis HN019 at a dose of 10 billion colony forming units (CFUs) per day, taken for 28 days, increased stool frequency and reduced straining in a subgroup of patients with functional constipation who reported three or fewer bowel movements per week at baseline.35 In another clinical trial of individuals with functional GI symptoms, B. lactis HN019 taken daily for 14 days was also shown to reduce transit time and relieve symptoms compared with placebo, with the higher daily dose of 17.2 billion CFUs being shown to be more effective than 1.8 billion CFUs.36 Additional studies evaluating B. lactis HN019 in combination with different Lactobacillus strains have shown positive effects on GI symptoms related to constipation in as little as two weeks.37,38
A combination of Lactobacillus paracasei IMC 502 and L. rhamnosus IMC 501 has been shown to improve bowel habits in clinical studies of healthy adults.39,40 Although participants in these studies were not diagnosed with IBS, questionnaire responses indicated their bowel habits improved after two weeks of consuming the probiotic supplement. Specifically, subjects reported increased stool regularity and volume.40 In another study, 20 billion CFUs of L. plantarum DSM 9843 were administered daily for four weeks to IBS sufferers. Flatulence resolved rapidly, and improvements in overall GI function remained long after supplementation was discontinued.41
A spore-forming bacteria known as Bacillus coagulans, which is extremely tolerant to harsh environments, including high temperature and stomach acidity,42 has also been shown to improve IBS symptoms in multiple clinical studies.43-46 Specifically, B. coagulans MTCC 5856 at a dose of 2 billion CFUs per day, taken for 90 days, was shown to significantly improve bloating, vomiting, diarrhea, abdominal pain, and stool frequency in patients with IBS-D compared with placebo, also improving disease severity and quality of life.46 A similarly designed study that tested 90 days of supplementation with B. coagulans MTCC 5856 in people with IBS and depression found improvements in depression and IBS symptoms compared with placebo.47
Saccharomyces boulardii is a well-studied probiotic yeast shown in several studies to improve diarrhea related to antibiotic use, traveler’s diarrhea, and recurrent Clostridium difficile infection.48,49 Although clinical data on improvement of specific IBS symptoms is not particularly strong for S. boulardii as a monotherapy, multiple studies have shown that treatment with this probiotic yeast is efficacious for improving IBS-related quality of life.50,51 Additionally, in people with IBS-D, S. boulardii has been shown to decrease the pro-inflammatory cytokines interleukin (IL)-8 and tumor necrosis factor-alpha (TNF-α) and increase levels of the anti-inflammatory cytokine IL-10 compared with placebo in blood and the upper rectum.50 Studies in other settings such as IBD have shown S. boulardii may play a role in resolving GI disease via improving the gut barrier integrity, a factor also implicated in IBS. It has also been shown to help resolve infectious diarrhea via increasing intestinal secretion of immunoglobulin A, which helps protect against infection.52
Artichoke & Ginger
Artichoke. Artichoke leaf has been used since Roman times as a traditional medicine that supports digestive function. It has been shown to promote the production of bile that helps digest dietary fats and reduce spasms and flatulence. An extract of artichoke, standardized to the biologically active cynarin, was shown in a randomized controlled trial to significantly increase volume of bile secretion at 120‒150 minutes after administration compared with placebo.53 Animal studies also support this traditional use of the plant.54,55
In one study, two capsules of 320 mg artichoke leaf extract taken three times daily almost completely eliminated abdominal pain, cramps, bloating, flatulence, and constipation in study participants with IBS who also had nonspecific GI discomfort.56 Further analysis revealed that artichoke leaf extract significantly improved quality of life in subjects with functional dyspepsia (ie, recurrent indigestion with no apparent cause).57 Another study found that consumption of 320 or 640 mg of artichoke leaf extract daily for two months significantly attenuated IBS symptoms and improved quality of life.58
Ginger. Ginger, often used to ease nausea and vomiting,59 may also improve constipation by stimulating activity of the migrating motor complex, the nerve and smooth muscle complex that promotes intestinal motility (peristalsis) and defecation.60 Acutely, treatment with 200 mg of ginger was shown to increase the activity of the migrating motor complex in response to a test meal.61 A lack of activity of the migrating motor complex may contribute to the state of dysbiosis known as SIBO, a factor in IBS for some.60 The gingerols and shogaols found in ginger also have anti-inflammatory properties.62
Combination (artichoke and ginger). Two clinical studies have looked at the effects of a specific combination of standardized extracts of artichoke and ginger on digestive health. In one multicenter, randomized, double-blind, placebo-controlled trial, the combination (containing 100 mg artichoke and 20 mg ginger extracts) was taken twice daily, before lunch and dinner, by individuals with functional dyspepsia.63 After 14 days of treatment, the group receiving the combination had a significant improvement in symptoms versus no change in the placebo group. By the end of the study (day 28), a significant improvement was seen in nausea, bloating, and epigastric fullness, and pain in the artichoke/ginger-supplemented group. In a pilot, randomized, crossover study looking at the effect of acute supplementation of the artichoke/ginger combination on gastric volume, subjects’ after-meal gastric area was 24% smaller, with one capsule before the meal, compared with placebo.64 A small group of these participants were evaluated at a later date and found to exhibit an even greater 38% reduction in post-meal gastric area compared with placebo after ingesting two capsules of the supplement prior to a meal.
Peppermint Oil/Caraway Oil
Peppermint oil is a natural antispasmodic. It is recommended by the ACG 2018 guidelines for the treatment of overall symptoms of IBS, shown in their analysis of seven randomized clinical trials to be significantly more effective than placebo with a number needed to treat of 4.29 The number needed to treat, or NNT, is the number of people who need to receive an intervention in order to realize one occurrence of the outcome under study (in this case, improvement of IBS symptoms). In one study, an enterically coated preparation of 225 mg peppermint oil taken twice daily was shown to reduce all IBS symptoms by over 50% in three-fourths of the patients, whereas only 38% of the placebo group improved.65 In another well-designed study, 187 mg of a similar peppermint oil product taken three times daily before meals for eight weeks led to a significant improvement over placebo with regard to abdominal discomfort, abdominal pain, and quality of life, but not in terms of diarrhea, constipation, or bloating.66 IBS patients treated with peppermint oil in yet another study reported benefits including decreased abdominal pain, less bloating and flatulence, decreased stomach growling, reduced stool frequency, and improved stool consistency.67 A meta-analysis of 12 randomized trials including 835 subjects concluded that peppermint oil improved global IBS symptoms and abdominal pain compared with placebo, with no difference in reported adverse effects.68
Peppermint oil has also been studied in combination with caraway oil. In a double-blind placebo-controlled clinical study of 45 patients with non-ulcer dyspepsia, about half of whom had IBS, subjects in the treatment group received one enteric coated capsule, containing a combination of peppermint and caraway oil, three times daily for four weeks. While all patients complained of moderate-to-severe pain before treatment, 42% of patients in the treatment group were pain-free two weeks after taking the combination therapy versus only one patient in the placebo group. After four weeks of treatment, 63% of those in the treatment group were pain-free versus 25% in the placebo group and 89% in the treatment group showed improvement in pain intensity versus 45% in the placebo group.69 A later trial by the same researchers found similar results—67% of functional dyspepsia patients given the peppermint/caraway oil mixture were described as much or very much improved after treatment for 29 days, compared to only 21% of those given placebo.70 A possible adverse effect of peppermint oil is heartburn as it may have relaxant effects on the esophageal muscle.29
Melatonin
Melatonin is a multifunctional hormone that exhibits a variety of beneficial effects in gastrointestinal disorders independent of its more widely known effects on sleep.71 Animal studies suggest production of melatonin in the GI tract by the enteroendocrine cells far exceeds pineal production of melatonin.72,73 Melatonin receptors in the gut have been shown to play a role in motility, inflammation, and pain.71 Melatonin has also been shown to influence the gut microbiota composition, reducing the dysbiosis induced by stress and sleep deprivation in an animal study.74
In one study of people with IBS and sleep disturbances, 3 mg melatonin taken prior to bedtime for two weeks significantly decreased abdominal pain and rectal sensitivity.75 These findings were supported by another double-blind, placebo-controlled, crossover study in which 3 mg melatonin reduced abdominal pain and bloating in women with IBS.76 In postmenopausal women, daily supplementation with melatonin, at a dose of 3 mg in the morning and 5 mg in the evening for a period of six months, significantly improved overall symptoms at four and six months compared with placebo in those with constipation-predominant IBS.77 In a small study that examined a wider array of symptoms, besides improving bowel function, melatonin was associated with a 43% improvement in overall quality of life compared with 14% in the placebo group. Overall IBS scores improved by 45% among those taking melatonin compared with 16% in those taking placebo.78
Vitamin D
Some evidence suggests vitamin D supplements may benefit some people with IBS, particularly those with vitamin D deficiency. In one study that randomized 88 people who satisfied the Rome IV criteria for IBS-D and who had vitamin D deficiency or insufficiency, subjects received either 1,250 mcg (50,000 IU) vitamin D3 or placebo weekly for nine weeks. All participants also received 135 mg of the antispasmodic drug mebeverine (Colofac) twice daily. Among 74 subjects who completed the study, IBS symptom severity and levels of the inflammatory mediator IL-6 decreased significantly in the vitamin D group compared with the placebo group and baseline.79 Another randomized controlled trial found that 1,250 mcg (50,000 IU) vitamin D3 every two weeks improved some biomarkers related to inflammation and oxidative stress relative to placebo. The effect on certain inflammatory biomarkers (TNF-α and IL-17) was evident only in subjects with IBS-D, but changes in other inflammatory markers and in those related to oxidative stress were apparent regardless of IBS subtype.80 In a six-month randomized controlled trial, 90 participants with IBS took either 1,250 mcg (50,000 IU) vitamin D or placebo every two weeks. Subjects who received vitamin D reported improved symptoms compared with those who received the placebo.81 A trial that enrolled 112 adolescents with IBS and low vitamin D levels found that supplementation with 50 mcg (2,000 IU) vitamin D daily for six months improved symptoms compared with placebo.82
On the other hand, a trial in which 135 participants with IBS and low vitamin D levels took 75 mcg (3,000 IU) vitamin D or placebo daily for 12 weeks found no differences in symptom severity at the end of study period, despite a significant increase in serum vitamin D levels.83
Although not all studies have found a benefit with vitamin D, supplementation is reasonable for those with IBS given the minimal cost and robust safety record of vitamin D. Supplementation may be especially pertinent for those with low vitamin D levels.84
Berberine
There is a multitude of mechanisms by which berberine, the yellow-orange alkaloid found in botanicals such as Oregon grape and goldenseal, may be useful in the setting of IBS. Berberine has well-documented antimicrobial effects and supports the integrity of the intestinal epithelial tight junctions.85-88 In animals, it also has been demonstrated to have antinociceptive effects, reducing visceral hypersensitivity.89
Berberine has been shown to be effective in human studies for the treatment of diarrhea of various etiologies. In patients with IBS-D, treatment with 400 mg of berberine hydrochloride twice daily for eight weeks significantly reduced the frequency of diarrhea, abdominal pain, and urgent need for defecation compared with placebo. Improvements were also seen in scores of IBS symptoms, IBS quality of life, and depression and anxiety versus placebo.90 A retrospective study of a combination including berberine, guar gum (a galactomannan), and melatonin also found treatment was associated with significant improvements in functional diarrhea or IBS-D. After 30 days, diarrhea events were reduced by 50‒70%, and by 90 days they were reduced by 70‒80% with more than half of the subjects reporting normalized Bristol Stool Scale scores.91 Berberine, in its sulfate form, has also been shown to be effective for acutely improving diarrhea due to enterotoxigenic Escherichia coli.92
A recent systemic review and meta-analysis out of China (a region in which berberine is widely used as a treatment for diarrhea) surveyed 38 randomized controlled trials in which berberine was used as a treatment for diarrhea of various origins, including infectious and non-infectious causes. Typically, berberine was given for a short period, similar in time to a standard course of antibiotics. Various other interventions were allowed in the studies selected and included antibiotics, probiotics, B vitamins, and montmorillonite (an absorbent clay) in addition to berberine. Overall, it was found that berberine improved clinical outcomes and shortened the duration of diarrhea compared to control interventions with no serious adverse events reported.93
Although berberine has been studied and shown to have a high level of safety in human clinical trials, concerns about its long-term use have been raised on the basis of preclinical studies that suggest long-term berberine use, especially at high doses, may impair cellular metabolism in specific types of cells.94-96 The implications of this preclinical research are yet to be determined by long-term human clinical trials, therefore Life Extension currently recommends short-term use of berberine when indicated.
Glutamine
Glutamine is the most abundant amino acid in the body and is an important fuel for the intestinal epithelial cells. Glutamine is considered a nonessential amino acid due to endogenous synthesis; however, in hypercatabolic states (such as severe burn wounds, infections, and surgery; and possibly also pregnancy, lactation, and substantial growth periods) it is often considered to be “conditionally essential.”97,98 In periods of high physical stress such as these, the rate of glutamine consumption by the small intestinal mucosa exceeds the rate of production.99 In these cases, glutamine has been shown to improve intestinal permeability.100,101
Glutamine has been shown to be of clinical benefit in settings where intestinal repair is important, such Crohn’s disease and post-infectious diarrhea.102,103 In individuals with IBS-D subsequent to enteric infection, a randomized controlled trial found that glutamine at a dose of 5 grams three times daily for eight weeks improved IBS severity scores meaningfully in almost 80% of individuals compared with only 5.8% of the placebo group achieving comparable improvement. Those in the glutamine group also saw highly significant improvements in daily bowel movement frequency, Bristol Stool Scale scores, and intestinal permeability. Intestinal permeability was normalized in the glutamine group but not in the control group.102
Exercise, particularly at intense levels or in hot temperatures, has been shown to increase intestinal permeability.104 In fact, 25‒50% of elite athletes are troubled by gastrointestinal symptoms.105 Extreme temperatures and inadequate hydration further contribute to dysregulated GI function.104 Glutamine has been shown in multiple clinical studies of athletes to help reduce or even prevent increased intestinal permeability related to intense exercise.106-108
Digestive Enzymes
According to a 2012 national survey, the use of digestive enzyme supplements by individuals with IBS is common.109 A comprehensive enzyme supplement commonly includes lactase for the breakdown of lactose, enzymes like xylanase, cellulase, and hemi-cellulase for the breakdown of plant substances, specific enzymes to facilitate the breakdown of gliadin, proteases to support digestion of proteins, lipase to improve digestion of fats, and alpha-galactosidase to help break down oligosaccharides found in foods like beans. Supplementation with specific digestive enzymes (eg, lactase) is a well-recognized intervention for the treatment of digestive symptoms associated with lactose intolerance.
Supplemental enzyme ingestion is also used as an intervention for symptoms associated with exocrine pancreatic insufficiency (EPI), which can have symptoms similar to IBS, particularly in its mild state.110 As EPI is typically diagnosed when pancreatic lipase falls below 10% of normal pancreatic output, many individuals may experience symptoms related to inadequate pancreatic output before this diagnostic threshold is breached.111 Several diseases have been linked to EPI—reduced pancreatic elastase production (<200 mcg/g) was identified in about 40% of individuals with longstanding diabetes, while celiac disease and alcohol use are other potential contributing factors.112-114 A comprehensive stool panel that includes assessment of digestive health measures (eg, elastase levels) and assesses for undigested fat can help determine if mild EPI is a contributing factor to mealtime symptoms attributed to IBS.
As there may be mild EPI, undiagnosed food intolerances or sensitivities, or sensitivities to high FODMAP foods, use of digestive enzymes as a dietary supplement may help improve mealtime-related symptoms of IBS.115,116
Magnesium
Magnesium is an osmotic laxative with a long history of use for treating constipation.117 In females with mild-to-moderate chronic constipation, 500 mg magnesium oxide taken three times daily for 28 days significantly improved the frequency of spontaneous bowel movements (SBM), colon transit time, and Bristol stool consistency scores compared with placebo.118 Symptoms overall and constipation-related quality of life were also significantly improved. In adults with chronic constipation, 28 days of treatment with 1,500 mg magnesium oxide or 1,000 mg of senna (a stimulant laxative) per day significantly improved symptoms overall (68% and 69%, respectively) compared with placebo (almost 12%).119 Significant improvements in SBM and complete SBM were also seen in the active treatment arms compared with placebo.
Because mild abdominal discomfort can occur with higher doses of magnesium, it is best to gradually increase and/or divide doses during the day. In healthy individuals, the kidneys filter 2,000–2,400 mg of magnesium daily,120 and there is little risk associated with the use of magnesium at doses commonly used for laxative purposes. However, excessive magnesium use, particularly by those with late-stage chronic kidney disease, can lead to hypermagnesemia.121 Those taking additional laxatives and using more than 1,000 mg magnesium per day are at greater risk of this complication.
Perilla Leaf Extract
Perilla frutescens is a plant in the mint family that is native to East Asia. Its leaves are rich in polyphenols and, in addition to being a flavorful and aromatic ingredient in many traditional foods, perilla leaf has been historically used in allergic, respiratory, and digestive ailments.122 In a randomized controlled trial, 47 participants with GI discomfort and reduced bowel movement frequency were treated with either perilla extract, 150 mg twice daily, or placebo for four weeks. All GI symptoms, including abdominal discomfort, flatulence, rumbling, fullness, and bloating, improved significantly in the perilla group. For bloating, the positive response rate in the perilla group was 83% compared with 57% in the placebo group.123
Perilla may have several modes of action that contribute to its beneficial effects for digestive symptoms. Preclinical studies indicate perilla stimulates motility of the intestinal tract124 and may relax intestinal muscle spasms.125 Other studies found perilla extract inhibited the inflammatory response generally126-128 and specifically in the large intestine in mice.129
Stress, anxiety, and depression are known to be associated with changes in pain sensitivity, GI motility, and intestinal permeability via a complex network that connects the GI tract, brain, immune system, and microbiota.130 In addition to preventing a stress-induced inflammatory response, an essential oil extract of perilla prevented depression-related behaviors in chronically stressed mice.131 Another study also noted a reduction in signs of stress-induced depression and a reversal of stress-induced changes in brain metabolism in mice treated with perilla essential oil. In this study, perilla extract compared favorably to fluoxetine (Prozac).132
Activated Charcoal
Activated charcoal can be a useful palliative agent, especially for post-infectious IBS-D. It is used to enhance the elimination of toxic substances via the GI tract.133,134 Due to its adsorbent nature, activated charcoal also helps the body eliminate immunogenic and toxic byproducts of infection, such as bacterial endotoxins.135,136 Activated charcoal has been suggested as a palliative agent in the treatment of diarrhea due to its low rate of side effects compared with anti-diarrheal treatments.137
A multicenter study of activated charcoal for the treatment of IBS, including approximately 60% who reported constipation as a symptom, found it was well tolerated and efficacious in nearly 80% of individuals after 12 weeks of treatment. Even the patients who began the study with severe and moderate constipation had substantial improvements. Flatulence, stool consistency, and abdominal distention were also reported to be improved.138 Activated charcoal has also been studied and shown to be supportive in reducing the severity and frequency of chemotherapy-induced diarrhea.139,140
Activated charcoal use may cause constipation in some individuals.141,142 Stools also appear black with activated charcoal use which can be mistaken for blood in the stool.143 Importantly, taking activated charcoal at the same time as prescription medications may reduce the amount of medication your body absorbs. Therefore, medications and activated charcoal should not be taken at the same time. If you are taking any medication, talk to your doctor before using activated charcoal.
