Maintaining a Healthy Microbiome
Maintaining a Healthy Microbiome
Last Section Update: 09/2025
Contributor(s): Maureen Williams, ND; Shayna Sandhaus, PhD; Chancellor Faloon, Health & Wellness Author
Table of Contents
- Overview
- Introduction
- Background
- Establishing and Shaping the Microbiome
- Dysbiosis
- Diet and Lifestyle Strategies for Supporting a Healthy Microbiome
- Novel And Emerging Therapies For Microbiome Health
- Introduction to Probiotics
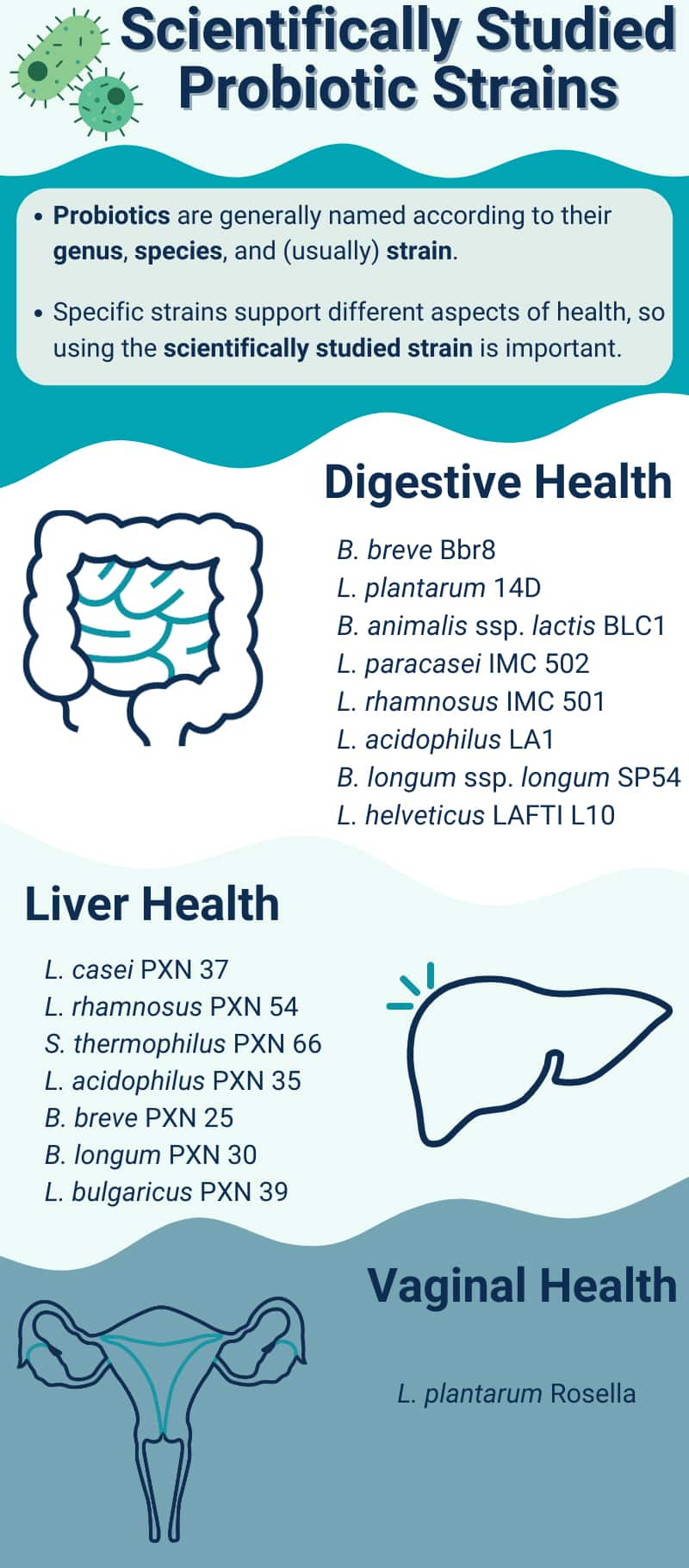
- Probiotics For Digestive Health
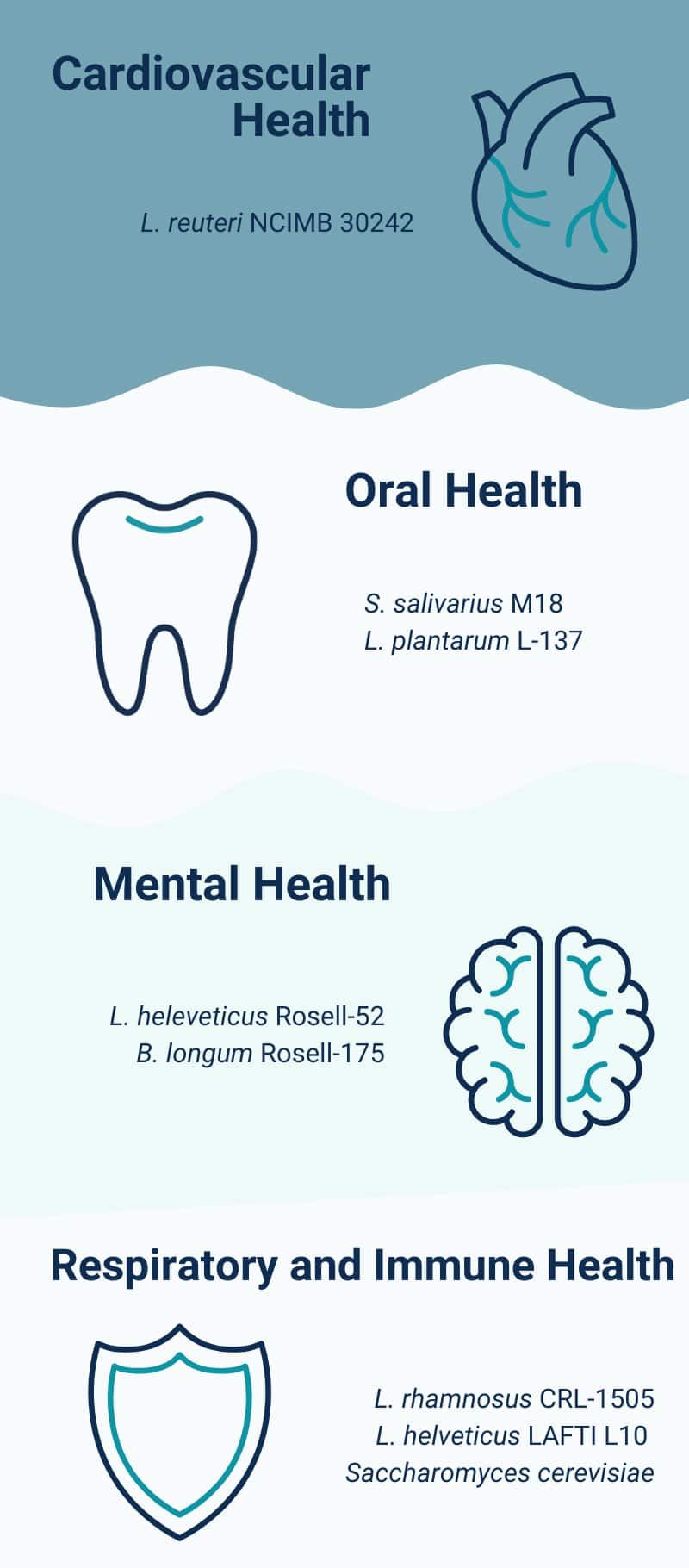
- Probiotics For Cardiovascular Health
- Probiotics For Oral Health
- Probiotics And Skin Health
- Probiotics For Vaginal And Urinary Health
- Probiotics For Respiratory And Immune Health
- Probiotics And Mood
- Update History
- References
1 Overview
Summary and Quick Facts for a Healthy Microbiome
- Most people are familiar with the importance of the microbiome for gut health but may not know that the microbiome can affect everything from mood to heart health.
- This protocol will teach you about the wide-ranging influence of the microbiome and what you can do to keep it healthy.
- Supplementation with probiotics and eating a diet that emphasizes unprocessed plant-based foods can go a long way toward improving the health of the microbiome and overall health.
What is the Microbiome?
The microbiome is the community, environment, and genetic material of microbes that live in the human body. There are estimated to be at least the same number of microbial cells in the body as human cells. People often associate the microbiome with gut health; however, research has demonstrated the microbiome’s role in many areas of human health.
Exploration of the microbiome has yielded startling discoveries: the microbes in our bodies are involved in gut, brain, oral, heart, and immune health, to name just a few. We are only recently discovering the implications of the microbiome on overall health.
Natural interventions such as probiotics and improved diet may help balance the microbiome and improve many aspects of health.
What Factors Contribute to Microbiome Development and Disturbances?
- Vaginal birth versus C-section
- Antibiotic use
- Dietary patterns
- Hormonal cycles, and more
What Health Effects are Associated with an Imbalanced Microbiome?
A shift in the microbiome, such as one that lowers beneficial species and/or boosts potentially harmful species, is known as dysbiosis. Dysbiosis of the gut has been linked with many chronic diseases and conditions, including:
- Allergies
- Cancer
- Obesity
- Depression
- Leaky gut and chronic inflammation
- Small intestinal bacterial overgrowth (SIBO)
- Cardiovascular disease, and more
What Dietary and Lifestyle Changes Can Be Beneficial for the Microbiome?
- Include more fiber (whole grains, legumes, fruits and vegetables) and less animal proteins and saturated fats in your diet
- Add omega-3 fatty acids and fermented foods such as yogurt, soy sauce, and miso to your diet
- Avoid excess alcohol
- Get plenty of sleep and try to manage stress efficiently
- Exercise regularly
What are Emerging Therapies for Microbiome Health?