Zinc Carnosine
Zinc carnosine is a form of zinc backed by multiple studies suggesting it supports the health of the gut lining, improving the integrity of the tight junctions and protecting the intestinal villi from damage.144,145 Increased intestinal permeability has been shown to be an issue in individuals with IBS-D and has been associated with visceral hypersensitivity.146,147 Zinc carnosine also has some anti-inflammatory and antioxidant effects, which may lend to its usefulness in IBS.148
In a crossover study in healthy volunteers, zinc carnosine at a dose of 37.5 mg twice daily was shown to ameliorate the increases in intestinal permeability induced by non-steroidal anti-inflammatory drug use.145 In a randomized, controlled, crossover trial, at the same dose, zinc carnosine was shown to significantly reduce the increase in intestinal permeability seen with heavy exercise. Effects were greatest at the end of the study, with a 71% decrease in permeability after 14 days of treatment.149
Collagen Peptides
Collagen is often purported to have a gut healing effect: numerous “gut healing” diets and collagen-enhanced products on supermarket shelves allude to this effect. However, human studies with collagen for this purpose are lacking as of mid-2021. Nevertheless, preclinical research from cellular and animal studies suggest collagen peptides may support gut integrity and reduce inflammation related to increased intestinal permeability. Specifically, in mice subjected to burn wounds, a setting known to increase intestinal permeability, at seven days post injury, treatment with collagen peptides had a greater effect than glutamine on reducing serum endotoxin levels (a marker of intestinal permeability) and the inflammatory markers TNF-α and IL-6.150 Other research with this same animal model found a reduction in burn-induced intestinal permeability and disruption of tight junctions when collagen peptides were supplemented early after injury.151 Findings from cellular studies with Caco-2 cell monolayers (a common model for studying intestinal permeability) also suggest collagen peptides reduce intestinal permeability induced by the inflammatory trigger TNF-α.152,153
Curcumin
Curcumin is a polyphenol extract of the plant Curcuma longa (turmeric). It is well-known for its anti-inflammatory properties and has been studied in the context of health conditions in which inflammation plays a role, including IBS and related gastrointestinal disorders.154,382
Curcumin has been shown to have benefits comparable to the proton-pump inhibitor omeprazole (Prilosec) for symptoms of functional dyspepsia (ie, recurrent indigestion with no apparent cause). In a randomized controlled trial, 151 people with functional dyspepsia received either 500 mg curcumin four times daily, 20 mg omeprazole, or a combination of the two for 28 days. Functional dyspepsia symptoms were assessed on days 28 and 56 by the Severity of Dyspepsia Assessment (SODA). On day 28, there were significant improvements in SODA scores for all three groups in the pain, non-pain, and satisfaction categories, and by day 56 these improvements had become more pronounced. Curcumin and omeprazole had comparable efficacy for functional dyspepsia without adverse effects, but there were no signs of a synergistic effect.383
Curcumin has also been used successfully in combination with fennel in the treatment of IBS. In a randomized controlled trial, 121 people with mild-to-moderate symptoms of IBS received 84 mg curcumin and 50 mg fennel together, or a placebo, twice daily for 30 days. After treatment, those that received the curcumin-fennel combination had a significant decrease in the symptom severity score of IBS compared with placebo. In addition, about 26% of people in the curcumin-fennel group became symptom free compared with only about 7% of those in the placebo group.384
In a randomized controlled trial, a total of 77 people with digestive complaints received either 500 mg of a standardized curcumin called Curcugen, which provided 50% curcuminoids, or a placebo daily for eight weeks. The researchers evaluated changes in gastrointestinal symptoms and intestinal microbiota. The results showed that, compared with placebo, those who took the curcumin supplement had improved scores of digestive complaints and anxiety levels on a self-reported questionnaire. There were no significant changes observed in the intestinal microbial profile or SIBO testing.385
Additional Support
Stress-modifying natural therapies. Several natural compounds, including adaptogenic and nervine herbs (eg, Bacopa, holy basil, lemon balm, and ashwagandha) may benefit IBS sufferers by mitigating stress.
Research on Bacopa monnieri indicates it has adaptogenic effects and can significantly decrease stress-related anxiety.156,157 In a 6-week, randomized, controlled trial, Bacopa (in combination with another herb) was found to be particularly beneficial in IBS-D.158 More stress-reduction strategies are discussed in Life Extension’s Stress Management protocol.
4 Dietary Considerations for Irritable Bowel Syndrome (IBS)
Dietary considerations, such as reducing daily intake of caffeine and fatty foods, may benefit individuals with IBS.159 Individuals with IBS are often aware of some foods that exacerbate symptoms; thus, they may be able to improve symptoms by avoiding those foods.160 If one is not aware of specific food triggers, a food and symptoms diary may be useful in identifying specific contributors to symptoms.161,162
Note: People who have ever had an eating disorder, among whom IBS is fairly common, should approach dietary restrictions judiciously as they may exacerbate eating disorder behavior.163
Low-FODMAP Diet
FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) are poorly absorbed carbohydrates that draw water into the small intestine and/or are fermented by bacteria in the large intestine, producing hydrogen gas—effects that may cause digestive symptoms.370,371 Although FODMAPs have been found to elicit the same gastrointestinal events in healthy people and IBS sufferers, heightened pain sensitivity in people with IBS is thought to cause them to experience symptoms like pain, distension, and bloating. Therefore, restricting these fermentable fibers may result in symptom relief.371 However, concerns have been raised that long-term restriction of FODMAPs may have detrimental effects on the gut microbiome since most prebiotics are FODMAPs, suggesting an elimination/reintroduction approach may be prudent.166
Table 1. High-FODMAP Foods and Low-FODMAP Alternatives370
|
High-FODMAP Foods | Low-FODMAP Alternatives |
|---|---|---|
Vegetables |
Artichokes, asparagus, cauliflower, garlic, green peas, mushrooms, onions |
Carrot, cucumber, lettuce, potato, eggplant, celery, green beans, pumpkin, squash |
Fruits |
Apples, cherries, mangoes, nectarines, peaches, pears, plums, watermelon, dried fruit |
Cantaloupe, kiwifruit, mandarin, orange, pineapple, blueberry |
Dairy & Alternatives |
Cow’s milk and soy milk products |
Almond milk, feta cheese, lactose-free milk, soy milk (made from soy protein) |
Protein |
Legumes, marinated meat and seafood, some processed meats |
Eggs, plain cooked meats/poultry/seafood, firm tofu, tempeh |
Breads & Cereals |
Wheat, rye, and barley products (eg, breads, pasta, and crackers), breakfast cereals |
Oatmeal, quinoa, rice, gluten-free products, sourdough spelt bread, polenta |
Sugars, Sweeteners |
Foods sweetened with high-fructose corn syrup, honey, artificial sweeteners with sugar alcohols |
Dark chocolate, maple syrup, table sugar, rice malt syrup |
Nuts & Seeds |
Cashews, pistachios |
Macadamias, peanuts, pumpkin seeds, walnuts |
A low-FODMAP diet is usually recommended as a three-phase program: foods high in FODMAPs are eliminated during the first phase; if symptoms improve, the second phase involves systematic food reintroductions to determine tolerance to the various types of FODMAPs; in the third phase, a personalized long-term diet that maximizes IBS symptom management and minimizes food restriction is developed.370,371
Multiple clinical trials and meta-analyses have shown a low-FODMAP diet can relieve symptoms including abdominal pain, distension, and bloating, and improve quality of life in people with IBS.372-375 In one meta-analysis of 13 randomized controlled trials that included a total of 944 participants with IBS, a low-FODMAP diet was found to be more effective for relieving IBS symptoms than habitual diet or other dietary interventions for treating IBS.376
A 2024 umbrella analysis pooled the findings of 11 meta-analyses and 24 randomized controlled trials with a total of 1,646 subjects with IBS. The analysis found a low-FODMAP diet377:
- Improved overall symptoms in patients with all subtypes of IBS
- Reduced most digestive symptoms within four weeks
- stool consistency improved with diet adherence longer than four weeks
- Increased quality of life
On the other hand, the umbrella analysis found a low-FODMAP diet did not decrease anxiety or depressive symptoms. Furthermore, it appeared to have negative impacts on the gut microbiome, reducing abundance of healthy gut bacteria, decreasing short-chain fatty acid production, and increasing (alkalinizing) stool pH.377 Other clinical trials using low-FODMAP diet interventions have shown this diet alters microbial balance in ways that may be detrimental to health, reducing Bifidobacterium species and increasing bacteria associated with dysbiosis.166
Because a low-FODMAP diet can negatively affect the gut microbiome, reintroducing foods high in prebiotic fibers, while maintaining symptom control, is an important goal.166 To this end, several studies have evaluated whether FODMAP elimination followed by judicious reintroduction may be a viable approach to achieving lasting symptom reduction for those with IBS.
In a study based on an online survey that received more than 20,000 responses from individuals with IBS who used a low-FODMAP diet intervention, 2,053 respondents answered questions about food reintroductions. According to these self-reports, the foods most commonly associated with symptom recurrence were wheat bread, wheat pasta, milk, onion, and garlic.378 A small clinical trial involving 18 patients with IBS found adhering to personalized food restrictions based on reactions to food reintroductions after a short-term low-FODMAP diet resulted in lasting symptom relief after one year in 12 of the participants; microbial analysis showed Bifidobacterium species were not reduced from baseline levels, but short-chain fatty acid production was diminished, suggesting some loss of beneficial bacteria.379
In a randomized controlled trial, 294 subjects with moderate-to-severe IBS were assigned to treatment with a low-FODMAP diet, a high-fiber/low-carbohydrate diet (~50 grams per day; also high in protein and fat), or optimized medical management. After four weeks, both diets relieved symptoms better than medical management, and the low-FODMAP diet was the more effective diet: 76% of those in the low-FODMAP diet group, 71% of those in the high-fiber/low-carbohydrate diet group, and 58% of those in the medical management group achieved a 50% or greater reduction in symptom scores. Furthermore, 68% of those in the low-FODMAP diet group and 60% of those in the high-fiber/low-carbohydrate diet group who were reached for a six-month follow-up reported lasting symptom relief despite partially returning to their usual diets.380
In a trial involving 50 patients with IBS, following a low-FODMAP diet for three months led to significant improvements in symptom severity and quality of life, and symptom improvement was maintained after reintroduction of some FODMAP-containing foods for the next three months. And there were no changes observed in the intestinal microbiome when participants adopted a low-FODMAP diet.381
Gluten-free Diet
While a gluten-free diet is required for people with celiac disease, there is a wide spectrum of non-celiac gluten sensitivity symptoms that may present like IBS.169 Gluten is found in grains (eg, wheat, barley, rye), breads, pasta, etc. Similar to the gluten-free diet, the low-FODMAP diet also restricts gluten. Both diets are used to manage food sensitivities, suggesting gluten sensitivity might be a more common contributor to IBS symptoms than previously thought.170 Following a gluten-free diet also reduces dietary consumption of fructans, a FODMAP, which one small study pointed to as a factor possibly leading to IBS symptoms rather than gluten.24
In one double-blind, randomized, placebo-controlled study of IBS sufferers who specifically did not have celiac disease, addition of gluten worsened abdominal pain, bloating, fatigue, stool consistency, and overall symptoms of IBS.171 However, as of mid-2021, only limited evidence supports adherence to a gluten-free diet as a strategy for the treatment of IBS symptoms.167,172
Bulking Agents
Bulking agents (ie, dietary fiber and fiber supplements) are frequently used to treat IBS. Dietary sources of fiber include whole grains, oats, fruit, vegetables, beans, lentils, and nuts and seeds; psyllium husk powder is a common source of supplemental fiber. Insoluble fiber facilitates defecation by reducing transit time (the time it takes for the remains of ingested food to be excreted) while soluble fiber increases stool viscosity. A 2018 review and meta-analysis by the ACG found that fiber improved overall IBS symptoms. However, when broken down by fiber type, it was found that psyllium (mostly soluble fiber), and not bran (mostly insoluble fiber), was effective in treating IBS.29
A potential side effect of fiber supplements is bloating and gas,5 which can exacerbate IBS for some individuals; thus, fiber intake should be increased gradually.
Additional Dietary Considerations for Irritable Bowel Syndrome (IBS)
In addition to the typical diet considerations discussed for the treatment of IBS thus far, certain additional consumables are also associated with IBS symptoms.
Both decaffeinated and regular coffee have been shown to stimulate colon motor activity.181 Individuals who regularly drink coffee were shown to be more likely to have IBS than those who never drink coffee. Higher intake of caffeine was also associated with increased rates of IBS compared with low intake, although when separated by gender, the relationship only held in women.182
Excessive consumption of alcohol was also associated with increased gastrointestinal symptoms in individuals with IBS, although only women were surveyed in this population study.183
5 Lifestyle Considerations for Irritable Bowel Syndrome (IBS)
Stress Reduction
Stress associated with early life adverse events is implicated in the etiology of IBS185; about 50% of individuals who seek IBS treatment have depression or anxiety.186 This relationship appears to be bidirectional, meaning IBS may cause stress and stress may contribute to IBS symptoms. This cycle may be partially attributed to enhanced sympathetic nervous system (“fight or flight”) signaling in people with IBS relative to healthy controls.187
IBS symptoms appear to respond positively to stress reduction. In one study, a meditation-based intervention known as mindfulness-based stress reduction (MBSR) reduced the severity of IBS and stress symptoms in IBS patients, although improvements in mood and quality of life were similar to those of a control group of IBS patients who were placed on a waiting list for MBSR.188 Furthermore, psychological therapies—including cognitive therapy, dynamic psychotherapy, and hypnotherapy—may be of some benefit to those with IBS.29 In a 2018 publication on the treatment of IBS, the ACG concluded, “IBS patients treated with psychological therapies were more likely to improve than patients not treated with psychological intervention,” finding a number needed to treat of 4. However, they noted significant design differences among the 36 randomized controlled trials surveyed.29
For more specific discussion on stress-reduction techniques and nutrients that may be of benefit, see Life Extension’s Stress Management protocol.
Cognitive behavioral therapy. To those unfamiliar with it, cognitive behavioral therapy (CBT) may seem intimidating and like a momentous undertaking. However, this form of therapy is focused on specific behaviors and thought patterns related to a complaint, whether it be IBS, insomnia, or a problematic behavior. Multiple studies have looked at different ways of applying CBT as a treatment for IBS, including via telephone, a web-based program, and with very minimal sessions.189,190 Treatment with home-based CBT was found to moderately to substantially improve gastroenterologist-rated IBS symptoms in almost 56% of individuals after two weeks, while IBS education only led to similar improvements in about 40% of participants.190 Six months after the end of treatment (which totaled four sessions over four weeks), a significantly greater improvement was still seen in the group that received home-based CBT than in those who received IBS education.