- Fecal microbiota transplantation
- Phage therapy (viruses to attack harmful bacteria while leaving helpful bacteria and human cells untouched)
What are Probiotics?
Probiotics are living microorganisms, such as bacteria, that can promote health when they are administered in sufficient quantities.(Hill, Guarner et al. 2014) Bacterial species from the Lactobacillus and Bifidobacterium genera are often found in probiotic supplements. Probiotics are generally safe; however, consultation with a qualified healthcare provider is advised before use of probiotics by very old, very young, or immunocompromised individuals (eg, cancer patients, patients on immunosuppressive drugs, etc.).
2 Introduction
The human body exists in a dynamic and interdependent relationship with microbes. The skin, digestive tract, airways, vagina, eyes, and ears each have unique microbial ecosystems (Sender 2016; Proctor 2017; Thursby 2017; Huang 2011; Martin 2012; Dibb 1990; Lu, Liu 2016). There is even evidence that regions of the body long thought to be sterile, such as the placenta, urinary tract, uterus, lungs, blood, and even the brain, can harbor communities of microbes (Falana 2015; Potgieter 2015; Branton 2013; Whiteside 2015; O'Dwyer 2016; Chen 2017; Thomas-White 2016). In healthy humans, these complex ecosystems include a range of microorganisms, from bacteria and viruses to fungi and eukaryotes (Lloyd-Price 2016). Rather than being viewed solely as a reservoir for infection, these communities of microorganisms are now recognized as essential to our health as individuals and as a species (Davenport 2017; Turnbaugh 2007).
Historically, the term microbiome has been used to refer to the genetic material of the microbes, while microbiota refers to a community of microbial species (Knight 2017; D'Argenio 2015). These terms have come to be used interchangeably. In this protocol, microbiome will be used to refer to both groups of microorganisms and to their combined genetic material.
The number of microbial cells we harbor is estimated to be at least equivalent to the number of human cells in the body (Sender 2016; Knight 2017). The gut microbiome, which is the largest subset of the human microbiome, includes over 1,000 different bacterial species (across individuals) (Koboziev 2014; Lloyd-Price 2016) and accounts for about 2‒4 pounds of body weight per person (D'Argenio 2015). Furthermore, about 5 million microbial genes have been found in the gut, which is vastly more than the approximately 22,000 protein-encoding genes in the entire human genome (Ursell 2012; D'Argenio 2015). Only recently have we begun to understand and appreciate the significance of our evolutionary relationship with our microbial ecosystems (Davenport 2017).
This protocol describes the microbiome as a whole and its regional subsets. It presents a brief summary of what scientists know about how the microbiome interacts with our own cells and influences our health. This protocol also summarizes how the microbiome is shaped and maintained, and presents dietary and lifestyle strategies for supporting healthy microbial communities, as well as novel and emerging technologies for manipulating the microbiome: fecal microbiota transplant and phage therapy. Finally, this protocol details current and emerging research about the use of probiotics—living beneficial microorganisms—to prevent and treat disease.
3 Background
Microbiome Research
The Human Microbiome Project (HMP) was launched in 2008 by the National Institutes of Health (NIH) as an extension of the Human Genome Project (Turnbaugh 2007; Huse 2012; Peterson 2009). It is an ongoing worldwide undertaking that seeks to understand microbial communities and their variations in and on healthy humans (Turnbaugh 2007), beginning with five major body regions: the gastrointestinal tract, skin, vagina, respiratory tract, and oral cavity (Gevers 2012). A major goal of the HMP is to create a resource for determining how altered microbial patterns may contribute to disease and how to restore healthy microbial balance (Ursell 2012; Gevers 2012; Foxman 2010). Thanks to advances in gene sequencing technologies, knowledge about the microbiome is expanding rapidly (Knight 2017).
The human microbiome is extremely diverse and surprisingly variable: while only approximately 0.5% of the human genome is variable between individuals, the microbiome can vary dramatically between individuals (Ursell 2012; Mayor 2007). Much of this variability is due to differences in diet, environment, genetics, antibiotic use, and microbial exposure early in life (Human Microbiome Project Consortium 2012; Lloyd-Price 2016; Wischmeyer 2016). The diversity and variability of the human microbiome makes its characterization extremely challenging (Ursell 2012; Lloyd-Price 2016; Johnson 2016; Lozupone 2012).
Beneficial Microbes
The largest and most heavily studied component of the human microbiome resides in the gut. Microbiome researchers have estimated that each person is host to about 160 distinct intestinal bacterial species (Lloyd-Price 2016). Lactobacillus and Bifidobacterium species, encountered during birth and present in breastmilk, are among the earliest colonizers of the infant gut, and they shape subsequent colonization with other species (Koboziev 2014). While Lactobacillus and Bifidobacterium species become less abundant in the intestinal microbiome of adults as compared with infants (Arboleya 2016; Sghir 2000), they have been the focus of much attention due to their beneficial health effects, modulation by diet and environment, and possible therapeutic effects when used as probiotic supplements (Evivie 2017; O'Callaghan 2016; Derrien 2015; Rodriguez 2015).
Functions of a Healthy Microbiome
Digestion
Microbes in the intestines produce enzymes that can break down certain food components that human digestive mechanisms alone cannot (Thursby 2017; Kau 2011). A healthy microbiome therefore allows us to tolerate otherwise indigestible plant foods and extract otherwise inaccessible nutrients from them (Turnbaugh 2007; Krajmalnik-Brown 2012).
Microbial enzymes are especially important for breaking down indigestible and partially digestible carbohydrates, including certain starches and fibers. This enhances the body’s ability to extract and absorb monosaccharides (single-unit sugars) that can be turned into energy (Flint 2012; Krajmalnik-Brown 2012). Gut bacteria metabolize and activate many plant polyphenols and other phytochemicals, which may have important implications for health (Ozdal 2016; Tomas-Barberan 2016; Theilmann 2017; Swanson 2015). For example, certain intestinal bacteria transform lignans and isoflavones into active compounds linked to improved female hormone signaling (Gaya 2016).
The gut microbiome is also equipped with enzymes to digest dietary proteins. Some microbes are more efficient protein-digesters than others. Some of the byproducts of microbial protein digestion can have toxic effects that may contribute to negative health outcomes. A Western-style diet, which is high in animal protein and low in indigestible fibers, has a considerable detrimental impact on gut microbiome composition by supporting species that can best adapt to a high-protein environment (Montemurno 2014; Graf 2015; David 2014).
Microbial Byproducts
As part of the metabolic process, bacteria secrete a variety of metabolites that can exert beneficial effects on the host (either directly or indirectly). Such metabolites have been termed “postbiotics,” and have drawn interest in recent years for their potential to modulate human health and the microbiome (Żółkiewicz 2020). A major metabolic pathway used by intestinal microbes to produce energy is fermentation, which is a metabolic process that does not require oxygen (Thursby 2017; Monda 2017). The main byproducts of microbial fermentation, primarily of dietary fibers, are short-chain fatty acids, including butyrate, acetate, and propionate. These postbiotic short-chain fatty acids are not only a major energy source for cells lining the large intestine, but also regulate immune activity, prevent infections, reduce inflammation, enhance the absorption of minerals such as calcium, have anti-cancer properties, and can influence cholesterol levels, appetite, and weight gain (Sharon 2014; Flint 2012; Krajmalnik-Brown 2012; Whisner 2017).
Byproducts of protein fermentation are somewhat different than those of carbohydrate fermentation and include a number of compounds that are potentially toxic. Some of these toxic byproducts are believed to contribute to health problems associated with diets high in animal protein, such as colon cancer and some chronic diseases (Montemurno 2014).
Bile acids are the main components of bile, which is secreted from the gallbladder into the small intestine during digestion (Taoka 2016). Microbial enzymes transform bile acids into a wide array of byproducts, some of which are reabsorbed (Ryan 2017). In addition to assisting in the digestion and assimilation of fats and fatty compounds, reabsorbed bile acids and their byproducts help regulate lipid and glucose metabolism and energy production (Taoka 2016; Ryan 2017). How bile acids modulate cardio-metabolic conditions such as obesity, diabetes, fatty liver, and heart disease is still being explored (Ryan 2017; Chiang 2017).
Interestingly, bile acids also contribute to shaping the gut microbiome via antimicrobial effects and interactions with the immune system (Ramirez-Perez 2017; Chiang 2017; Ridlon 2014; Nie 2015). Bile acids may be one of the ways to connect diet, which influences the types of bile acids produced, to the composition of the gut microbial populations, which may be supported or suppressed by the presence of certain bile acids (Ridlon 2014; Islam 2011; Wahlstrom 2016).
Other byproducts of microbial activity that benefit human hosts include B vitamins, vitamin K, and amino acids such as tryptophan (Jandhyala 2015; Etienne-Mesmin 2017; Conly 1992; Morowitz 2011; Paiva 1998). Several tryptophan-derived compounds have important impacts on the functions of the immune and nervous systems (Cervenka 2017; Jenkins 2016). For example, approximately 90% of the body’s serotonin is made in the gastrointestinal tract from tryptophan (Sharon 2014; Kato 2013; Mawe 2013). Bacteria that colonize the intestines may also produce other neurotransmitters, such as acetylcholine, norepinephrine, dopamine, and gamma-aminobutyric acid (GABA) (Clark 2016; Galland 2014). These neurotransmitters exert a host of peripheral effects, such as modulation of the stress response, which ultimately influences brain function and mood (Galland 2014; Jenkins 2016; Robson 2017).
Gut microbes play an important role in metabolizing some medications, in some cases activating them prior to absorption (Noh 2017). Some of these medications, such as the anti-diabetes drug metformin, appear to rely at least in part on altering the gut microbiome to achieve their therapeutic effect (Wu 2017; Rena 2017). In one case report, targeting the growth of beneficial microbes with a food-based supplement improved both metformin’s tolerability and efficacy (Greenway 2014). Based on this intriguing case report, researchers carried out a small clinical study on 10 diabetic individuals. They found supplementation with the microbiome-targeted nutrients agave inulin, oat beta-glucan, and blueberry polyphenols improved metformin’s tolerability and efficacy (Burton 2015). Gut microbes also help process and detoxify harmful environmental compounds such as carcinogens (Turnbaugh 2007; Moon 2016).