Hypnotherapy. Hypnotherapy is often misrepresented as a single session in which one is “reprogrammed” to not engage in a behavior; however, it is actually a specific psychology practice that occurs over many sessions. IBS happens to be one medical condition for which this therapy has been studied as the mind-body connection is well established and recognized even by traditional gastroenterologists.29 Similar to CBT, hypnotherapy also has the possibility of being performed remotely, and even can be done in a group.191,192 In IBS patients who still had symptoms despite previous treatment attempts, 12 weeks of weekly hypnotherapy sessions (applied in a psychology practice setting) significantly improved IBS-related symptoms at three months compared with controls. Additionally, the improvements seen at three months were maintained at one year.193
In a three-arm study comparing individual or group hypnotherapy to control (IBS educational support), both forms of hypnotherapy were significantly more effective than control for adequate relief of symptoms at three months when treatment ended, as well as at nine months later. For each intervention, six sessions occurred biweekly during the treatment period. Interestingly, in the per-protocol analysis, more participants in both hypnotherapy groups reported adequate relief at 12 months versus three months, when the sessions had just been completed.194 Findings from both studies suggest the improvements were maintained after the course of treatment despite the fact that IBS is typically a chronic or recurrent condition.194,195
Exercise
Although particularly intense exercise, especially in hot environments, can be a contributing factor to IBS (as discussed in section on causes and risk factors for IBS), exercise can also be beneficial for IBS patients. In one study, subjects who engaged in 20–60 minutes of moderate-to-vigorous physical activity 3‒5 days per week experienced a marked improvement in quality of life that was associated with reduced IBS severity.196 Aerobic exercise at a lower intensity threshold and of shorter duration has also been shown in clinical studies to benefit those with IBS. Thirty minutes of treadmill exercise three times a week for six weeks significantly improved IBS symptoms and IBS-related quality of life compared with control (no prescribed exercise) and baseline in one small trial.197 Even walking regularly has been shown to improve symptoms of IBS, and it additionally had a positive effect on mood.199
Yoga is another form of exercise that has been studied in the context of IBS, as well as numerous other stress-associated conditions. Compared with adhering to a low-FODMAP diet, engaging in a yoga session twice weekly led to similar improvements in IBS symptoms at 12 and 24 weeks. Of note, both groups were given guidance to only follow the prescribed intervention for 12 weeks; however, statistically significant benefits were still seen at 24 weeks.200 Another study compared three arms: yoga with limited conventional treatment, yoga with conventional treatment, and the control group. Yoga sessions occurred three days per week. Significant improvements were seen in IBS symptom severity and quality of life scores in both yoga groups compared with control. In the yoga groups, there also were significant improvements in several other parameters, including flexibility and autonomic function, which correlated with a reduction in medication and supplement use.201 A systemic review and meta-analysis of six trials considering yoga as a treatment for IBS also found a beneficial effect over conventional treatment, with improvements in bowel symptoms, IBS severity, and anxiety, as well as other parameters. However, the authors noted major flaws in the study methods that prevented them from making a strong conclusion that yoga should be recommended for individuals with IBS.202
Acupuncture
Several clinical trials suggest acupuncture may alleviate IBS symptoms.203-207 A multicenter, randomized, controlled trial found acupuncture (18 sessions) was significantly more effective than standard pharmaceutical treatments (polyethylene glycol [PEG] given at 20 g/d for constipation-predominant IBS or pinaverium bromide given at 150 mg/d for IBS-D) for reducing IBS symptom severity scores on all domains after six weeks of treatment. Post-hoc analysis also suggested the responder rate with acupuncture remained higher than among those receiving pharmaceutical treatment 12 weeks after the intervention was completed.203 Another study compared treatment with acupuncture at specific points (noted to be key for treatment of diarrhea) to treatment with pinaverium bromide in people with IBS-D. After six weeks of treatment and at the three-month follow up, IBS-quality of life and symptom severity scores in the acupuncture group were significantly better than baseline. At both six weeks and three months, symptom severity scores were significantly lower in the acupuncture group than those treated with pinaverium bromide, while quality of life scores were significantly higher in the acupuncture group at three months.204 Two meta-analyses also found acupuncture to be more effective than pharmaceutical treatment of IBS, and to have the least severe side effects; however, there was no difference in symptom severity or quality of life when comparing acupuncture to sham acupuncture.208,209
6 Irritable Bowel Syndrome (IBS) Causes & Risk Factors
The cause(s) of IBS are not clear.210 Stress, GI tract hypersensitivity, altered gut microbiota, intestinal inflammation, genetics, and food sensitivities may all be involved.211-213 One theory proposes that altered serotonin metabolism within the GI tract and/or abnormalities in pain perception pathways cause hypersensitivity to abdominal sensations or pain.186 Other hypotheses suggest stress-induced inflammation, gastroenteritis, and a history of traumatic events may contribute to the development of IBS.185,213,214
Disrupted Gut-Brain Communication
Some evidence suggests alterations in the bidirectional communication between the brain and gut may contribute to IBS symptoms.215-218 The mechanisms behind these phenomena are unclear, but some studies have identified altered autonomic and central nervous system function in individuals with IBS.219-221 Studies also point to the gut microbiome as a mediating factor in altered gut-brain communication in the context of IBS.215 Additional studies employing magnetic resonance imaging to examine the brains of people with IBS have identified some structural changes and heightened visceral stimuli response that may contribute to enteric hypersensitivity.222-224
Stress and anxiety appear to contribute to gut hypersensitivity via modulation of neural pain-processing pathways by glucocorticoid hormones, which are also called “stress hormones.”225 Moreover, alterations of the microbiome have been observed in mood disorders, which frequently also present with IBS symptoms; these microbiome changes may contribute to both IBS and mood disorders.226-229 Persistent systemic and neuroinflammation may be another factor in altered gut-brain communication and IBS symptoms.212
Several studies suggest impaired gut-brain communication may stem from altered levels of chemical messengers called neurotransmitters. Levels and activity of the neurotransmitter serotonin in particular appear to be somewhat abnormal in people with IBS.230,231 Serotonin affects intestinal motility and may be a factor in visceral hypersensitivity and intestinal permeability.232-236 Enteric serotonin levels are regulated by the serotonin reuptake transporter (SERT). An increase in SERT expression leads to lower enteric serotonin levels and contributes to constipation-predominant IBS (IBS-C), while a decrease in SERT expression increases serotonin levels in the gut and can be a factor in IBS-D.237
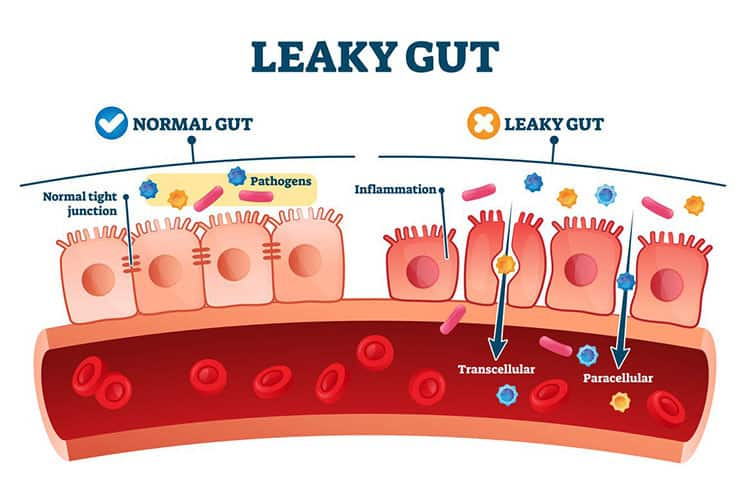
Dysbiosis
One consistent finding associated with both IBS-D and IBS-C is dysbiosis —a pathogenic alteration in the gut microbiota which can cause or exacerbate IBS symptoms in a variety of ways.31,238 Dysbiosis is associated with increased intestinal permeability (often referred to as “leaky gut”) whereby pathogens, toxins, or undigested foods that are not usually absorbed are able to pass into the bloodstream. This can trigger abdominal pain and altered bowel habits. Dysbiosis can also lead to aberrant immune system activation, resulting in the release of cytokines that increase abdominal pain perception and alter bowel habits.239
People with IBS produce an abnormally high amount of gas in response to certain foods, particularly those high in fermentable carbohydrates, and this may be attributed to dysbiosis.240 Increased gas formation results in an increase in abdominal bloating, abdominal pain, and flatulence that can often be reversed by avoiding those foods.168 Studies have also determined that the gut microbiota may alter levels of neurotransmitters and steroid hormones, another mechanism possibly linking anxiety and depression with IBS.241
Small intestinal bacterial overgrowth (SIBO) is a condition characterized by overgrowth of microbes in the small intestine. As a result, fermentation of food begins before it has been thoroughly digested and absorbed, which can lead to gas formation.242,243 The formation of gases (typically hydrogen, but also methane) after consumption of a fixed amount of carbohydrates and their exhalation via respiration is typically used for the diagnosis of SIBO; however, the gold standard for diagnosing SIBO is microbial investigation of fluids collected from the small intestine.244 SIBO is more common in people with motility disturbances, low stomach acid production, and bowel obstruction.243 The prevalence of SIBO in IBS varies across studies, but estimates range from about 20‒84%.10-12 A 2018 systematic review and meta-analysis found SIBO to exist in IBS 38% of the time, being more common in females, older people, and those with IBS-D.245
Obesity is a risk factor for the development of SIBO, and the link has been shown to be unrelated to alterations in bowel transit time or stomach pH.246,247 Other risk factors for SIBO include proton pump inhibitor use, chronic pancreatitis, immunodeficiencies, altered anatomy, small intestinal disease (including IBD and celiac disease), and chronic liver disease.248,249
Intestinal Inflammation
Historically, the presence of intestinal inflammation was considered a differentiator between IBS and IBD, only being a factor in the pathology of IBD. Now, intestinal inflammation is recognized as a factor in IBS. However, inflammation in IBS is distinctly different from the inflammatory profiles of IBD.210,212,250 Increased numbers of lymphocytes, mast cells, and eosinophils have been shown in different regions of the intestine in individuals with IBS, while elevated levels of proinflammatory cytokines have also been shown both locally in the gut and systemically.210,251-254 These activated immune cells release inflammatory mediators including nitric oxide, histamine, proteases, and tryptase, which studies suggest contribute to increased intestinal permeability, pain, and other IBS symptoms.210,255,256
Food Intolerances & Sensitivities
Food intolerances and sensitivities may have a role in IBS. Please see the “Dietary Considerations” section of this protocol for further exploration of this topic.
Gluten sensitivity. Gluten is a protein component of some grains, especially wheat. Sensitivity to gluten is common and is associated with a spectrum of symptoms ranging in severity from minor skin conditions to severe GI compromise in the case of celiac disease, which should be ruled out as a cause of symptoms.257-260 Some evidence suggests gluten sensitivity potentially contributes to IBS symptoms; however, gluten-containing foods also often contain fructans, which may cause the symptoms.24,260,261 A population survey of 1,002 individuals in the UK self-reporting gluten sensitivity (with the possibility of celiac disease ruled out) found that 20% of them met the Rome III criteria for IBS compared with 3.9% of those not reporting gluten sensitivity.262 Although evidence is not yet strong enough to support a recommendation that all IBS patients avoid gluten, findings from at least one study indicate that using a blood test to detect IgG antibodies against components of wheat may help identify patients with IBS-D who are likely to respond positively to a gluten-free diet.263 Individuals who are HLA-DQ2 or HLA-DQ8 positive (at risk for celiac disease but not diagnosed with the condition) have also been shown to have altered bowel movement frequency and increased intestinal permeability with gluten consumption and may want to avoid gluten.264
Carbohydrate malabsorption. Lactose, the sugar found in dairy products, is broken down by the enzyme lactase, found on the mucosal lining of the intestine. Reduced levels of this enzyme lead to lactose intolerance, which manifests with symptoms similar to IBS after the consumption of lactose-containing dairy. Lactose intolerance can be present from birth, develop with increasing age, or arise secondary to GI infection or other stress that affects the brush border lining of the intestine.265
Fructose, a sugar naturally found in fruits, can also contribute to IBS-type symptoms if poorly absorbed. In one study of 209 individuals with IBS symptoms, of the 80 individuals diagnosed with IBS by the Rome II criteria, 31 were found to have fructose intolerance by a positive fructose breath test.266 High-fructose foods include fruit juices, apples, grapes, watermelon, and honey. Processed foods sometimes contain added fructose; this added fructose may be listed on labels under various names, including high-fructose corn syrup, agave syrup, or simply “fructose.”
Sugar alcohols are also a common trigger for IBS symptoms. These include xylitol, sorbitol, mannitol, and erythritol. Sugar alcohols have a sweet taste and a lower caloric value than sugar, so they are commonly added to foods intended to be lower calorie or to support healthy blood sugar. At high doses, sugar alcohols can lead to IBS-type symptoms in both individuals with IBS and healthy subjects.267
Post-infectious IBS
It is not uncommon for IBS to arise following a GI infection. This is called post-infectious IBS, or PI-IBS, and occurs in up to about 30% of individuals who contract an acute GI infection.268,269 PI-IBS is not limited to bacterial and parasitic infections, but also can be secondary to viral gastroenteritis.270 Symptoms of PI-IBS typically resemble IBS-D and can be present for several months after the infection.269,270
Irritable bowel symptoms are thought to arise following enteric infection due to inflammatory damage to the gut epithelium, which increases intestinal permeability and alters levels and activity of digestive enzymes found in the brush border lining. Additional factors that may contribute to development of PI-IBS include alterations in intestinal flora, bile malabsorption, and altered levels of immune responders that influence serotonin levels.268,270-274 Some estimates suggest as many as one-third of all IBS cases may arise post-infection.275
Sex Hormones
As women are twice as likely as men to be affected by IBS, numerous studies have looked at the role sex hormones may play in the condition.276 In general, intestinal transit times may be slower in women, even more so during the luteal phase (after ovulation) of the menstrual cycle, and women are more likely to experience constipation-predominant IBS.3,277 Women also often experience worsening of IBS symptoms around menstruation, which coincides with natural changes in sex hormone levels.278 Evidence regarding the effect of menopause and associated changes in hormone levels on IBS frequency and severity is mixed.279-282
There are several possible mechanisms by which hormonal variations could influence IBS susceptibility or severity. For instance, differing expression of estrogen receptors in the GI tract among women compared with men may be a factor, as estrogen may relax gastric muscle cells.283 Also, testosterone may have antinociceptive effects and may influence pain and temperature sensation in the GI tract. Sex steroids influence the GI microbiome, which may play a role as well.284
Studies assessing the effects of hormone replacement therapy (HRT) in women with IBS are generally lacking. However, some observational evidence suggests HRT may be associated with increased IBS risk.285 More studies are needed to clarify the potential role of sex hormones in IBS and assess the influence of HRT in this context.
Exercise
Although physical activity is often prescribed as a lifestyle intervention for treating IBS, intense exercise can be a factor contributing to it. In endurance athletes, IBS often goes undiagnosed, and symptoms are worse during training and competition.8 Factors that can contribute to IBS in athletes are increased intestinal permeability due to heat and/or intense exercise, acute psychological stress, and immune activation.104,286,287
Medications that may Contribute to IBS
Certain medications may contribute to the development of IBS. Proton-pump inhibitors (eg, omeprazole), which are used to treat heartburn, can alter intestinal barrier function and affect the intestinal microbiota; and these drugs are known to have a positive association with IBS.288 Similarly, many common analgesics, such as non-steroidal anti-inflammatory drugs, can damage the intestinal epithelium, an important barrier against harmful substances. This tissue damage may compromise intestinal permeability.289,290 Although broad-spectrum antibiotics are designed to target systemic infections, antibiotics are known to alter the colonic microbiota. Indeed, a retrospective study showed that the use of broad-spectrum antibiotics, particularly macrolides (eg, erythromycin) or tetracyclines (eg, tetracycline, doxycycline), was associated with IBS development.291
Although medications that interact with serotonin receptors are at times used for the treatment of IBS, they also can contribute to it. For example, tricyclic antidepressant drugs like amitriptyline inhibit peristalsis and may worsen IBS-C.292 Antidepressant medications also can contribute to diarrhea.293 Opiate pain medications are also well-known for their constipating effects.294
7 Symptoms & Diagnosis of Irritable Bowel Syndrome (IBS)
The cardinal symptoms of IBS are abdominal pain (typically related to defecation) and altered bowel habits.295,296 Pain or discomfort associated with IBS typically “flares” for several days intermittently.
Other symptoms not directly associated with the GI tract have been reported in some IBS patients, including headache, backache, anxiety or depression, and lethargy.297,298
Diverticulitis (inflammation/infection of small pouches in the digestive tract) is more common with IBS and can be problematic if not appropriately treated; however, IBS does not increase risk for serious conditions like colon cancer nor is it associated with increased mortality.186,299,300
Diagnosing IBS is complex and often involves multiple tests to rule out several other diseases that may be associated with IBS-like symptoms, such as30,160,301-303:
- hyper- or hypothyroidism
- celiac disease
- lactose or fructose malabsorption
- IBD
- giardia or other enteric infection
- microscopic colitis
- diverticulitis
- colon cancer and/or pancreatic cancer
A complete blood count and blood chemistry panel may be ordered as well to assess for anemia or other abnormalities.
The specific criteria used to diagnose IBS have changed over the years and currently the Rome IV criteria is most widely used. The Rome IV criteria are more restrictive than previous versions, so not as many people meet the diagnostic criteria for IBS as used to be the case.1 The Rome IV criteria does not include abdominal discomfort and requires a higher frequency of abdominal pain (at least one day per week vs. three days per month) than Rome III for diagnosis. Although bloating is also a very common symptom, it is not required for diagnosis.30
According to the Rome IV criteria, a diagnosis of IBS requires recurrent abdominal pain, (on average, at least one day per week during the past three months) with at least two of the following characteristics:
- related to defecation
- associated with a change in stool frequency
- associated with a change in stool appearance
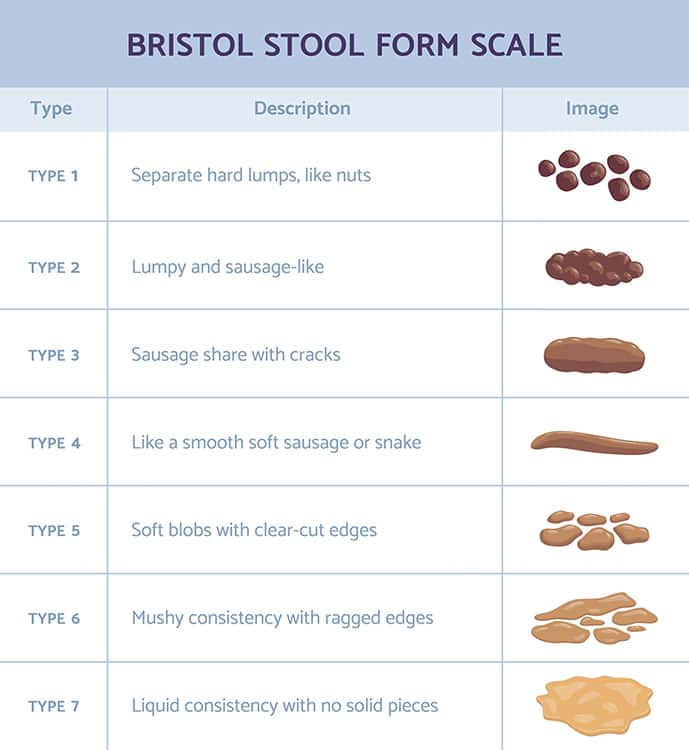
As different treatments may be indicated for those with constipation-predominant IBS (IBS-C) versus diarrhea-predominant IBS (IBS-D), knowledge of which subtype a patient experiences can be useful. By the Rome IV criteria, IBS is considered IBS-C if more than 25% of bowel movements are classified as Bristol Stool Scale Types 1-2 and less than 25% are Types 6-7. The subtype IBS-D is the opposite of this with more than 25% of bowel movements classified as Bristol Stool Scale Types 6-7 and less than 25% being Types 1-2. In Rome IV classification, these criteria are only applied during days with abnormal bowel movements (ie, during flare-ups).304
8 Treatment of Irritable Bowel Syndrome (IBS)
IBS treatment aims to alleviate predominant symptoms, such as diarrhea, constipation, or abdominal cramping.5 Dietary and lifestyle strategies, discussed at length in their respective sections of this protocol, are usually suggested as first-line interventions in the conventional treatment of IBS, particularly in those with mild or intermittent symptoms.305 The therapeutic options discussed in the remainder of this section may be implemented in addition to dietary and lifestyle changes based upon the characteristics of each case.
Laxatives
Laxatives that draw water into the colon are often used by clinicians to treat constipation-predominant IBS (IBS-C).5,306 These treatments typically provide rapid relief, but are not recommended for long-term use as they can cause electrolyte imbalances by enhancing the excretion of fluids.307 The most common laxatives work by osmosis, that is, they draw fluid into the intestine producing softer stools that are easier to pass.
Polyethylene glycol. Polyethylene glycol (PEG, commonly prescribed as MiraLAX) is one of the most studied laxatives; it has a better side effect profile compared with lactulose (another osmotic laxative).308 However, possible side effects of PEG are bloating and abdominal pain, and dosing should be gradually increased to help lessen their likelihood. Treatment of patients with IBS-C with PEG has been shown to significantly improve spontaneous bowel movements, stool consistency, and severity of straining within 28 days compared with placebo.309
Lubiprostone. Lubiprostone (Amitiza) is a prostaglandin E1 analog that stimulates fluid secretion into the intestine by directly and selectively acting on the chloride receptor ClC-2.310 Lubiprostone is indicated for IBS-C in the United States.306,311 Lubiprostone has been shown to act quickly to facilitate defecation, relieve discomfort, and resolve abdominal pain.310,312 A meta-analysis of studies including 1,366 patients with IBS-C found that lubiprostone improved spontaneous bowel movements at all time points assessed between one week and three months, also significantly improving abdominal pain especially after one month of treatment initiation.313
Linaclotide. Linaclotide (Linzess) activates a receptor in cells on the intestinal surface called the guanylate cyclase 2C receptor, which stimulates intestinal fluid secretion and softens the stool making it easier to pass. Linaclotide is effective in attenuating IBS-C, chronic constipation, and abdominal discomfort. Linaclotide was approved by the Food and Drug Administration (FDA) in 2012 for the treatment of IBS-C; however, as long-term risks are unknown, it is limited to use in patients unresponsive to PEG. In two large randomized clinical trials, linaclotide safely and effectively treated bowel and abdominal symptoms associated with chronic constipation.314 In patients with IBS-C, linaclotide was shown to significantly improve IBS-C including symptoms of abdominal pain, complete and spontaneous bowel movements, bloating, straining, and stool consistency. Diarrhea was the most common adverse effect, leading to study discontinuation by 5.7% of participants.315
Tegaserod. Tegaserod (Zelnorm, Zelmac) is a 5-hydroxytryptamine-4 (5-HT4) partial agonist that has been studied and shown to accelerate small bowel transit time and colonic emptying. 5-HT4 receptor agonists stimulate colonic motility by increasing peristalsis and the release of neurotransmitters, which also can modulate visceral hypersensitivity.316 In patients with IBS-C, tegaserod taken twice daily was shown to significantly improve abdominal discomfort, bloating, and constipation compared with placebo.317,318 Common adverse events are diarrhea, headache, abdominal pain, and flatulence.319
Anti-diarrheal Medications
There are several different medications used to mitigate the diarrhea of IBS. These medications rely on a variety of mechanisms to decrease peristalsis and prolong transit time, including interacting with opioid receptors, acting as a bile acid sequestrant, and antagonizing serotonin receptors.
Eluxadoline. Eluxadoline (Viberzi) is a mixed opioid receptor agonist and antagonist approved for the treatment of diarrhea-predominant IBS (IBS-D), although it is contraindicated in individuals who do not have a gallbladder and have a history of biliary disorders, pancreatitis, or alcohol use disorder, among other things. A review of three clinical trials, including 3,235 patients with IBS-D, found eluxadoline was significantly superior to placebo for the treatment of IBS-D and significantly improved stool consistency.29,30
Loperamide. Loperamide (Imodium, among others) is another antidiarrheal agent that interacts with opioid receptors to inhibit peristalsis and increase transit time. It also has antisecretory activity and reduces fluid and electrolyte loss.320,321 Loperamide has been shown to be effective for reducing stool frequency and improving consistency; however, the results are mixed with regard to its impact on pain.322,323 In two ACG position papers (2018 and 2021) on the management of IBS, loperamide is not recommended as a first-line therapy due to the limited trials and small number of patients included, as well as its inability to improve global IBS symptoms.29,30
Bile acid sequestrants. Bile acid sequestrants (eg, cholestyramine [Prevalite], colestipol [Colestid]) also can be effective for the treatment of diarrhea. It has been shown that a substantial percentage of individuals with IBS-D have bile acid malabsorption which can be the cause of functional diarrhea.305 In a randomized controlled trial of 24 patients with IBS-D, the bile acid sequestrant colesevelam (Welchol) significantly increased ascending colon emptying time by four hours compared with placebo; however, overall colon transit time was not significantly increased. Treatment with colesevelam significantly improved ease of stool passage and was associated with a somewhat firmer stool consistency.324 In an eight-week, randomized, open-label, controlled trial of patients with varied subtypes of IBS, treatment with colestipol significantly improved IBS symptoms.325
Alosetron. Alosetron (Lotronex), an antagonist of the 5-HT3 serotonin receptor, is used in females to treat severe IBS-D that is unresponsive to other treatments.5,186,326 By blocking the 5-HT3 receptor, alosetron modulates visceral sensation and reduces colonic motility.327 A meta-analysis of 14 randomized controlled trials found that alosetron or cilansetron, another 5-HT3 antagonist, were more effective than placebo or mebeverine (an anti-spasmodic agent) for improving abdominal pain and discomfort and IBS symptoms overall in patients with non-constipated IBS or IBS-D.328 In one randomized clinical trial in women with severe IBS-D, alosetron was found to have significant beneficial effects versus placebo on numerous markers of quality of life (eg, emotional and mental health, sleep, energy, etc.) as well as workplace productivity.326 However, alosetron may cause significant side effects including severe constipation and loss of blood flow to the colon, although the latter is fairly uncommon.328-330
Medications for Abdominal Pain & Bloating
Antispasmodic medications. Antispasmodic medications can help reduce the pain and discomfort associated with IBS and may be used on an as-needed basis, but generally are not taken long-term. Antispasmodics relax the intestinal smooth muscle of the lower GI tract and thus may reduce not only pain but also fecal urgency.331 There are several different commonly used medications that fall into this category. The 2018 ACG position paper on the management of IBS recommends the use of antispasmodics for overall symptom management, finding from the review of 26 randomized controlled trials that the use of antispasmodics (including 13 different medications) significantly improved IBS symptoms with the number needed to treat being 5. Of the different antispasmodic medications, otilonium bromide (Spasmomen, among others) and pinaverium bromide were the most studied with both being shown to be effective for treatment of IBS with a low number needed to treat of 5 and 4, respectively. While the other antispasmodic medications were less studied, all were found to be more effective than placebo with a low number needed to treat.29 However, in their 2021 guidelines, the ACG recommends against antispasmodics available in the United States, namely dicyclomine (Bentyl), hyoscyamine (Levsin, among others), and hyoscine (Scopolamine), due to lack of evidence supporting their efficacy.30
Antidepressants. Although best known for the treatment of mood disorders, antidepressants are another class of therapeutic agents that can be used in IBS treatment.306 As previously described, serotonergic signaling can affect not only motility but also visceral hypersensitivity. Tricyclic antidepressants (TCAs) have central analgesic effects as well as peripheral anticholinergic effects which also may slow transit time; thus, they may not be an appropriate choice for constipation.332 In addition to TCAs, selective serotonin reuptake inhibitors (SSRIs) are the second-most-studied category of antidepressants used in the treatment of IBS. The 2018 and 2021 ACG position papers recommend TCA use for overall symptom improvement, while SSRIs are only weakly suggested in the 2018 guidelines, based on the low quality of evidence for their use.29,30
While a 2015 meta-analysis of 12 randomized controlled trials found treatment with antidepressants improved global IBS symptoms, a subgroup analysis found only TCAs, not SSRIs, were effective.333 However, in the analysis of seven randomized controlled trials discussed in the 2018 ACG position paper it was found that SSRIs were effective for reducing IBS symptoms although there was significant differences in study design in the trials assessed.29 The ACG noted that due to the high rate of mood disorders in this population, providers still may elect to use these medications. There is a fairly high rate of adverse effects associated with antidepressant medications, particularly TCAs, which includes drowsiness and dry mouth. That said, lower doses of these medications are typically used to treat IBS than are used in mood disorders, which may lessen these effects.29,334
Antibiotics. Although not broadly applied as a general treatment for IBS, the non-absorbable antibiotic rifaximin (Xifaxan) has become recognized in recent years as a treatment for non-constipated IBS patients—particularly when bloating is a symptom.29 Rifaximin has been substantially studied and shown to be effective as a treatment for SIBO, which has been shown to be present in a high percentage of patients with IBS.245,335 A meta-analysis of five trials in which rifaximin was used for the treatment of IBS found that, compared with placebo, it was effective for reducing global symptoms of IBS, and significantly improved symptoms of bloating. However, the number needed to treat was fairly high at approximately 10. Adverse events (including diarrhea) and discontinuation rates were similar between the treatment and placebo groups, and there were no reports of C. difficile -associated diarrhea.336 The 2021 ACG position paper recommends rifaximin use in IBS-D due to its improvement of abdominal pain and stool consistency and reduction of global IBS symptoms.30
9 Novel & Emerging Therapies
Evidence points to low-level inflammation and immune system activation as possible factors in IBS pathology.210,253,254,337,338 Thus, medications directed at addressing these and related factors have been investigated as possible treatments for IBS.
Mesalazine
The aspirin-like anti-inflammatory drug mesalazine (also known as mesalamine and 5-aminosalicylic acid [5-ASA]), which is used in the treatment of IBD,339 has successfully relieved IBS symptoms in clinical trials.340 In a trial of 360 subjects with varying types of IBS who were treated with 500 mg mesalazine four times daily or standard therapy for 28 days, mesalazine led to significant reductions in pain and symptom duration in most IBS subtypes. In addition, the treatment normalized stool patterns among subjects with IBS-D and lessened infiltration of immune cells into bowel mucosa.341 In a placebo-controlled study of 148 patients with diarrhea-predominant IBS (IBS-D), treatment with 1,500 mg mesalazine daily was associated with a significantly greater portion of responders (defined as patients having improvements in both abdominal pain and stool consistency for ≥2 months) at three months.342 However, in a 2016 study where the dosing was 2,000 mg twice daily, no benefit was seen for improving abdominal pain or stool consistency in patients with IBS-D.343 In the 2018 ACG position paper, the use of 5-ASAs, based on low quality of evidence, was weakly recommended against for overall symptom management in IBS; however, they also suggested these medications should be further studied based on existing clinical findings.29
Mast Cell Stabilizers
Research suggests increased levels of mast cells and their activation and release of intracellular molecules (including histamine, tryptase and other proteases, serotonin, nitric oxide, and numerous cytokines and chemokines) may play a role in the pathogenesis of IBS.344 Thus, medications known as mast cell stabilizers have been investigated as a treatment for IBS in preclinical and clinical research with positive findings. Due to the various compounds they release when activated, mast cells are thought to be a factor in diarrhea and increased visceral sensitivity.345 One clinical trial assessed this specifically, finding that treatment with ketotifen, a mast cell inhibitor and antihistamine, for eight weeks increased the threshold for discomfort in IBS patients with increased visceral sensitivity. Ketotifen also significantly decreased abdominal pain and other IBS symptoms and improved quality of life scores.346
Multiple clinical studies with different cromoglycates (eg, disodium cromoglycate, cromolyn) have also shown improvements in IBS symptoms, mostly in patients with IBS-D. Findings of interest, in addition to improvement in overall symptoms, stool consistency, and abdominal pain, were that these mast cell stabilizers may decrease intestinal permeability and mucosal tryptase release, as well as alter the expression of genes associated with immune response and mucosal barrier function.347 However, most of the clinical studies were small and uncontrolled. In one large, unblinded, uncontrolled study of patients with IBS-D, improvement in symptoms with cromolyn were similar to that of individuals following an elimination diet (67% vs. 60% improvement, respectively). It was noted that response rates in both groups were greater in those having positive skin tests for dietary antigens.348
Numerous nutritional interventions, including essential fatty acids, vitamin D, carotenoids (eg, astaxanthin), palmitoylethanolamide (PEA), quercetin, and curcumin also help reduce mast cell activity and thus may be helpful in certain individuals with IBS.349
Serine Protease Inhibitors
Proteases (enzymes that break peptide bonds in proteins) are released by mast cells as well as other lymphocytes, intestinal cells, and the gut microbiota. The majority of these proteases are classified as serine proteases.350 The gut microbiota also produces protease inhibitors, exerting a regulatory effect on protease activity in the gut.351 High levels of serine protease activity have been seen in fecal samples of IBD patients as well as individuals with IBS.256,352-354 In mice, application of fecal supernatants from IBS-D patients increased perception of pain and colonic paracellular permeability, both of which were prevented by serine protease inhibitors.256 High fecal protease activity in IBS patients has been shown to be associated with symptom severity, higher intestinal permeability, and reduced microbial diversity.353 Additional animal models have also shown serine protease inhibitors have promise for improving this potential factor that may contribute to the development of IBS as well as its symptoms.355,356
Transcutaneous Vagal Nerve Stimulation
Transcutaneous vagal nerve stimulation (tVNS) is a novel non-invasive technique by which the FDA-approved practice of vagus nerve stimulation is being applied to a variety of common conditions. Studies have been done showing its efficacy in conditions including migraines, symptoms of lupus, post-stroke motor recovery, and atrial fibrillation.357-360 Its origins lie in the medical practice of invasive vagal nerve stimulation which is used in managing severe, treatment-resistant depression and epilepsy.361 Human studies have also shown it has potential to intersect with the stress axis and thinking patterns, both of which may impact symptoms of IBS.362-364
One small, open-label, pilot study looked at the impact of tVNS on IBS. In women with IBS (nine who completed the trial), the application of tVNS was shown to significantly improve symptoms of IBS at three and six months, although there were no changes in measured variables (transit time, blood cytokines, and fecal calprotectin).365 Specific to GI function, tVNS also has been shown in humans to induce gastric slowing by reducing myoelectrical signaling, reduce the development of and reverse established acid-induced esophageal hypersensitivity, improve gastrointestinal symptoms in Parkinson’s disease, and improve biomarkers related to postoperative ileus.366-369
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. Oct 2020;5(10):908-917. doi:10.1016/S2468-1253(20)30217-X
- Aziz I, Palsson OS, Tornblom H, Sperber AD, Whitehead WE, Simren M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study. Lancet Gastroenterol Hepatol. Apr 2018;3(4):252-262. doi:10.1016/S2468-1253(18)30003-7
- Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. The American journal of gastroenterology. Jul 2012;107(7):991-1000. doi:10.1038/ajg.2012.131
- Wald A. Clinical manifestations and diagnosis of irritable bowel syndrome in adults. UpToDate. Updated 2/24/2021. Accessed 8/18/2021, https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-irritable-bowel-syndrome-in-adults?source=history_widget
- Mayer EA. Clinical practice. Irritable bowel syndrome. The New England journal of medicine. Apr 17 2008;358(16):1692-9. doi:10.1056/NEJMcp0801447
- Okobi OE, Udoete IO, Fasehun OO, et al. A Review of Four Practice Guidelines of Inflammatory Bowel Disease. Cureus. Aug 2021;13(8):e16859. doi:10.7759/cureus.16859
- Grad S, Dumitrascu DL. Irritable Bowel Syndrome Subtypes: New Names for Old Medical Conditions. Digestive diseases (Basel, Switzerland). 2020;38(2):122-127. doi:10.1159/000505287
- Killian LA, Lee SY. Irritable bowel syndrome is underdiagnosed and ineffectively managed among endurance athletes. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. Dec 2019;44(12):1329-1338. doi:10.1139/apnm-2019-0261
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain research. Mar 2 2001;893(1-2):135-42. doi:10.1016/s0006-8993(00)03305-9
- Reddymasu SC, Sostarich S, McCallum RW. Small intestinal bacterial overgrowth in irritable bowel syndrome: are there any predictors? BMC gastroenterology. Feb 22 2010;10:23. doi:10.1186/1471-230x-10-23
- Yakoob J, Abbas Z, Khan R, Hamid S, Awan S, Jafri W. Small intestinal bacterial overgrowth and lactose intolerance contribute to irritable bowel syndrome symptomatology in Pakistan. Saudi journal of gastroenterology : official journal of the Saudi Gastroenterology Association. Nov-Dec 2011;17(6):371-375. doi:10.4103/1319-3767.87176
- Sachdeva S, Rawat AK, Reddy RS, Puri AS. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. Journal of gastroenterology and hepatology. Apr 2011;26 Suppl 3:135-8. doi:10.1111/j.1440-1746.2011.06654.x
- Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. Oct 2004;53(10):1459-64. doi:10.1136/gut.2003.037697
- Lydiard RB, Falsetti SA. Experience with anxiety and depression treatment studies: implications for designing irritable bowel syndrome clinical trials. Am J Med. Nov 8 1999;107(5a):65s-73s. doi:10.1016/s0002-9343(99)00082-0
- Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? The Journal of clinical psychiatry. 2001;62 Suppl 8:38-45; discussion 46-7.
- Asahina S, Hasegawa K, Tsuboi K. [Depression in patients of irritable bowel syndrome]. Nihon rinsho Japanese journal of clinical medicine. Aug 2006;64(8):1527-31.
- Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. Mar 2011;140(3):761-5. doi:10.1053/j.gastro.2011.01.032
- Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. American journal of physiology Gastrointestinal and liver physiology. Apr 2001;280(4):G519-24. doi:10.1152/ajpgi.2001.280.4.G519
- Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. Mar 2009;58(3):367-78. doi:10.1136/gut.2008.163162
- Zijdenbos IL, de Wit NJ, van der Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. The Cochrane database of systematic reviews. Jan 21 2009;(1):Cd006442. doi:10.1002/14651858.CD006442.pub2
- Hayee B, Forgacs I. Psychological approach to managing irritable bowel syndrome. BMJ (Clinical research ed). May 26 2007;334(7603):1105-9. doi:10.1136/bmj.39199.679236.AE
- Bohmer CJ, Tuynman HA. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: a 5-year follow-up study. European journal of gastroenterology & hepatology. Aug 2001;13(8):941-4. doi:10.1097/00042737-200108000-00011
- Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. The American journal of gastroenterology. Dec 2016;111(12):1824-1832. doi:10.1038/ajg.2016.434
- Skodje GI, Sarna VK, Minelle IH, et al. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology. Feb 2018;154(3):529-539 e2. doi:10.1053/j.gastro.2017.10.040
- Harvie RM, Chisholm AW, Bisanz JE, et al. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J Gastroenterol. Jul 7 2017;23(25):4632-4643. doi:10.3748/wjg.v23.i25.4632
- myupchar.com. Depiction of a person suffering from Irritable Bowel Syndrome (IBS). Wikimedia Commons. Accessed 08/25/2021, https://commons.wikimedia.org/wiki/File:Depiction_of_a_person_suffering_from_Irritable_Bowel_Syndrome_(IBS).png
- Grassi M, Petraccia L, Mennuni G, et al. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutr Hosp. Jul-Aug 2011;26(4):659-68. doi:10.1590/s0212-16112011000400001
- Ciorba MA. A gastroenterologist's guide to probiotics. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Sep 2012;10(9):960-8. doi:10.1016/j.cgh.2012.03.024
- Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. The American journal of gastroenterology. Jun 2018;113(Suppl 2):1-18. doi:10.1038/s41395-018-0084-x
- Lacy BE, Pimentel M, Brenner DM, et al. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. The American journal of gastroenterology. Jan 1 2021;116(1):17-44. doi:10.14309/ajg.0000000000001036
- Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Alimentary pharmacology & therapeutics. Apr 2012;35(7):828-38. doi:10.1111/j.1365-2036.2012.05007.x
- Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Jun 2012;24(6):513-20, e246-7. doi:10.1111/j.1365-2982.2012.01893.x
- Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. Jul 1982;5(3):185-94.