Infection Control
A critical function of the microbiome is preventing the overgrowth of harmful microbes. Some mechanisms by which it achieves this are: maintaining an acidic pH; secreting antimicrobial substances; inhibiting microbial toxicity; competing for nutrients and resources; preventing harmful organisms from adhering to superficial cells; and activating local immune cells (Tosh 2012; Surendran Nair 2017; Knaus 2017).
Immune Regulation
The microbiome plays an integral role in immune regulation (Gulden 2017). Almost all tissues and organs, whether in direct contact with or further away from the microbiota, are influenced by the microbiota. Microbes and their products communicate with the immune system, promote its development and maturation, and guide immune responsiveness (Brown 2017; Shi 2017; Correa-Oliveira 2016; Shibata 2017; Belkaid 2014).
The immune regulatory role of the microbiome also helps prevent the dysfunctional immune activity that can lead to allergies, autoimmune diseases, and chronic inflammatory conditions (Brown 2017; Fujimura 2015). Microbiome disturbances have been linked to chronic inflammatory conditions such as periodontal disease, bacterial vaginosis, atopic dermatitis, inflammatory bowel disease, rheumatoid arthritis, and obesity (Correa-Oliveira 2016; Yamazaki 2017). Furthermore, interactions between the gut microbiome and immune system are important for stimulating the renewal and repair of cells lining the gut epithelium where the gut microbiome resides (Turnbaugh 2007).
Metabolism
The microbiome, particularly in the gut, appears to play a substantial role in regulating metabolism. From the very beginning of life, factors that shape the microbiome have been shown to be indicators of the risk of obesity or metabolic diseases. For example, Cesarean section (C-section) birth, a formula-based diet, illness, and antibiotic use early in life have all been correlated with higher rates of childhood overweight and obesity (Rosenbaum 2015; Mueller 2015; Korpela 2016; Li 2017). Maternal antibiotic use during pregnancy or breastfeeding has also been shown to impact the infant microbiome and has been correlated with childhood obesity risk (Cassidy-Bushrow 2017; Lemas 2016; Mor 2015).
Patterns of gut microbial imbalance have been seen in adults with metabolic diseases such as obesity, non-alcoholic fatty liver disease, and type 2 diabetes (Sedighi 2017; He 2017; Lippert 2017; Ma 2017). Certain dietary changes, such as consuming more fiber or capsaicin from chili peppers, calorie restriction, and weight loss, have been associated with beneficial changes in the gut microbiome, which may contribute to improvements in metabolic disturbances. Diets high in fat, protein, and sugar, on the other hand, can disrupt microbiome health (Vinke 2017; Rosenbaum 2015; Yang 2017; Ley 2006; Kang, Wang 2017; Singh 2017; Kang 2016; Turnbaugh 2009).
Circadian Rhythms
The intestinal microbiome undergoes daily shifts in composition that are driven in part by dietary patterns. In an animal model, interruptions in circadian rhythms intended to mimic jet lag or night-shift work altered the normal cycling of the microbiome, leading to metabolic disturbances (Thaiss 2014; Zarrinpar 2016). Emerging evidence further indicates that the relationship between circadian rhythms and the microbiome may be bidirectional, so that imbalances in the microbiome can disrupt circadian signaling, resulting in a cycle of disordered daily biological rhythms and metabolic perturbations (Thaiss 2014; Leone 2015; Wang, Kuang 2017).
4 Establishing and Shaping the Microbiome
The infant microbiome plays a foundational role in educating the immune system and is therefore an important determinant of lifelong health (Dominguez-Bello 2016). Its composition is shaped by multiple factors, including mode of birth, human genetic factors, geography and culture, diet and lifestyle, physiology, illnesses, surroundings, and contact with other people and pets (van der Meulen 2016; Turnbaugh 2007; Song 2013; Goodrich 2014; Yatsunenko 2012; Tasnim 2017). In addition, the use of medications such as antibiotics and proton pump inhibitors can influence the microbiome (van der Meulen 2016).
Our first exposure to the microbiome is at birth (Falana 2015; Koboziev 2014). During vaginal delivery, the newborn becomes colonized with microorganisms that originate from the maternal birth canal and skin (Knight 2017; Krajmalnik-Brown 2012; Koboziev 2014). Compared with babies born vaginally, the microbiomes of those born by C-section have been found to have more microbes that originate from the mother's skin and fewer microbes that originate from the vaginal canal (Krajmalnik-Brown 2012; Dominguez-Bello 2016; Rutayisire 2016). Bifidobacterium bacteria, as well as Lactobacillus species, are especially important early colonizers of the infant gut, where they help guide the development of a healthy microbiome and the maturation of the immune system (Koboziev 2014; Mueller 2015).
The mother's vaginal microbiome is critical in establishing the initial gut microbiome in the newborn during birth, and it helps shape immune system maturation and metabolism. This microbiome transmission from the mother to the newborn occurs during a critical period of the newborn brain development. Environmental factors, such as maternal stress during pregnancy, can change the vaginal microbiome and its transmission to the infant, and may affect neurological development of the infant later in life (Jasarevic, Rodgers 2015; Jasarevic, Howerton 2015; Jasarevic 2017).
Breastfeeding is another opportunity for the transfer of Lactobacillus and Bifidobacterium species, as well as other bacterial species, to the infant (Soto 2014; Mueller 2015; Urbaniak 2012). In addition, certain prebiotic carbohydrates found in human breast milk (called human milk oligosaccharides) support the growth of Bifidobacterium species, which are important in the infant's gut, where they promote the healthy development and function of the intestinal mucosal surface and the immune system (Knight 2017; Mueller 2015).
The differences in microbiome composition between babies born vaginally versus by C-section may explain the increased risks of allergies and asthma, obesity, type 1 diabetes, and neurodevelopmental disorders associated with C-section birth (Ho 2015; Moya-Perez 2017). Introducing C-section-born infants to vaginal microbes by swabbing them with maternal vaginal fluids at birth is a new strategy being explored to help establish a healthy microbiome (Dominguez-Bello 2016). Formula feeding also disrupts the healthy infant microbiome and has been associated with an increased risk for immunologic and metabolic disorders later in life. Even small amounts of supplemental formula can alter the gut microbial populations (Mueller 2015).
The infant gut microbiome undergoes changes in the first two to four years of life and then becomes more similar to the one found in adults (Chan 2013). Changes in diet and the body contribute to this shift in the microbial profile. Exposure to animals, such as pets, during these younger years is associated with a lower risk of allergies. Microbial patterns can also be altered by antibiotic use, microbes in the surroundings, and illness (Xu 2015; Francino 2015; Ojima 2016).
After this time of rapid diversification and expansion, the microbiome becomes relatively stable, and changes are more gradual (Knight 2017; Krajmalnik-Brown 2012). The relationship between aging and the microbiome is still being explored, but evidence suggests gut microbial profiles of the elderly are different from those of younger adults and are correlated with measures of frailty, poor diet, and health problems (Claesson 2011; Claesson 2012).
While a healthy microbiome is generally resistant to changes in its environment, and returns to the previous state afterwards (Lloyd-Price 2016), it is nonetheless susceptible to certain disrupting factors. Antibiotics, for example, can have dramatic effects on the microbiome, and the ability to recover to the pre-antibiotic microbial balance differs between individuals. Changes in eating habits can also cause profound and rapid alteration in the microbiome that can be brief or persistent. Hormonal cycles, travel, illness, and aging are other examples of factors that shape the microbiome (Krajmalnik-Brown 2012; D'Argenio 2015; Xu 2015; Zapata 2015).
5 Dysbiosis
A shift in the microbial balance away from a pattern associated with health is known as dysbiosis (Wischmeyer 2016; Lloyd-Price 2016; Chan 2013). A healthy microbiome is highly diverse and characteristically resilient to physiological stress; on the other hand, a dysbiotic microbiome is generally marked by smaller numbers of beneficial species, the presence of more species with disease-causing potential, and lower species diversity (Chan 2013).
Dysbiosis in the gut increases the risk of immune perturbations both inside and outside of the digestive tract (Brown 2017). In addition, gut dysbiosis has been associated with a growing list of chronic diseases and conditions that includes—but is not limited to—the following (Lloyd-Price 2016; Wang, Du 2017; Morris 2016; Potgieter 2015; Knight 2017; Strati 2017; Marasco 2016; Chen 2018; Distrutti 2016; Flowers 2015):
- allergies
- asthma
- autism
- autism spectrum disorders
- autoimmune diseases
- cancer
- cardiovascular disease
- celiac disease
- chronic fatigue syndrome
- depression
- type 1 and type 2 diabetes
- inflammatory bowel disease
- irritable bowel syndrome
- multiple sclerosis
- neurological diseases
- obesity
- osteoporosis
- cognitive and emotional health
Dysbiosis, Leaky Gut, and Systemic Inflammation
Imbalanced intestinal microflora can trigger chronic mucosal inflammation, which increases intestinal permeability and creates a condition known as leaky gut (van der Meulen 2016; Morris 2016; Nagpal 2017). Intestinal permeability is controlled by interactions between proteins on the surfaces of adjacent cells lining the intestinal wall, which form structures known as tight junctions. Zonulin is a protein that modulates these tight junctions, and increased zonulin levels in the circulation are considered to indicate an impaired intestinal barrier (Ohlsson 2017; Fasano 2012; Moreno-Navarrete 2012).