- Wong CB, Odamaki T, Xiao J-z. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. Journal of functional foods. 2019;54:506-519.
- Ibarra A, Latreille-Barbier M, Donazzolo Y, Pelletier X, Ouwehand AC. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes. 2018;9(3):236-251. doi:10.1080/19490976.2017.1412908
- Waller PA, Gopal PK, Leyer GJ, et al. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scandinavian journal of gastroenterology. Sep 2011;46(9):1057-64. doi:10.3109/00365521.2011.584895
- Airaksinen K, Yeung N, Lyra A, et al. The effect of a probiotic blend on gastrointestinal symptoms in constipated patients: a double blind, randomised, placebo controlled 2-week trial. Benef Microbes. Jul 10 2019;10(6):617-627. doi:10.3920/BM2018.0163
- Magro DO, de Oliveira LM, Bernasconi I, et al. Effect of yogurt containing polydextrose, Lactobacillus acidophilus NCFM and Bifidobacterium lactis HN019: a randomized, double-blind, controlled study in chronic constipation. Nutr J. Jul 24 2014;13:75. doi:10.1186/1475-2891-13-75
- Silvi S, Verdenelli MC, Cecchini C, et al. Probiotic-enriched foods and dietary supplement containing SYNBIO positively affects bowel habits in healthy adults: an assessment using standard statistical analysis and Support Vector Machines. International journal of food sciences and nutrition. Dec 2014;65(8):994-1002. doi:10.3109/09637486.2014.940284
- Verdenelli MC, Silvi S, Cecchini C, Orpianesi C, Cresci A. Influence of a combination of two potential probiotic strains, Lactobacillus rhamnosus IMC 501(R) and Lactobacillus paracasei IMC 502(R) on bowel habits of healthy adults. Lett Appl Microbiol. Jun 2011;52(6):596-602. doi:10.1111/j.1472-765X.2011.03042.x
- Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. The American journal of gastroenterology. May 2000;95(5):1231-8. doi:10.1111/j.1572-0241.2000.02015.x
- Majeed M, Majeed S, Arumugam S, Ali F, Beede K. Comparative evaluation for thermostability and gastrointestinal survival of probiotic Bacillus coagulans MTCC 5856. Bioscience, biotechnology, and biochemistry. Mar 24 2021;85(4):962-971. doi:10.1093/bbb/zbaa116
- Gupta AK, Maity C. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome: A prospective, interventional, randomized, double-blind, placebo-controlled clinical study [CONSORT Compliant]. Medicine. Jan 22 2021;100(3):e23641. doi:10.1097/MD.0000000000023641
- Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med. Mar 2009;121(2):119-24. doi:10.3810/pgm.2009.03.1984
- Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods and findings in experimental and clinical pharmacology. Dec 2009;31(10):655-9. doi:10.1358/mf.2009.31.10.1441078
- Majeed M, Nagabhushanam K, Natarajan S, et al. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: a double blind randomized placebo controlled pilot clinical study. Nutrition Journal. 2015;15(1):1-10.
- Majeed M, Nagabhushanam K, Arumugam S, Majeed S, Ali F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr Res. 2018;62doi:10.29219/fnr.v62.1218
- McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. May 14 2010;16(18):2202-22.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. Jun 22-29 1994;271(24):1913-8.
- Abbas Z, Yakoob J, Jafri W, et al. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: a randomized trial. European journal of gastroenterology & hepatology. Jun 2014;26(6):630-9. doi:10.1097/MEG.0000000000000094
- Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. Journal of clinical gastroenterology. Sep 2011;45(8):679-83. doi:10.1097/MCG.0b013e318204593e
- Terciolo C, Dapoigny M, Andre F. Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical disorders associated with intestinal barrier disruption. Clin Exp Gastroenterol. 2019;12:67-82. doi:10.2147/CEG.S181590
- Kirchhoff R, Beckers C, Kirchhoff GM, Trinczek-Gartner H, Petrowicz O, Reimann HJ. Increase in choleresis by means of artichoke extract. Phytomedicine. Sep 1994;1(2):107-15. doi:10.1016/S0944-7113(11)80027-9
- Saenz Rodriguez T, Garcia Gimenez D, de la Puerta Vazquez R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine. Dec 2002;9(8):687-93. doi:10.1078/094471102321621278
- Wegener T, Fintelmann V. [Pharmacological properties and therapeutic profile of artichoke (Cynara scolymus L.)]. Wien Med Wochenschr. 1999;149(8-10):241-7. Pharmakologische Eigenschaften und therapeutisches Profil der Artischocke (Cynara scolymus L.).
- Walker AF, Middleton RW, Petrowicz O. Artichoke leaf extract reduces symptoms of irritable bowel syndrome in a post-marketing surveillance study. Phytother Res. Feb 2001;15(1):58-61.
- Holtmann G, Liebregts T, Collet W, Windeck T. Functional dyspepsia and irritable bowel syndrome—Treatment effects of artichoke-leaf-extract: A placebo-controlled, randomised, multicenter trial. Gastroenterology. 04/01 2003;124doi:10.1016/S0016-5085(03)80907-1
- Bundy R, Walker AF, Middleton RW, Marakis G, Booth JC. Artichoke leaf extract reduces symptoms of irritable bowel syndrome and improves quality of life in otherwise healthy volunteers suffering from concomitant dyspepsia: a subset analysis. Journal of alternative and complementary medicine (New York, NY). Aug 2004;10(4):667-9. doi:10.1089/acm.2004.10.667
- Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. British journal of anaesthesia. Mar 2000;84(3):367-71. doi:10.1093/oxfordjournals.bja.a013442
- Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. Mar 27 2012;9(5):271-85. doi:10.1038/nrgastro.2012.57
- Micklefield GH, Redeker Y, Meister V, Jung O, Greving I, May B. Effects of ginger on gastroduodenal motility. International journal of clinical pharmacology and therapeutics. Jul 1999;37(7):341-6.
- Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. Journal of ethnopharmacology. Feb 3 2010;127(2):515-20. doi:10.1016/j.jep.2009.10.004
- Giacosa A, Guido D, Grassi M, et al. The Effect of Ginger (Zingiber officinalis) and Artichoke (Cynara cardunculus) Extract Supplementation on Functional Dyspepsia: A Randomised, Double-Blind, and Placebo-Controlled Clinical Trial. Evid Based Complement Alternat Med. 2015;2015:915087. doi:10.1155/2015/915087
- Lazzini S, Polinelli W, Riva A, Morazzoni P, Bombardelli E. The effect of ginger (Zingiber officinalis) and artichoke (Cynara cardunculus) extract supplementation on gastric motility: a pilot randomized study in healthy volunteers. European review for medical and pharmacological sciences. 2016;20(1):146-9.
- Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo-controlled randomized trial. Dig Liver Dis. Jun 2007;39(6):530-6. doi:10.1016/j.dld.2007.02.006
- Merat S, Khalili S, Mostajabi P, Ghorbani A, Ansari R, Malekzadeh R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig Dis Sci. May 2010;55(5):1385-90. doi:10.1007/s10620-009-0854-9
- Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. Journal of gastroenterology. Dec 1997;32(6):765-8. doi:10.1007/bf02936952
- Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC complementary and alternative medicine. 2019;19(1):21-21. doi:10.1186/s12906-018-2409-0
- May B, Kuntz HD, Kieser M, Kohler S. Efficacy of a fixed peppermint oil/caraway oil combination in non-ulcer dyspepsia. Arzneimittel-Forschung. Dec 1996;46(12):1149-53.
- May B, Köhler S, Schneider B. Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia. Alimentary pharmacology & therapeutics. 2000;14(12):1671-1677. doi: https://doi.org/10.1046/j.1365-2036.2000.00873.x
- Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. Sep 14 2011;17(34):3888-98. doi:10.3748/wjg.v17.i34.3888
- Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. Aug 2008;59 Suppl 2:33-51.
- Pal PK, Sarkar S, Chattopadhyay A, Tan DX, Bandyopadhyay D. Enterochromaffin cells as the source of melatonin: key findings and functional relevance in mammals. Melatonin Research. 2019;2(4):61-82.
- Park YS, Kim SH, Park JW, et al. Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation. Intestinal research. Jul 2020;18(3):325-336. doi:10.5217/ir.2019.00093
- Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. Oct 2005;54(10):1402-7. doi:10.1136/gut.2004.062034
- Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Alimentary pharmacology & therapeutics. Nov 15 2005;22(10):927-34. doi:10.1111/j.1365-2036.2005.02673.x
- Chojnacki C, Walecka-Kapica E, Lokiec K, et al. Influence of melatonin on symptoms of irritable bowel syndrome in postmenopausal women. Endokrynologia Polska. 2013;64(2):114-20.
- Saha L, Malhotra S, Rana S, Bhasin D, Pandhi P. A preliminary study of melatonin in irritable bowel syndrome. Journal of clinical gastroenterology. Jan 2007;41(1):29-32. doi:10.1097/MCG.0b013e31802df84c
- Khalighi Sikaroudi M, Mokhtare M, Janani L, et al. Vitamin D3 Supplementation in Diarrhea-Predominant Irritable Bowel Syndrome Patients: The Effects on Symptoms Improvement, Serum Corticotropin-Releasing Hormone, and Interleukin-6 - A Randomized Clinical Trial. Complement Med Res. 2020;27(5):302-309. Vitamin-D3-Supplementierung bei Patienten mit diarrhödominantem Reizdarmsyndrom: Auswirkungen auf die Symptomverbesserung und die Serumkonzentration von Corticotropin-Releasing Hormon und Interleukin-6 – Eine randomisierte klinische Studie. doi:10.1159/000506149
- Amani R, Abbasnezhad A, Hajiani E, Cheraghian B, Abdoli Z, Choghakhori R. Vitamin D3 Induced Decrease in IL-17 and Malondialdehyde, and Increase in IL-10 and Total Antioxidant Capacity Levels in Patients with Irritable Bowel Syndrome. Iran J Immunol. Sep 2018;15(3):186-196. doi:10.22034/iji.2018.39388
- Abbasnezhad A, Amani R, Hajiani E, Alavinejad P, Cheraghian B, Ghadiri A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: a randomized double-blind clinical trial. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Oct 2016;28(10):1533-44. doi:10.1111/nmo.12851
- El Amrousy D, Hassan S, El Ashry H, Yousef M, Hodeib H. Vitamin D supplementation in adolescents with irritable bowel syndrome: Is it useful? A randomized controlled trial. Saudi J Gastroenterol. Mar-Apr 2018;24(2):109-114. doi:10.4103/sjg.SJG_438_17
- Williams CE, Williams EA, Corfe BM. Vitamin D supplementation in people with IBS has no effect on symptom severity and quality of life: results of a randomised controlled trial. European journal of nutrition. Jul 30 2021;doi:10.1007/s00394-021-02633-w
- Barbalho SM, Goulart RA, Araújo AC, Guiguer É L, Bechara MD. Irritable bowel syndrome: a review of the general aspects and the potential role of vitamin D. Expert review of gastroenterology & hepatology. Apr 2019;13(4):345-359. doi:10.1080/17474124.2019.1570137
- Cernakova M, Kostalova D. Antimicrobial activity of berberine--a constituent of Mahonia aquifolium. Folia microbiologica. 2002;47(4):375-8. doi:10.1007/BF02818693
- Slobodnikova L, Kost'alova D, Labudova D, Kotulova D, Kettmann V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother Res. Aug 2004;18(8):674-6. doi:10.1002/ptr.1517
- Gu L, Li N, Gong J, Li Q, Zhu W, Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis. Jun 1 2011;203(11):1602-12. doi:10.1093/infdis/jir147
- Li N, Gu L, Qu L, et al. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. Apr 16 2010;40(1):1-8. doi:10.1016/j.ejps.2010.02.001
- Tang QL, Lai ML, Zhong YF, Wang AM, Su JK, Zhang MQ. Antinociceptive effect of berberine on visceral hypersensitivity in rats. World J Gastroenterol. Jul 28 2013;19(28):4582-9. doi:10.3748/wjg.v19.i28.4582
- Chen C, Tao C, Liu Z, et al. A Randomized Clinical Trial of Berberine Hydrochloride in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Phytother Res. Nov 2015;29(11):1822-7. doi:10.1002/ptr.5475
- Di Pierro F, Bertuccioli A, Giuberti R, Saponara M, Ivaldi L. Role of a berberine-based nutritional supplement in reducing diarrhea in subjects with functional gastrointestinal disorders. Minerva gastroenterologica e dietologica. Mar 2020;66(1):29-34. doi:10.23736/S1121-421X.19.02649-7
- Rabbani GH, Butler T, Knight J, Sanyal SC, Alam K. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. J Infect Dis. May 1987;155(5):979-84. doi:10.1093/infdis/155.5.979
- Yu M, Jin X, Liang C, et al. Berberine for diarrhea in children and adults: a systematic review and meta-analysis. Therapeutic advances in gastroenterology. 2020;13:1756284820961299. doi:10.1177/1756284820961299
- Kysenius K, Brunello CA, Huttunen HJ. Mitochondria and NMDA receptor-dependent toxicity of berberine sensitizes neurons to glutamate and rotenone injury. PLoS One. 2014;9(9):e107129. doi:10.1371/journal.pone.0107129
- Mikes V, Yaguzhinskij LS. Interaction of fluorescent berberine alkyl derivatives with respiratory chain of rat liver mitochondria. J Bioenerg Biomembr. Feb 1985;17(1):23-32. doi:10.1007/BF00744986
- Mikes V, Dadak V. Berberine derivatives as cationic fluorescent probes for the investigation of the energized state of mitochondria. Biochim Biophys Acta. May 27 1983;723(2):231-9. doi:10.1016/0005-2728(83)90122-6
- Watford M. Glutamine and glutamate: Nonessential or essential amino acids? Anim Nutr. Sep 2015;1(3):119-122. doi:10.1016/j.aninu.2015.08.008
- Buchman AL. Glutamine: commercially essential or conditionally essential? A critical appraisal of the human data. Am J Clin Nutr. Jul 2001;74(1):25-32. doi:10.1093/ajcn/74.1.25
- Rao R, Samak G. Role of Glutamine in Protection of Intestinal Epithelial Tight Junctions. J Epithel Biol Pharmacol. Jan 2012;5(Suppl 1-M7):47-54. doi:10.2174/1875044301205010047
- Li J, Langkamp-Henken B, Suzuki K, Stahlgren LH. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability. JPEN Journal of parenteral and enteral nutrition. Jul-Aug 1994;18(4):303-7. doi:10.1177/014860719401800404
- Ding LA, Li JS. Effects of glutamine on intestinal permeability and bacterial translocation in TPN-rats with endotoxemia. World J Gastroenterol. Jun 2003;9(6):1327-32. doi:10.3748/wjg.v9.i6.1327
- Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. Jun 2019;68(6):996-1002. doi:10.1136/gutjnl-2017-315136
- Benjamin J, Makharia G, Ahuja V, et al. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn's disease: a randomized controlled trial. Dig Dis Sci. Apr 2012;57(4):1000-12. doi:10.1007/s10620-011-1947-9
- Pires W, Veneroso CE, Wanner SP, et al. Association Between Exercise-Induced Hyperthermia and Intestinal Permeability: A Systematic Review. Sports medicine (Auckland, NZ). Jul 2017;47(7):1389-1403. doi:10.1007/s40279-016-0654-2
- de Oliveira EP, Burini RC. The impact of physical exercise on the gastrointestinal tract. Current opinion in clinical nutrition and metabolic care. Sep 2009;12(5):533-8. doi:10.1097/MCO.0b013e32832e6776
- Pugh JN, Sage S, Hutson M, et al. Glutamine supplementation reduces markers of intestinal permeability during running in the heat in a dose-dependent manner. European journal of applied physiology. Dec 2017;117(12):2569-2577. doi:10.1007/s00421-017-3744-4
- Zuhl M, Dokladny K, Mermier C, Schneider S, Salgado R, Moseley P. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell stress & chaperones. Jan 2015;20(1):85-93. doi:10.1007/s12192-014-0528-1
- Zuhl MN, Lanphere KR, Kravitz L, et al. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol (1985). Jan 15 2014;116(2):183-91. doi:10.1152/japplphysiol.00646.2013
- Nguyen L. Complementary and Alternative Medicine for the Management of Irritable Bowel Syndrome. Gastroenterol Hepatol (N Y). Sep 2018;14(9):536-538.
- Capurso G, Traini M, Piciucchi M, Signoretti M, Arcidiacono PG. Exocrine pancreatic insufficiency: prevalence, diagnosis, and management. Clin Exp Gastroenterol. 2019;12:129-139. doi:10.2147/CEG.S168266
- Struyvenberg MR, Martin CR, Freedman SD. Practical guide to exocrine pancreatic insufficiency - Breaking the myths. BMC Med. Feb 10 2017;15(1):29. doi:10.1186/s12916-017-0783-y
- Hardt PD, Hauenschild A, Nalop J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1,021 diabetic patients. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2003;3(5):395-402. doi:10.1159/000073655
- Leeds JS, Hopper AD, Hurlstone DP, et al. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Alimentary pharmacology & therapeutics. Feb 1 2007;25(3):265-71. doi:10.1111/j.1365-2036.2006.03206.x
- Pezzilli R, Caputo F, Testino G, et al. Alcohol-related chronic exocrine pancreatic insufficiency: diagnosis and therapeutic management. A proposal for treatment by the Italian Association for the Study of the Pancreas (AISP) and the Italian Society of Alcohology (SIA). Minerva Med. Oct 2019;110(5):425-438. doi:10.23736/S0026-4806.19.06043-9
- Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr Drug Metab. 2016;17(2):187-93. doi:10.2174/138920021702160114150137
- Ido H, Matsubara H, Kuroda M, et al. Combination of Gluten-Digesting Enzymes Improved Symptoms of Non-Celiac Gluten Sensitivity: A Randomized Single-blind, Placebo-controlled Crossover Study. Clin Transl Gastroenterol. Sep 19 2018;9(9):181. doi:10.1038/s41424-018-0052-1
- Mori H, Tack J, Suzuki H. Magnesium Oxide in Constipation. Nutrients. Jan 28 2021;13(2)doi:10.3390/nu13020421
- Mori S, Tomita T, Fujimura K, et al. A Randomized Double-blind Placebo-controlled Trial on the Effect of Magnesium Oxide in Patients With Chronic Constipation. J Neurogastroenterol Motil. Oct 30 2019;25(4):563-575. doi:10.5056/jnm18194
- Morishita D, Tomita T, Mori S, et al. Senna Versus Magnesium Oxide for the Treatment of Chronic Constipation: A Randomized, Placebo-Controlled Trial. The American journal of gastroenterology. Jan 1 2021;116(1):152-161. doi:10.14309/ajg.0000000000000942
- Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clinical journal of the American Society of Nephrology : CJASN. Jul 7 2015;10(7):1257-72. doi:10.2215/CJN.09750913
- Mori H, Suzuki H, Hirai Y, et al. Clinical features of hypermagnesemia in patients with functional constipation taking daily magnesium oxide. Journal of clinical biochemistry and nutrition. Jul 2019;65(1):76-81. doi:10.3164/jcbn.18-117
- Asif M. Phytochemical study of polyphenols in Perilla Frutescens as an antioxidant. Avicenna journal of phytomedicine. Fall 2012;2(4):169-178.