A leaky gut allows the absorption of small food components and bacteria, which can then trigger systemic immune activation and inflammation (van der Meulen 2016). Gut microbes and microbial products and toxins can also cross a leaky gut (Slyepchenko 2017; van der Meulen 2016). This can lead to immune and inflammatory activation in distant tissues and organs, and is thought to be an important mechanism in the development of autoimmune diseases and diseases related to systemic inflammation (Morris 2016; Slyepchenko 2017; Nagpal 2017; van der Meulen 2016).
Small Intestinal Bacterial Overgrowth
While the colon (part of the large intestine) has a complex and diverse microbial community, comprising more than 70% of gut microbes (Ghoshal 2017), the healthy small intestine has a more limited microbiome (Ponziani 2016). Small intestinal bacterial overgrowth (SIBO) is a pattern of dysbiosis that is increasingly recognized as a cause of common digestive symptoms such as indigestion, bloating, flatulence, and disordered bowel function, and as a possible underlying feature of irritable bowel syndrome (Ghoshal 2017; Thompson 2016; Sachdev 2013). The most commonly studied antibiotic treatment for patients with SIBO is rifaximin (Xifaxan), a non-absorbable antibiotic. In addition, preliminary evidence suggests probiotic therapy, alone or in combination with rifaximin, may be helpful. Promising results have been reported from trials using probiotics containing Saccharomyces boulardii, or rifaximin together with Lactobacillus casei (Ghoshal 2017; Chedid 2014).
6 Diet and Lifestyle Strategies for Supporting a Healthy Microbiome
The link between the gut microbiome, diet, and overall health has become increasingly apparent in recent years (Amato 2015). The gut microbiome is in constant evolution in response to dietary and other stimuli. Consistently eating a healthy, fiber-rich diet is one of the most effective ways to support a healthy gut microbiome (Xu 2015; Vanamala 2015; Davenport 2017).
Western vs. High-Fiber Diet
A high-fat diet, especially one including large amounts of saturated fats, has been shown to induce gut dysbiosis, intestinal permeability, and inflammation (Araujo 2017; Yang 2017; Silva Figueiredo 2017). A diet high in complex carbohydrates and fiber, on the other hand, supports the growth of microbial populations that are efficient carbohydrate fermenters. These bacteria produce short-chain fatty acids and other fermentation products that support the health of the intestinal lining and have positive effects on immune and metabolic functions (Montemurno 2014; Yang 2017).
A Western-type diet in particular—high in animal proteins and fat and low in fiber—selects for bacteria that metabolize proteins efficiently and those that can best tolerate bile acids secreted in response to high-fat meals. Microbial metabolism of proteins and certain bile acids creates byproducts that appear to contribute to the development of diseases known to be closely connected to a Western diet, including colon cancer, cardiovascular disease, and metabolic disorders (Montemurno 2014; Ridlon 2014). Conversely, in one study, a Mediterranean-style diet (with 35% of calories from fat) and a low-fat diet (<30% total fat) each improved gut microbial communities in subjects with obesity and metabolic dysfunction after two years (Haro 2017).
Prebiotics are carbohydrates that are completely or partially indigestible and that promote the growth of beneficial bacterial populations. These include small carbohydrates called pectin-oligosaccharides, large and small fructose-based carbohydrates known as inulins, and resistant starch (Ferrario 2017; Kelly 2008). Legumes, fruits and vegetables, nuts, and whole grains are especially high in prebiotic fibers (Vinke 2017; Dahl 2015; Slavin 2013; Lamuel-Raventos 2017). These foods have long been recognized as the cornerstones of a healthy, high-fiber diet, including the Mediterranean diet, known to show benefits regarding a number of metabolic and inflammatory health problems (Fabbiano 2017; Montemurno 2014).
7 Novel And Emerging Therapies For Microbiome Health
Fecal Microbiota Transplantation
One of the most exciting areas of microbiome research has been fecal microbiota transplantation, which involves the transfer of a fecal suspension, containing gut microbes, from a healthy subject to an unhealthy subject for therapeutic purposes. The procedure has generally been performed through enema or a tube that is introduced into the stomach or duodenum. However, in a 2017 study on patients with recurring Clostridium difficile infections, an oral capsule of freeze-dried fecal microbes was comparably effective to delivery via colonoscopy in preventing recurrences (Gallo 2016; Kao 2017).
Fecal microbiota transplantation has become an important treatment option in recurrent intractable C. difficile infections, for which its efficacy is close to 90% (Liubakka 2016; Cammarota 2017; Matijasic 2016). Clinical research, though in its early days, suggests it may also have value in treating a number of inflammatory and metabolic conditions associated with dysbiosis, such as inflammatory bowel disease (Matijasic 2016), irritable bowel syndrome (Distrutti 2016), obesity and type 2 diabetes (Marotz 2016), and autism spectrum disorders (Kang, Adams 2017). Furthermore, fecal microbiota transplantation so far appears to have an excellent safety profile (Cammarota 2017).