- Buchwald-Werner S, Fujii H, Reule C, Schoen C. Perilla extract improves gastrointestinal discomfort in a randomized placebo controlled double blind human pilot study. BMC Complement Altern Med. May 27 2014;14:173. doi:10.1186/1472-6882-14-173
- Koezuka Y, Honda G, Tabata M. An Intestinal Propulsion Promoting Substance from Perilla frutescens and Its Mechanism of Action. Planta Med. Dec 1985;51(6):480-2. doi:10.1055/s-2007-969568
- Verspohl EJ, Fujii H, Homma K, Buchwald-Werner S. Testing of Perilla frutescens extract and Vicenin 2 for their antispasmodic effect. Phytomedicine. Mar 15 2013;20(5):427-31. doi:10.1016/j.phymed.2012.12.018
- Chen CY, Leu YL, Fang Y, et al. Anti-inflammatory effects of Perilla frutescens in activated human neutrophils through two independent pathways: Src family kinases and Calcium. Sci Rep. Dec 14 2015;5:18204. doi:10.1038/srep18204
- Lee H-A, Han J-S. Anti-inflammatory Effect of Perilla frutescens (L.) Britton var. frutescens Extract in LPS-stimulated RAW 264.7 Macrophages. Preventive nutrition and food science. 2012;17(2):109-115. doi:10.3746/pnf.2012.17.2.109
- Oh HA, Park CS, Ahn HJ, Park YS, Kim HM. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Experimental biology and medicine (Maywood, NJ). Jan 2011;236(1):99-106. doi:10.1258/ebm.2010.010252
- Urushima H, Nishimura J, Mizushima T, Hayashi N, Maeda K, Ito T. Perilla frutescens extract ameliorates DSS-induced colitis by suppressing proinflammatory cytokines and inducing anti-inflammatory cytokines. American journal of physiology Gastrointestinal and liver physiology. Jan 1 2015;308(1):G32-41. doi:10.1152/ajpgi.00294.2014
- Moloney RD, Johnson AC, O'Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS neuroscience & therapeutics. Feb 2016;22(2):102-17. doi:10.1111/cns.12490
- Ji WW, Li RP, Li M, et al. Antidepressant-like effect of essential oil of Perilla frutescens in a chronic, unpredictable, mild stress-induced depression model mice. Chinese journal of natural medicines. Oct 2014;12(10):753-9. doi:10.1016/s1875-5364(14)60115-1
- Yi LT, Li J, Geng D, et al. Essential oil of Perilla frutescens-induced change in hippocampal expression of brain-derived neurotrophic factor in chronic unpredictable mild stress in mice. Journal of ethnopharmacology. May 2 2013;147(1):245-53. doi:10.1016/j.jep.2013.03.015
- Isbister GK, Kumar VV. Indications for single-dose activated charcoal administration in acute overdose. Current opinion in critical care. Aug 2011;17(4):351-7. doi:10.1097/MCC.0b013e328348bf59
- Jurgens G, Hoegberg LC, Graudal NA. The effect of activated charcoal on drug exposure in healthy volunteers: a meta-analysis. Clin Pharmacol Ther. May 2009;85(5):501-5. doi:10.1038/clpt.2008.278
- Pegues AS, Sofer SS, McCallum RE, Hinshaw LB. The removal of 14C labeled endotoxin by activated charcoal. Int J Artif Organs. May 1979;2(3):153-8.
- Kodama M, Hanasawa K, Tani T. Blood purification for critical care medicine: endotoxin adsorption. Ther Apher. Aug 1997;1(3):224-7. doi:10.1111/j.1744-9987.1997.tb00142.x
- Senderovich H, Vierhout MJ. Is there a role for charcoal in palliative diarrhea management? Current medical research and opinion. Jul 2018;34(7):1253-1259. doi:10.1080/03007995.2017.1416345
- Kurti F, Duni A, Plefka E, Çina T. Treatment of irritable bowel syndrome symptoms and the role of compounded charcoal-A multicentre study. Albanian Med J. 2014;4:66-72.
- Sergio GC, Felix GM, Luis JV. Activated charcoal to prevent irinotecan-induced diarrhea in children. Pediatr Blood Cancer. Jul 2008;51(1):49-52. doi:10.1002/pbc.21491
- Michael M, Brittain M, Nagai J, et al. Phase II study of activated charcoal to prevent irinotecan-induced diarrhea. J Clin Oncol. Nov 1 2004;22(21):4410-7. doi:10.1200/JCO.2004.11.125
- Farrell KS, Burkitt-Creedon JM, Osborne LG, Gibson EA, Massie AM. Gastrointestinal obstruction secondary to activated charcoal granule impaction in a dog. J Vet Emerg Crit Care (San Antonio). Jul 2020;30(4):461-466. doi:10.1111/vec.12980
- Chyka PA, Seger D, Krenzelok EP, et al. Position paper: Single-dose activated charcoal. Clinical toxicology (Philadelphia, Pa). 2005;43(2):61-87. doi:10.1081/clt-200051867
- Sato RL, Wong JJ, Sumida SM, Yamamoto LG. Adverse effects of superactivated charcoal administered to healthy volunteers. Hawaii Med J. Nov 2002;61(11):251-3.
- Watari I, Oka S, Tanaka S, et al. Effectiveness of polaprezinc for low-dose aspirin-induced small-bowel mucosal injuries as evaluated by capsule endoscopy: a pilot randomized controlled study. BMC gastroenterology. 2013;13:108. doi:10.1186/1471-230x-13-108
- Mahmood A, FitzGerald AJ, Marchbank T, et al. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. Feb 2007;56(2):168-75. doi:10.1136/gut.2006.099929
- Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Alimentary pharmacology & therapeutics. Aug 2014;40(3):288-97. doi:10.1111/apt.12829
- Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. Nov 2009;146(1-2):41-6. doi:10.1016/j.pain.2009.06.017
- Choi HS, Lim JY, Chun HJ, et al. The effect of polaprezinc on gastric mucosal protection in rats with ethanol-induced gastric mucosal damage: comparison study with rebamipide. Life Sci. Jul 30 2013;93(2-3):69-77. doi:10.1016/j.lfs.2013.05.019
- Davison G, Marchbank T, March DS, Thatcher R, Playford RJ. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am J Clin Nutr. Aug 2016;104(2):526-36. doi:10.3945/ajcn.116.134403
- Chen Q, Hou H, Wang S, Zhao X, Li B. Effects of early enteral nutrition supplemented with collagen peptides on post-burn inflammatory responses in a mouse model. Food Funct. May 24 2017;8(5):1933-1941. doi:10.1039/c7fo00181a
- Chen Q, Gao X, Zhang H, Li B, Yu G, Li B. Collagen peptides administration in early enteral nutrition intervention attenuates burn-induced intestinal barrier disruption: Effects on tight junction structure. Journal of Functional Foods. 2019;55:167-174.
- Song W, Chen Q, Wang Y, Han Y, Zhang H, Li B. Identification and Structure-Activity Relationship of Intestinal Epithelial Barrier Function Protective Collagen Peptides from Alaska Pollock Skin. Mar Drugs. Jul 31 2019;17(8)doi:10.3390/md17080450
- Chen Q, Chen O, Martins IM, et al. Collagen peptides ameliorate intestinal epithelial barrier dysfunction in immunostimulatory Caco-2 cell monolayers via enhancing tight junctions. Food Funct. Mar 22 2017;8(3):1144-1151. doi:10.1039/c6fo01347c
- Ng QX, Soh AYS, Loke W, Venkatanarayanan N, Lim DY, Yeo WS. A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS). J Clin Med. Sep 22 2018;7(10)doi:10.3390/jcm7100298
- Singh RH, Lallan S. Studies on the anti-anxiety effect of the medhya rasayana drug brahmi (Bacopa moniera Wettst), part 1. Journal of Research in Ayurveda and Siddha. 1981;1:138-148.
- Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. Aug 2013;16(4):313-26. doi:10.1089/rej.2013.1431
- Yadav SK, Jain AK, Tripathi SN, Gupta JP. Irritable bowel syndrome: therapeutic evaluation of indigenous drugs. The Indian journal of medical research. Dec 1989;90:496-503.
- Cozma-Petruţ A, Loghin F, Miere D, Dumitraşcu DL. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J Gastroenterol. Jun 7 2017;23(21):3771-3783. doi:10.3748/wjg.v23.i21.3771
- Torpy JM, Golub RM. JAMA patient page. Irritable bowel syndrome. Jama. Oct 5 2011;306(13):1501. doi:10.1001/jama.306.13.1501
- Wright-McNaughton M, Ten Bokkel Huinink S, Frampton CMA, et al. Measuring Diet Intake and Gastrointestinal Symptoms in Irritable Bowel Syndrome: Validation of the Food and Symptom Times Diary. Clin Transl Gastroenterol. Dec 2019;10(12):e00103. doi:10.14309/ctg.0000000000000103
- Rosa K, Delgado-Herrera L, Zeiher B, et al. Psychometric assessment of the IBS-D Daily Symptom Diary and Symptom Event Log. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. Dec 2016;25(12):3197-3208. doi:10.1007/s11136-016-1335-1
- Mari A, Hosadurg D, Martin L, Zarate-Lopez N, Passananti V, Emmanuel A. Adherence with a low-FODMAP diet in irritable bowel syndrome: are eating disorders the missing link? European journal of gastroenterology & hepatology. Feb 2019;31(2):178-182. doi:10.1097/MEG.0000000000001317
- Vandeputte D, Joossens M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms. Oct 23 2020;8(11)doi:10.3390/microorganisms8111638
- Dionne J, Ford AC, Yuan Y, et al. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. The American journal of gastroenterology. Sep 2018;113(9):1290-1300. doi:10.1038/s41395-018-0195-4
- Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. Oct 2011;24(5):487-95. doi:10.1111/j.1365-277X.2011.01162.x
- Volta U, De Giorgio R. New understanding of gluten sensitivity. Nat Rev Gastroenterol Hepatol. Feb 28 2012;9(5):295-9. doi:10.1038/nrgastro.2012.15
- Carroccio A, Mansueto P, Iacono G, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. The American journal of gastroenterology. Dec 2012;107(12):1898-906; quiz 1907. doi:10.1038/ajg.2012.236
- Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. The American journal of gastroenterology. Mar 2011;106(3):508-14; quiz 515. doi:10.1038/ajg.2010.487
- Bellini M, Tonarelli S, Mumolo MG, et al. Low Fermentable Oligo- Di- and Mono-Saccharides and Polyols (FODMAPs) or Gluten Free Diet: What Is Best for Irritable Bowel Syndrome? Nutrients. Nov 1 2020;12(11)doi:10.3390/nu12113368
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. Dec 2016;16(12):751-765. doi:10.1038/nri.2016.111
- Gocki J, Bartuzi Z. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy dermatologii i alergologii. Aug 2016;33(4):253-6. doi:10.5114/ada.2016.61600
- Karakula-Juchnowicz H, Szachta P, Opolska A, et al. The role of IgG hypersensitivity in the pathogenesis and therapy of depressive disorders. Nutritional neuroscience. Feb 2017;20(2):110-118. doi:10.1179/1476830514Y.0000000158
- Geiselman JF. The Clinical Use of IgG Food Sensitivity Testing with Migraine Headache Patients: a Literature Review. Curr Pain Headache Rep. Aug 27 2019;23(11):79. doi:10.1007/s11916-019-0819-4
- Drisko J, Bischoff B, Hall M, McCallum R. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J Am Coll Nutr. Dec 2006;25(6):514-22. doi:10.1080/07315724.2006.10719567
- Pinto-Sanchez MI, Nardelli A, Borojevic R, et al. Gluten-Free Diet Reduces Symptoms, Particularly Diarrhea, in Patients With Irritable Bowel Syndrome and Antigliadin IgG. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Aug 19 2020;doi:10.1016/j.cgh.2020.08.040
- Fritscher-Ravens A, Pflaum T, Mosinger M, et al. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated With Immunoglobulin E. Gastroenterology. Jul 2019;157(1):109-118 e5. doi:10.1053/j.gastro.2019.03.046
- Shanahan F, Whorwell PJ. IgG-mediated food intolerance in irritable bowel syndrome: a real phenomenon or an epiphenomenom? The American journal of gastroenterology. Jul 2005;100(7):1558-9. doi:10.1111/j.1572-0241.2005.50009.x
- Rao SS, Welcher K, Zimmerman B, Stumbo P. Is coffee a colonic stimulant? European journal of gastroenterology & hepatology. Feb 1998;10(2):113-8. doi:10.1097/00042737-199802000-00003
- Koochakpoor G, Salari-Moghaddam A, Keshteli AH, Esmaillzadeh A, Adibi P. Association of Coffee and Caffeine Intake With Irritable Bowel Syndrome in Adults. Frontiers in nutrition. 2021;8:632469. doi:10.3389/fnut.2021.632469
- Reding KW, Cain KC, Jarrett ME, Eugenio MD, Heitkemper MM. Relationship between patterns of alcohol consumption and gastrointestinal symptoms among patients with irritable bowel syndrome. The American journal of gastroenterology. Feb 2013;108(2):270-6. doi:10.1038/ajg.2012.414
- UW Integrative Medicine DoFM. An Integrative Approach for Treating Irritable Bowel Syndrome. University of Wisconsin School of Medicine and Public Health. https://www.fammed.wisc.edu/files/webfm-uploads/documents/outreach/im/module_IBS_patient.pdf
- Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Apr 2012;10(4):385-90.e1-3. doi:10.1016/j.cgh.2011.12.018
- Spiller R. Clinical update: irritable bowel syndrome. Lancet. May 12 2007;369(9573):1586-8. doi:10.1016/s0140-6736(07)60726-0
- Berman SM, Suyenobu BY, Naliboff BD, et al. Evidence for altered central noradrenergic modulation in irritable bowel syndrome (IBS). Gastroenterology. 2009;136(5):A170.
- Zernicke KA, Campbell TS, Blustein PK, et al. Mindfulness-based stress reduction for the treatment of irritable bowel syndrome symptoms: a randomized wait-list controlled trial. International journal of behavioral medicine. Sep 2013;20(3):385-96. doi:10.1007/s12529-012-9241-6
- Everitt HA, Landau S, O'Reilly G, et al. Assessing telephone-delivered cognitive-behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut. Sep 2019;68(9):1613-1623. doi:10.1136/gutjnl-2018-317805
- Lackner JM, Jaccard J, Keefer L, et al. Improvement in Gastrointestinal Symptoms After Cognitive Behavior Therapy for Refractory Irritable Bowel Syndrome. Gastroenterology. Jul 2018;155(1):47-57. doi:10.1053/j.gastro.2018.03.063
- Krouwel M, Jolly K, Greenfield S. How do people with refractory irritable bowel syndrome perceive hypnotherapy?: Qualitative study. Complementary therapies in medicine. Aug 2019;45:65-70. doi:10.1016/j.ctim.2019.05.020
- Hasan SS, Pearson JS, Morris J, Whorwell PJ. SKYPE HYPNOTHERAPY FOR IRRITABLE BOWEL SYNDROME: Effectiveness and Comparison with Face-to-Face Treatment. The International journal of clinical and experimental hypnosis. Jan-Mar 2019;67(1):69-80. doi:10.1080/00207144.2019.1553766
- Lindfors P, Unge P, Arvidsson P, et al. Effects of gut-directed hypnotherapy on IBS in different clinical settings-results from two randomized, controlled trials. The American journal of gastroenterology. Feb 2012;107(2):276-85. doi:10.1038/ajg.2011.340
- Flik CE, Laan W, Zuithoff NPA, et al. Efficacy of individual and group hypnotherapy in irritable bowel syndrome (IMAGINE): a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. Jan 2019;4(1):20-31. doi:10.1016/S2468-1253(18)30310-8
- El-Serag HB, Pilgrim P, Schoenfeld P. Systemic review: Natural history of irritable bowel syndrome. Alimentary pharmacology & therapeutics. Apr 15 2004;19(8):861-70. doi:10.1111/j.1365-2036.2004.01929.x
- Johannesson E, Simrén M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. The American journal of gastroenterology. May 2011;106(5):915-22. doi:10.1038/ajg.2010.480
- Fani M, Mostamand J, Fani M, Chitsaz N, Feizi A. The effect of aerobic exercises among women with mild and moderate irritable bowel syndrome: A pilot study. J Bodyw Mov Ther. Jan 2019;23(1):161-165. doi:10.1016/j.jbmt.2018.02.003
- Shahabi L, Naliboff BD, Shapiro D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: a pilot study. Psychol Health Med. 2016;21(2):176-88. doi:10.1080/13548506.2015.1051557
- Schumann D, Langhorst J, Dobos G, Cramer H. Randomised clinical trial: yoga vs a low-FODMAP diet in patients with irritable bowel syndrome. Alimentary pharmacology & therapeutics. Jan 2018;47(2):203-211. doi:10.1111/apt.14400
- Kavuri V, Selvan P, Malamud A, Raghuram N, Selvan SR. Remedial yoga module remarkably improves symptoms in irritable bowel syndrome patients: a 12-week randomized controlled trial. European journal of integrative medicine. 2015;7(6):595-608.
- Schumann D, Anheyer D, Lauche R, Dobos G, Langhorst J, Cramer H. Effect of Yoga in the Therapy of Irritable Bowel Syndrome: A Systematic Review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Dec 2016;14(12):1720-1731. doi:10.1016/j.cgh.2016.04.026
- Pei L, Geng H, Guo J, et al. Effect of Acupuncture in Patients With Irritable Bowel Syndrome: A Randomized Controlled Trial. Mayo Clin Proc. Aug 2020;95(8):1671-1683. doi:10.1016/j.mayocp.2020.01.042
- Guo J, Sun JH, Chen L, et al. [Correlation between curative effect and 5-HTTLPR polymorphism in treatment of diarrhea-predominant irritable bowel syndrome with acupuncture for regulating shen and strengthening spleen]. Zhongguo zhen jiu = Chinese acupuncture & moxibustion. Apr 12 2021;41(4):365-70. doi:10.13703/j.0255-2930.20200313-k0002
- Stuardi T, MacPherson H. Acupuncture for irritable bowel syndrome: diagnosis and treatment of patients in a pragmatic trial. Journal of alternative and complementary medicine (New York, NY). Nov 2012;18(11):1021-7. doi:10.1089/acm.2011.0670
- MacPherson H, Tilbrook H, Bland JM, et al. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC gastroenterology. Oct 24 2012;12:150. doi:10.1186/1471-230x-12-150
- Shi ZM, Zhu YS, Wang QX, Lei MN. [Comparative study on irritable bowel syndrome treated with acupuncture and western medicine]. Zhongguo zhen jiu = Chinese acupuncture & moxibustion. Jul 2011;31(7):607-9.
- Manheimer E, Wieland LS, Cheng K, et al. Acupuncture for irritable bowel syndrome: systematic review and meta-analysis. The American journal of gastroenterology. Jun 2012;107(6):835-47; quiz 848. doi:10.1038/ajg.2012.66
- Zhu L, Ma Y, Ye S, Shu Z. Acupuncture for Diarrhoea-Predominant Irritable Bowel Syndrome: A Network Meta-Analysis. Evidence-Based Complementary and Alternative Medicine. 2018/05/27 2018;2018:2890465. doi:10.1155/2018/2890465
- Lazaridis N, Germanidis G. Current insights into the innate immune system dysfunction in irritable bowel syndrome. Annals of Gastroenterology : Quarterly Publication of the Hellenic Society of Gastroenterology. Mar-Apr 2018;31(2):171-187. doi:10.20524/aog.2018.0229
- Major G, Pritchard S, Murray K, et al. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. Jan 2017;152(1):124-133.e2. doi:10.1053/j.gastro.2016.09.062
- Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345-349. doi:10.2147/JIR.S174982
- Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. Jul 2012;18(3):258-68. doi:10.5056/jnm.2012.18.3.258
- Fedor A, Bojanowski I, Korzeniewski K. Gastrointestinal infections in returned travelers. Int Marit Health. 2019;70(4):244-251. doi:10.5603/IMH.2019.0039
- Quigley EMM. The Gut-Brain Axis and the Microbiome: Clues to Pathophysiology and Opportunities for Novel Management Strategies in Irritable Bowel Syndrome (IBS). J Clin Med. Jan 3 2018;7(1)doi:10.3390/jcm7010006
- Fichna J, Storr MA. Brain-Gut Interactions in IBS. Frontiers in pharmacology. 2012;3:127-127. doi:10.3389/fphar.2012.00127
- Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. Journal of gastroenterology. Nov 2012;47(11):1177-85. doi:10.1007/s00535-012-0627-7
- Mach T. The brain-gut axis in irritable bowel syndrome--clinical aspects. Med Sci Monit. Jun 2004;10(6):Ra125-31.
- Jarrett ME, Burr RL, Cain KC, Hertig V, Weisman P, Heitkemper MM. Anxiety and depression are related to autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. Feb 2003;48(2):386-94. doi:10.1023/a:1021904216312
- Azpiroz F. Gastrointestinal perception: pathophysiological implications. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Jun 2002;14(3):229-39. doi:10.1046/j.1365-2982.2002.00324.x
- Orr WC, Crowell MD, Lin B, Harnish MJ, Chen JD. Sleep and gastric function in irritable bowel syndrome: derailing the brain-gut axis. Gut. Sep 1997;41(3):390-3. doi:10.1136/gut.41.3.390
- Hall GB, Kamath MV, Collins S, et al. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Mar 2010;22(3):276-e80. doi:10.1111/j.1365-2982.2009.01436.x
- Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. Apr 2010;59(4):489-95. doi:10.1136/gut.2008.175000
- Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. Jan 8 2008;70(2):153-4. doi:10.1212/01.wnl.0000295509.30630.10
- Meerveld BG, Johnson AC. Mechanisms of Stress-induced Visceral Pain. J Neurogastroenterol Motil. Jan 30 2018;24(1):7-18. doi:10.5056/jnm17137
- El-Bakry S, Michael V, El Hamady M, Awd MS. A study of psychiatric comorbidities in irritable bowel syndrome. Original Article. Egyptian Journal of Psychiatry. September 1, 2018 2018;39(3):140-149. doi:10.4103/ejpsy.ejpsy_9_18
- Staudacher HM, Mikocka-Walus A, Ford AC. Common mental disorders in irritable bowel syndrome: pathophysiology, management, and considerations for future randomised controlled trials. Lancet Gastroenterol Hepatol. May 2021;6(5):401-410. doi:10.1016/S2468-1253(20)30363-0
- Vinberg M, Ottesen NM, Meluken I, et al. Remitted affective disorders and high familial risk of affective disorders associate with aberrant intestinal microbiota. Acta psychiatrica Scandinavica. Feb 2019;139(2):174-184. doi:10.1111/acps.12976
- Capuco A, Urits I, Hasoon J, et al. Gut Microbiome Dysbiosis and Depression: a Comprehensive Review. Curr Pain Headache Rep. Jun 6 2020;24(7):36. doi:10.1007/s11916-020-00871-x
- Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. Jan 2006;130(1):34-43. doi:10.1053/j.gastro.2005.09.031
- Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Apr 2005;3(4):349-57. doi:10.1016/s1542-3565(04)00726-8
- Ge X, Pan J, Liu Y, Wang H, Zhou W, Wang X. Intestinal Crosstalk between Microbiota and Serotonin and its Impact on Gut Motility. Current pharmaceutical biotechnology. 2018;19(3):190-195. doi:10.2174/1389201019666180528094202
- Wang L, Martinez V, Kimura H, Tache Y. 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. American journal of physiology Gastrointestinal and liver physiology. Jan 2007;292(1):G419-28. doi:10.1152/ajpgi.00289.2006
- Chen J, Li Q, Saliuk G, Bazhanov S, Winston JH. Estrogen and serotonin enhance stress-induced visceral hypersensitivity in female rats by up-regulating brain-derived neurotrophic factor in spinal cord. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Mar 11 2021:e14117. doi:10.1111/nmo.14117
- Keszthelyi D, Troost FJ, Jonkers DM, et al. Visceral hypersensitivity in irritable bowel syndrome: evidence for involvement of serotonin metabolism--a preliminary study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Aug 2015;27(8):1127-37. doi:10.1111/nmo.12600
- Dong Y, Wang Z, Qin Z, Cao J, Chen Y. Role of serotonin in the intestinal mucosal epithelium barrier in weaning mice undergoing stress-induced diarrhea. J Mol Histol. Feb 2018;49(1):85-97. doi:10.1007/s10735-017-9749-9
- Vahora IS, Tsouklidis N, Kumar R, Soni R, Khan S. How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome. Cureus. Aug 19 2020;12(8):e9871. doi:10.7759/cureus.9871
- Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Jun 2012;24(6):521-30, e248. doi:10.1111/j.1365-2982.2012.01891.x
- Barbara G, Zecchi L, Barbaro R, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. Journal of clinical gastroenterology. Oct 2012;46 Suppl:S52-5. doi:10.1097/MCG.0b013e318264e918
- Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. Journal of gastroenterology and hepatology. Aug 2010;25(8):1366-73. doi:10.1111/j.1440-1746.2010.06370.x
- Lee Y, Kim YK. Understanding the Connection Between the Gut-Brain Axis and Stress/Anxiety Disorders. Curr Psychiatry Rep. Mar 12 2021;23(5):22. doi:10.1007/s11920-021-01235-x
- Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, Koussoulas V, Barbatzas C, Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: relationship with irritable bowel syndrome. Dig Dis Sci. May 2012;57(5):1321-9. doi:10.1007/s10620-012-2033-7
- Yamini D, Pimentel M. Irritable bowel syndrome and small intestinal bacterial overgrowth. Journal of clinical gastroenterology. Nov-Dec 2010;44(10):672-5. doi:10.1097/MCG.0b013e3181ef3476
- Erdogan A, Rao SS, Gulley D, Jacobs C, Lee YY, Badger C. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Apr 2015;27(4):481-9. doi:10.1111/nmo.12516
- Chen B, Kim JJ, Zhang Y, Du L, Dai N. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. Journal of gastroenterology. Jul 2018;53(7):807-818. doi:10.1007/s00535-018-1476-9
- Wijarnpreecha K, Werlang ME, Watthanasuntorn K, et al. Obesity and Risk of Small Intestine Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Dig Dis Sci. May 2020;65(5):1414-1422. doi:10.1007/s10620-019-05887-x
- Roland BC, Lee D, Miller LS, et al. Obesity increases the risk of small intestinal bacterial overgrowth (SIBO). Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Mar 2018;30(3)doi:10.1111/nmo.13199
- Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. Journal of gastroenterology. Jan 2018;53(1):27-36. doi:10.1007/s00535-017-1371-9
- Quigley EMM. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO). Current gastroenterology reports. Jan 15 2019;21(1):3. doi:10.1007/s11894-019-0671-z
- Moraes L, Magnusson MK, Mavroudis G, et al. Systemic Inflammatory Protein Profiles Distinguish Irritable Bowel Syndrome (IBS) and Ulcerative Colitis, Irrespective of Inflammation or IBS-Like Symptoms. Inflammatory bowel diseases. May 12 2020;26(6):874-884. doi:10.1093/ibd/izz322
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. Jun 2002;122(7):1778-83. doi:10.1053/gast.2002.33579
- Tornblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. Dec 2002;123(6):1972-9. doi:10.1053/gast.2002.37059
- Guilarte M, Santos J, de Torres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. Feb 2007;56(2):203-9. doi:10.1136/gut.2006.100594
- Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. Mar 2007;132(3):913-20. doi:10.1053/j.gastro.2007.01.046
- Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. Mar 2004;126(3):693-702. doi:10.1053/j.gastro.2003.11.055
- Gecse K, Roka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. May 2008;57(5):591-9. doi:10.1136/gut.2007.140210
- Usai P, Manca R, Cuomo R, Lai MA, Boi MF. Effect of gluten-free diet and co-morbidity of irritable bowel syndrome-type symptoms on health-related quality of life in adult coeliac patients. Dig Liver Dis. Sep 2007;39(9):824-8. doi:10.1016/j.dld.2007.05.017
- Lundin KE, Alaedini A. Non-celiac gluten sensitivity. Gastrointestinal endoscopy clinics of North America. Oct 2012;22(4):723-34. doi:10.1016/j.giec.2012.07.006
- Di Sabatino A, Corazza GR. Nonceliac gluten sensitivity: sense or sensibility? Ann Intern Med. Feb 21 2012;156(4):309-11. doi:10.7326/0003-4819-156-4-201202210-00010
- Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN Journal of parenteral and enteral nutrition. Jan 2012;36(1 Suppl):68s-75s. doi:10.1177/0148607111426276
- Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the "no man's land" of gluten sensitivity. The American journal of gastroenterology. Jun 2009;104(6):1587-94. doi:10.1038/ajg.2009.188
- Aziz I, Lewis NR, Hadjivassiliou M, et al. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. European journal of gastroenterology & hepatology. Jan 2014;26(1):33-9. doi:10.1097/01.meg.0000435546.87251.f7
- Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Jul 2007;5(7):844-50; quiz 769. doi:10.1016/j.cgh.2007.03.021
- Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. May 2013;144(5):903-911.e3. doi:10.1053/j.gastro.2013.01.049
- Bhatnagar S, Aggarwal R. Lactose intolerance. BMJ (Clinical research ed). Jun 30 2007;334(7608):1331-2. doi:10.1136/bmj.39252.524375.80
- Choi YK, Kraft N, Zimmerman B, Jackson M, Rao SS. Fructose intolerance in IBS and utility of fructose-restricted diet. Journal of clinical gastroenterology. Mar 2008;42(3):233-8. doi:10.1097/MCG.0b013e31802cbc2f
- Lenhart A, Chey WD. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv Nutr. Jul 2017;8(4):587-596. doi:10.3945/an.117.015560
- Thabane M, Marshall JK. Post-infectious irritable bowel syndrome. World journal of gastroenterology. 2009;15(29):3591-3596. doi:10.3748/wjg.15.3591
- Ghoshal UC, Ranjan P. Post-infectious irritable bowel syndrome: the past, the present and the future. Journal of gastroenterology and hepatology. Apr 2011;26 Suppl 3:94-101. doi:10.1111/j.1440-1746.2011.06643.x
- Zanini B, Ricci C, Bandera F, et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. The American journal of gastroenterology. Jun 2012;107(6):891-9. doi:10.1038/ajg.2012.102
- Niaz SK, Sandrasegaran K, Renny FH, Jones BJ. Postinfective diarrhoea and bile acid malabsorption. J R Coll Physicians Lond. Jan-Feb 1997;31(1):53-6.
- Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. Dec 2000;47(6):804-11. doi:10.1136/gut.47.6.804
- Serghini M, Karoui S, Boubaker J, Filali A. [Post-infectious irritable bowel syndrome]. La Tunisie medicale. Mar 2012;90(3):205-13. Une nouvelle entité clinique: le syndrome de l'intestin irritable postinfectieux.
- Pallotti F, Fogacci E, Frisoni C, et al. [Post-infectious irritable bowel syndrome]. La Clinica terapeutica. 2011 Mar-Apr 2011;162(2):157-161.
- Schwille-Kiuntke J, Frick JS, Zanger P, Enck P. Post-infectious irritable bowel syndrome--a review of the literature. Z Gastroenterol. Aug 2011;49(8):997-1003. doi:10.1055/s-0031-1281581
- Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World J Gastroenterol. Jun 14 2014;20(22):6725-43. doi:10.3748/wjg.v20.i22.6725
- Jung HK, Kim DY, Moon IH. Effects of gender and menstrual cycle on colonic transit time in healthy subjects. The Korean journal of internal medicine. Sep 2003;18(3):181-6. doi:10.3904/kjim.2003.18.3.181
- Pati GK, Kar C, Narayan J, et al. Irritable Bowel Syndrome and the Menstrual Cycle. Cureus. 2021;13(1):e12692-e12692. doi:10.7759/cureus.12692
- Triadafilopoulos G, Finlayson M, Grellet C. Bowel dysfunction in postmenopausal women. Women Health. 1998;27(4):55-66. doi:10.1300/J013v27n04_04
- Weaver KR, Boulineaux CM, Robinson JM, Butler K, Heitkemper MM, Henderson WA. Sex Hormones, BDNF, Leptin, and TGF-beta1 in Females With IBS: A Pilot Investigation. Biological research for nursing. Apr 2021;23(2):231-237. doi:10.1177/1099800420948589
- Cui N, Wu BP, Wu SZ. [Association of peripheral blood estradiol, progesterone and testosterone levels with irritable bowel syndrome]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. Mar 2006;26(3):367-8.
- Mulak A, Tache Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. Mar 14 2014;20(10):2433-48. doi:10.3748/wjg.v20.i10.2433
- Al-Shboul OA, Nazzal MS, Mustafa AG, et al. Estrogen relaxes gastric muscle cells via a nitric oxide- and cyclic guanosine monophosphate-dependent mechanism: A sex-associated differential effect. Experimental and therapeutic medicine. Sep 2018;16(3):1685-1692. doi:10.3892/etm.2018.6406
- So SY, Savidge TC. Sex-Bias in Irritable Bowel Syndrome: Linking Steroids to the Gut-Brain Axis. Frontiers in endocrinology. 2021;12:684096. doi:10.3389/fendo.2021.684096
- Ruigómez A, García Rodríguez LA, Johansson S, Wallander MA. Is hormone replacement therapy associated with an increased risk of irritable bowel syndrome? Maturitas. Feb 25 2003;44(2):133-40. doi:10.1016/s0378-5122(02)00321-3
- Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. Aug 2014;63(8):1293-9. doi:10.1136/gutjnl-2013-305690
- Halasa M, Maciejewska D, Ryterska K, Baskiewicz-Halasa M, Safranow K, Stachowska E. Assessing the Association of Elevated Zonulin Concentration in Stool with Increased Intestinal Permeability in Active Professional Athletes. Medicina (Kaunas, Lithuania). Oct 21 2019;55(10)doi:10.3390/medicina55100710
- Keszthelyi D, Dackus GH, Masclee GM, Kruimel JW, Masclee AAM. Increased proton pump inhibitor and NSAID exposure in irritable bowel syndrome: results from a case-control study. BMC gastroenterology. 2012;12:121-121. doi:10.1186/1471-230X-12-121
- Kerckhoffs AP, Akkermans LM, de Smet MB, et al. Intestinal permeability in irritable bowel syndrome patients: effects of NSAIDs. Dig Dis Sci. Mar 2010;55(3):716-23. doi:10.1007/s10620-009-0765-9
- Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. Journal of gastroenterology. 2009;44 Suppl 19:23-9. doi:10.1007/s00535-008-2266-6
- Villarreal AA, Aberger FJ, Benrud R, Gundrum JD. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. Wmj. Feb 2012;111(1):17-20.
- Bet PM, Hugtenburg JG, Penninx BW, Hoogendijk WJ. Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol. Nov 2013;23(11):1443-51. doi:10.1016/j.euroneuro.2013.05.001
- Crawford AA, Lewis S, Nutt D, et al. Adverse effects from antidepressant treatment: randomised controlled trial of 601 depressed individuals. Psychopharmacology. Aug 2014;231(15):2921-31. doi:10.1007/s00213-014-3467-8
- Kushnir VM, Bhat P, Chokshi RV, et al. The impact of opiate pain medications and psychoactive drugs on the quality of colon preparation in outpatient colonoscopy. Dig Liver Dis. Jan 2014;46(1):56-61. doi:10.1016/j.dld.2013.07.020
- Khan S, Chang L. Diagnosis and management of IBS. Nat Rev Gastroenterol Hepatol. Oct 2010;7(10):565-81. doi:10.1038/nrgastro.2010.137
- Di Palma JA, Herrera JL. The role of effective clinician-patient communication in the management of irritable bowel syndrome and chronic constipation. Journal of clinical gastroenterology. Oct 2012;46(9):748-51. doi:10.1097/MCG.0b013e31825a2ff2
- Lee C, Doo E, Choi JM, et al. The Increased Level of Depression and Anxiety in Irritable Bowel Syndrome Patients Compared with Healthy Controls: Systematic Review and Meta-analysis. J Neurogastroenterol Motil. Jul 30 2017;23(3):349-362. doi:10.5056/jnm16220
- Agrawal A, Khan MH, Whorwell PJ. Irritable bowel syndrome in the elderly: An overlooked problem? Dig Liver Dis. Oct 2009;41(10):721-4. doi:10.1016/j.dld.2009.03.011
- Hsu SM, Lin HJ, Lin MC, Huang ST. Increased incidence and recurrence rates of acute diverticulitis in patients with irritable bowel syndrome. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. Dec 2020;22(12):2181-2190. doi:10.1111/codi.15325
- Staller K, Olen O, Soderling J, et al. Mortality Risk in Irritable Bowel Syndrome: Results From a Nationwide Prospective Cohort Study. The American journal of gastroenterology. May 2020;115(5):746-755. doi:10.14309/ajg.0000000000000573
- Longstreth GF, Wong C, Chen Q. Misdiagnosis of Diverticulitis After a Prior Diagnosis of Irritable Bowel Syndrome (IBS). J Am Board Fam Med. Jul-Aug 2020;33(4):549-560. doi:10.3122/jabfm.2020.04.190328
- Mearin F, Lacy BE. Diagnostic criteria in IBS: useful or not? Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Sep 2012;24(9):791-801. doi:10.1111/j.1365-2982.2012.01992.x
- Spiegel BM, Farid M, Esrailian E, Talley J, Chang L. Is irritable bowel syndrome a diagnosis of exclusion?: a survey of primary care providers, gastroenterologists, and IBS experts. The American journal of gastroenterology. Apr 2010;105(4):848-58. doi:10.1038/ajg.2010.47
- Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. Apr 30 2017;23(2):151-163. doi:10.5056/jnm16214
- Cangemi DJ, Lacy BE. Management of irritable bowel syndrome with diarrhea: a review of nonpharmacological and pharmacological interventions. Therapeutic advances in gastroenterology. 2019;12:1756284819878950. doi:10.1177/1756284819878950
- Trinkley KE, Nahata MC. Treatment of irritable bowel syndrome. Journal of Clinical Pharmacy and Therapeutics. 2011;36:275-282.
- Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs. Aug 20 2010;70(12):1487-503. doi:10.2165/11898640-000000000-00000
- Attar A, Lémann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. Feb 1999;44(2):226-30. doi:10.1136/gut.44.2.226
- Chapman RW, Stanghellini V, Geraint M, Halphen M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. The American journal of gastroenterology. Sep 2013;108(9):1508-15. doi:10.1038/ajg.2013.197
- Lacy BE, Levy LC. Lubiprostone: a novel treatment for chronic constipation. Clin Interv Aging. 2008;3(2):357-64. doi:10.2147/cia.s2938
- Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci. Apr 2010;55(4):1090-7. doi:10.1007/s10620-009-1068-x
- Ambizas E, Ginzburg R. Lubiprostone: A Chloride Channel Activator for Treatment of Chronic Constipation. The Annals of pharmacotherapy. 07/01 2007;41:957-64. doi:10.1345/aph.1K047
- Passos M, Takemoto MLS, Corradino GC, Guedes LS. Systematic review with meta-analysis: lubiprostone efficacy on the treatment of patients with constipation. Arq Gastroenterol. Oct-Dec 2020;57(4):498-506. doi:10.1590/S0004-2803.202000000-83
- Lembo AJ, Schneier HA, Shiff SJ, et al. Two Randomized Trials of Linaclotide for Chronic Constipation. New England Journal of Medicine. 2011/08/11 2011;365(6):527-536. doi:10.1056/NEJMoa1010863
- Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. Nov 2012;107(11):1714-24; quiz p.1725. doi:10.1038/ajg.2012.255
- Kale-Pradhan PB, Wilhelm SM. Tegaserod for constipation-predominant irritable bowel syndrome. Pharmacotherapy. Feb 2007;27(2):267-77. doi:10.1592/phco.27.2.267
- Scott LJ, Perry CM. Tegaserod. Drugs. Sep 1999;58(3):491-6; discussion 497-8. doi:10.2165/00003495-199958030-00013
- Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. Mar 2000;118(3):463-8. doi:10.1016/s0016-5085(00)70251-4
- Tougas G, Snape WJ, Jr., Otten MH, et al. Long-term safety of tegaserod in patients with constipation-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. Oct 2002;16(10):1701-8. doi:10.1046/j.1365-2036.2002.01347.x
- Hughes S, Higgs NB, Turnberg LA. Loperamide has antisecretory activity in the human jejunum in vivo. Gut. Sep 1984;25(9):931-5. doi:10.1136/gut.25.9.931
- Ooms LA, Degryse AD, Janssen PA. Mechanisms of action of loperamide. Scandinavian journal of gastroenterology Supplement. 1984;96:145-55.
- Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scandinavian journal of gastroenterology. May 1996;31(5):463-8. doi:10.3109/00365529609006766
- Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scandinavian journal of gastroenterology Supplement. 1987;130:81-4. doi:10.3109/00365528709091004
- Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Feb 2010;8(2):159-65. doi:10.1016/j.cgh.2009.10.020
- Bajor A, Tornblom H, Rudling M, Ung KA, Simren M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. Jan 2015;64(1):84-92. doi:10.1136/gutjnl-2013-305965
- Cremonini F, Nicandro JP, Atkinson V, Shringarpure R, Chuang E, Lembo A. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Alimentary pharmacology & therapeutics. 2012;36(5):437-448. doi:10.1111/j.1365-2036.2012.05208.x
- Mayer EA, Bradesi S. Alosetron and irritable bowel syndrome. Expert opinion on pharmacotherapy. Nov 2003;4(11):2089-98. doi:10.1517/14656566.4.11.2089
- Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. May 2008;6(5):545-55. doi:10.1016/j.cgh.2007.12.015
- Gallo-Torres H, Brinker A, Avigan M. Alosetron: ischemic colitis and serious complications of constipation. The American journal of gastroenterology. May 2006;101(5):1080-3. doi:10.1111/j.1572-0241.2006.00650.x
- Butt I, Kasmin F. Alosetron. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Annahazi A, Roka R, Rosztoczy A, Wittmann T. Role of antispasmodics in the treatment of irritable bowel syndrome. World J Gastroenterol. May 28 2014;20(20):6031-43. doi:10.3748/wjg.v20.i20.6031
- Talley NJ. Evaluation of drug treatment in irritable bowel syndrome. Br J Clin Pharmacol. Oct 2003;56(4):362-9. doi:10.1046/j.1365-2125.2003.01966.x
- Xie C, Tang Y, Wang Y, et al. Efficacy and Safety of Antidepressants for the Treatment of Irritable Bowel Syndrome: A Meta-Analysis. PLoS One. 2015;10(8):e0127815. doi:10.1371/journal.pone.0127815
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. Apr 7 2009;15(13):1548-53. doi:10.3748/wjg.15.1548
- Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Alimentary pharmacology & therapeutics. Mar 2017;45(5):604-616. doi:10.1111/apt.13928
- Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. The American journal of gastroenterology. Jan 2012;107(1):28-35; quiz 36. doi:10.1038/ajg.2011.355
- Hauser G, Tkalcic M, Pletikosic S, Grabar N, Stimac D. Erythrocyte sedimentation rate - possible role in determining the existence of the low grade inflammation in Irritable Bowel Syndrome patients. Med Hypotheses. Jun 2012;78(6):818-20. doi:10.1016/j.mehy.2012.03.020
- Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. American journal of physiology Gastrointestinal and liver physiology. Oct 2012;303(7):G775-85. doi:10.1152/ajpgi.00155.2012
- Klotz U. The pharmacological profile and clinical use of mesalazine (5-aminosalicylic acid). Arzneimittel-Forschung. Feb 2012;62(2):53-8. doi:10.1055/s-0031-1299685
- Bafutto M, Almeida JR, Leite NV, Oliveira EC, Gabriel-Neto S, Rezende-Filho J. Treatment of postinfectious irritable bowel syndrome and noninfective irritable bowel syndrome with mesalazine. Arq Gastroenterol. Jan-Mar 2011;48(1):36-40. doi:10.1590/s0004-28032011000100008
- Dorofeyev AE, Kiriyan EA, Vasilenko IV, Rassokhina OA, Elin AF. Clinical, endoscopical and morphological efficacy of mesalazine in patients with irritable bowel syndrome. Clin Exp Gastroenterol. 2011;4:141-53. doi:10.2147/ceg.S18381
- Aron J, Lin M, Yu J, Paterson C, Bortey E, Forbes W. Mesalamine Granules 1500 mg Once Daily for 12 Weeks Provides Adequate Relief of IBS Symptoms in Irritable Bowel Syndrome with Diarrhea: Results from a Phase 2 Trial: 1749. Official journal of the American College of Gastroenterology | ACG. 2012;107:S711-S712.
- Lam C, Tan W, Leighton M, et al. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut. Jan 2016;65(1):91-9. doi:10.1136/gutjnl-2015-309122
- Krammer L, Sowa AS, Lorentz A. Mast Cells in Irritable Bowel Syndrome: A Systematic Review. Journal of gastrointestinal and liver diseases : JGLD. Dec 9 2019;28(4):463-472. doi:10.15403/jgld-229
- Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta. Jan 2012;1822(1):85-92. doi:10.1016/j.bbadis.2011.06.004
- Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. Sep 2010;59(9):1213-21. doi:10.1136/gut.2010.213108
- Barbara G, Stanghellini V, Cremon C, et al. Aminosalicylates and other anti-inflammatory compounds for irritable bowel syndrome. Digestive diseases (Basel, Switzerland). 2009;27 Suppl 1:115-21. doi:10.1159/000268131
- Stefanini GF, Saggioro A, Alvisi V, et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scandinavian journal of gastroenterology. Jun 1995;30(6):535-41. doi:10.3109/00365529509089786
- Uranga JA, Martinez V, Abalo R. Mast Cell Regulation and Irritable Bowel Syndrome: Effects of Food Components with Potential Nutraceutical Use. Molecules (Basel, Switzerland). Sep 20 2020;25(18)doi:10.3390/molecules25184314
- Hedstrom L. Serine protease mechanism and specificity. Chem Rev. Dec 2002;102(12):4501-24. doi:10.1021/cr000033x
- Hou JJ, Wang X, Li Y, Su S, Wang YM, Wang BM. The relationship between gut microbiota and proteolytic activity in irritable bowel syndrome. Microbial pathogenesis. Aug 2021;157:104995. doi:10.1016/j.micpath.2021.104995
- Jablaoui A, Kriaa A, Mkaouar H, et al. Fecal Serine Protease Profiling in Inflammatory Bowel Diseases. Frontiers in cellular and infection microbiology. 2020;10:21. doi:10.3389/fcimb.2020.00021
- Edogawa S, Edwinson AL, Peters SA, et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut. Jan 2020;69(1):62-73. doi:10.1136/gutjnl-2018-317416
- Roka R, Rosztoczy A, Leveque M, et al. A pilot study of fecal serine-protease activity: a pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. May 2007;5(5):550-5. doi:10.1016/j.cgh.2006.12.004
- Ceuleers H, Hanning N, Heirbaut J, et al. Newly developed serine protease inhibitors decrease visceral hypersensitivity in a post-inflammatory rat model for irritable bowel syndrome. Br J Pharmacol. Sep 2018;175(17):3516-3533. doi:10.1111/bph.14396
- Hanning N, De Bruyn M, Ceuleers H, et al. Local Colonic Administration of a Serine Protease Inhibitor Improves Post-Inflammatory Visceral Hypersensitivity in Rats. Pharmaceutics. May 29 2021;13(6)doi:10.3390/pharmaceutics13060811
- Zhang Y, Huang Y, Li H, et al. Transcutaneous auricular vagus nerve stimulation (taVNS) for migraine: an fMRI study. Regional anesthesia and pain medicine. Feb 2021;46(2):145-150. doi:10.1136/rapm-2020-102088
- Aranow C, Atish-Fregoso Y, Lesser M, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. Feb 2021;80(2):203-208. doi:10.1136/annrheumdis-2020-217872
- Wu D, Ma J, Zhang L, Wang S, Tan B, Jia G. Effect and Safety of Transcutaneous Auricular Vagus Nerve Stimulation on Recovery of Upper Limb Motor Function in Subacute Ischemic Stroke Patients: A Randomized Pilot Study. Neural plasticity. 2020;2020:8841752. doi:10.1155/2020/8841752
- Stavrakis S, Stoner JA, Humphrey MB, et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin Electrophysiol. Mar 2020;6(3):282-291. doi:10.1016/j.jacep.2019.11.008
- Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front Neurosci. 2020;14:284. doi:10.3389/fnins.2020.00284
- Gurel NZ, Huang M, Wittbrodt MT, et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. Jan - Feb 2020;13(1):47-59. doi:10.1016/j.brs.2019.08.002
- Burger AM, Van der Does W, Thayer JF, Brosschot JF, Verkuil B. Transcutaneous vagus nerve stimulation reduces spontaneous but not induced negative thought intrusions in high worriers. Biol Psychol. Mar 2019;142:80-89. doi:10.1016/j.biopsycho.2019.01.014
- Colzato LS, Ritter SM, Steenbergen L. Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuropsychologia. Mar 2018;111:72-76. doi:10.1016/j.neuropsychologia.2018.01.003
- Mion F, Pellissier S, Garros A, Damon H, Roman S, Bonaz B. Transcutaneous auricular vagus nerve stimulation for the treatment of irritable bowel syndrome: A pilot, open-label study. Bioelectronics in Medicine. 2020;3(1):5-12.
- Teckentrup V, Neubert S, Santiago JCP, Hallschmid M, Walter M, Kroemer NB. Non-invasive stimulation of vagal afferents reduces gastric frequency. Brain Stimul. Mar - Apr 2020;13(2):470-473. doi:10.1016/j.brs.2019.12.018
- Farmer AD, Albusoda A, Amarasinghe G, et al. Transcutaneous vagus nerve stimulation prevents the development of, and reverses, established oesophageal pain hypersensitivity. Alimentary pharmacology & therapeutics. Sep 2020;52(6):988-996. doi:10.1111/apt.15869
- Kaut O, Janocha L, Weismuller TJ, Wullner U. Transcutaneous vagal nerve stimulation improves gastroenteric complaints in Parkinson's disease patients. NeuroRehabilitation. Dec 18 2019;45(4):449-451. doi:10.3233/NRE-192909
- Hong GS, Pintea B, Lingohr P, et al. Effect of transcutaneous vagus nerve stimulation on muscle activity in the gastrointestinal tract (transVaGa): a prospective clinical trial. International journal of colorectal disease. Mar 2019;34(3):417-422. doi:10.1007/s00384-018-3204-6
- Bertin L, Zanconato M, Crepaldi M, et al. The Role of the FODMAP Diet in IBS. Nutrients. Jan 26 2024;16(3)doi:10.3390/nu16030370. https://pubmed.ncbi.nlm.nih.gov/38337655/
- O'Brien L, Kasti A, Halmos EP, Tuck C, Varney J. Evolution, adaptation, and new applications of the FODMAP diet. JGH Open. May 2024;8(5):e13066. doi:10.1002/jgh3.13066. https://pubmed.ncbi.nlm.nih.gov/38770353/
- van Lanen AS, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. European journal of nutrition. Sep 2021;60(6):3505-3522. doi:10.1007/s00394-020-02473-0. https://pubmed.ncbi.nlm.nih.gov/33585949/
- Hahn J, Choi J, Chang MJ. Effect of Low FODMAPs Diet on Irritable Bowel Syndromes: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients. Jul 19 2021;13(7)doi:10.3390/nu13072460. https://pubmed.ncbi.nlm.nih.gov/34371973/
- Jent S, Bez NS, Haddad J, et al. The efficacy and real-world effectiveness of a diet low in fermentable oligo-, di-, monosaccharides and polyols in irritable bowel syndrome: A systematic review and meta-analysis. Clin Nutr. Jun 2024;43(6):1551-1562. doi:10.1016/j.clnu.2024.05.014. https://pubmed.ncbi.nlm.nih.gov/38754307/
- Wang J, Yang P, Zhang L, Hou X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Frontiers in nutrition. 2021;8:683191. doi:10.3389/fnut.2021.683191. https://pubmed.ncbi.nlm.nih.gov/34490319/
- Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. Jun 2022;71(6):1117-1126. doi:10.1136/gutjnl-2021-325214. https://pubmed.ncbi.nlm.nih.gov/34376515/
- Khalighi Sikaroudi M, Soltani S, Ghoreishy SM, Ebrahimi Z, Shidfar F, Dehnad A. Effects of a low FODMAP diet on the symptom management of patients with irritable bowel syndrome: a systematic umbrella review with the meta-analysis of clinical trials. Food Funct. May 20 2024;15(10):5195-5208. doi:10.1039/d3fo03717g. https://pubmed.ncbi.nlm.nih.gov/38711328/
- Dimidi E, Belogianni K, Whelan K, Lomer MCE. Gut Symptoms during FODMAP Restriction and Symptom Response to Food Challenges during FODMAP Reintroduction: A Real-World Evaluation in 21,462 Participants Using a Mobile Application. Nutrients. Jun 9 2023;15(12)doi:10.3390/nu15122683. https://pubmed.ncbi.nlm.nih.gov/37375587/
- Staudacher HM, Rossi M, Kaminski T, et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. Apr 2022;34(4):e14241. doi:10.1111/nmo.14241. https://pubmed.ncbi.nlm.nih.gov/34431172/
- Nybacka S, Törnblom H, Josefsson A, et al. A low FODMAP diet plus traditional dietary advice versus a low-carbohydrate diet versus pharmacological treatment in irritable bowel syndrome (CARIBS): a single-centre, single-blind, randomised controlled trial. The Lancet Gastroenterology & Hepatology. 2024;9(6):507-520. doi:10.1016/S2468-1253(24)00045-1. https://pubmed.ncbi.nlm.nih.gov/38643782/
- Harvie RM, Chisholm AW, Bisanz JE, et al. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J Gastroenterol. Jul 7 2017;23(25):4632-4643. doi:10.3748/wjg.v23.i25.4632. https://pubmed.ncbi.nlm.nih.gov/28740352/
- Kotha RR, Luthria DL. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules (Basel, Switzerland). Aug 13 2019;24(16)doi:10.3390/molecules24162930
- Kongkam P, Khongkha W, Lopimpisuth C, et al. Curcumin and proton pump inhibitors for functional dyspepsia: a randomised, double blind controlled trial. BMJ Evid Based Med. Nov 22 2023;28(6):399-406. doi:10.1136/bmjebm-2022-112231
- Portincasa P, Bonfrate L, Scribano ML, et al. Curcumin and Fennel Essential Oil Improve Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome. Journal of gastrointestinal and liver diseases : JGLD. Jun 2016;25(2):151-7. doi:10.15403/jgld.2014.1121.252.ccm
- Lopresti AL, Smith SJ, Rea A, Michel S. Efficacy of a curcumin extract (Curcugen™) on gastrointestinal symptoms and intestinal microbiota in adults with self-reported digestive complaints: a randomised, double-blind, placebo-controlled study. BMC Complement Med Ther. Jan 21 2021;21(1):40. doi:10.1186/s12906-021-03220-6