Phage Therapy
Bacteriophages, or phages, are viruses that infect specific bacteria but do not infect human cells (Doss 2017). Phages, believed to be the most abundant organisms on earth, are an integral part of the human microbiome, where they are thought to manage bacterial communities by limiting target species (Mirzaei 2017; Keen 2015). Phage therapy is being explored as an alternative to antibiotic therapy for certain conditions and, potentially, for eliminating harmful bacteria from the body's microbial communities (McCarville 2016; Lin, Koskella 2017).
An abundant and diverse community of phages inhabits the oral cavity, and specific phages or their enzymes have shown promise for treating periodontal disease and preventing dental plaque (Szafranski 2017). Phages that target infection-causing gastrointestinal bacteria such as Vibrio cholerae (the cause of cholera) (Yen 2017), Helicobacter pylori (Wan 2011), C. difficile (Nale 2016), Shigella dysenteriae (Mai 2015), and some harmful Escherichia coli strains (Dalmasso 2016) have been identified and hold therapeutic potential. Older reports of phage therapy being used successfully to prevent and treat various other types of bacterial infections, including those of the skin, urinary tract, and eyes, have emerged and provide a basis for future research (Chanishvili 2012).
8 Introduction to Probiotics
Probiotics are living microorganisms that promote health when ingested in sufficient amounts (Shi 2016). Most probiotic supplements contain bacteria, and commonly include species from the Lactobacillus and Bifidobacterium genera (Ciorba 2012; Islam 2016; Saini 2010). Several species of fungal Saccharomyces are also used in probiotic supplements (Fakruddin 2017). Probiotic supplements are usually measured in colony forming units (CFUs), which represent the number of viable cells (Weese 2011; Wallace 2017).
Microorganisms are classified in a hierarchy of families, genera, species, and strains. The genus is indicated by an organism's first name; the species is its second name, and is followed in some cases by the strain designation (Khalighi 2016). For example, Lactobacillus acidophilus La-14 is a well-described probiotic (Stahl 2013) of the genus Lactobacillus, species acidophilus, and strain La-14 (Salvetti 2012).
A growing body of probiotic research is establishing the safety and therapeutic efficacy of many individual strains (Sanders 2010; Shi 2016).
9 Probiotics For Digestive Health
Probiotic therapy has been shown to be safe and effective in the prevention and treatment of a wide range of digestive ailments and is likely to become increasingly important as challenges related to antibiotic resistance grow (Pamer 2016; Wilkins 2017). Probiotics likely preserve digestive health through their anti-microbial, anti-inflammatory, and immune-modulating actions on the intestinal lining (Surendran Nair 2017; Wilkins 2017). In a randomized, double-blind, placebo-controlled clinical trial on elderly volunteers, a probiotic combination that contained Bifidobacterium bifidum BB-02, B. lactis BL-01, and oligofructose, led to beneficial changes on size and diversity of the gut bacterial populations, which are often affected during aging (Bartosch 2005).
Diarrhea
A systematic review and meta-analysis of clinical trials reported that Lactobacillus reuteri DSM 17938, at doses of 100–400 million CFUs per day for five to seven days, decreased the duration of diarrhea in children (Urbanska 2016). In another systematic review and meta-analysis, L. rhamnosus GG, in doses between 400 million and 120 billion CFUs per day, effectively prevented antibiotic-associated diarrhea in children and adults (Szajewska 2015b).
Traveler's diarrhea is a common form of acute infectious diarrhea that is estimated to affect 25% of travelers in their first two weeks abroad (Giddings 2016). Although trials have shown mixed results, supplements containing the probiotic yeast Saccharomyces boulardii (5‒10 billion CFUs per day) have demonstrated promising effects in preventing traveler's diarrhea (Giddings 2016; Ouwehand 2017). Other helpful probiotics include L. rhamnosus GG, L. acidophilus, and B. bifidum (Giddings 2016).
Antibiotic-associated Diarrhea
Several probiotic supplements have shown promise in preventing antibiotic-associated diarrhea. S. boulardii in particular was found to reduce the risk of antibiotic-associated diarrhea by 53% in a systematic review and meta-analysis of 21 randomized controlled trials with a total of 4,780 antibiotic-treated participants (Szajewska 2015b). In another systematic review and meta-analysis of 12 randomized controlled clinical trials with a total of 1,499 antibiotic-treated participants, L. rhamnosus GG reduced treatment-related diarrhea risk by 51% (Szajewska 2015a). The most recent systematic review and meta-analysis, with 17 randomized controlled trials and a combined total of 3,631 non-hospitalized antibiotic-treated patients, found that those receiving any probiotics were 51% less likely to develop antibiotic-associated diarrhea. In this analysis, supplements containing L. rhamnosus GG and S. boulardii in daily doses higher that 5 billion CFUs appeared to be more protective (Blaabjerg 2017).
Clostridium difficile Diarrhea
C. difficile is a spore-forming bacterium that can cause inflammation of the large intestine (colitis) and diarrhea in vulnerable individuals (Heinlen 2010). It is the most common cause of diarrhea acquired in hospitals (Ofosu 2016). The main risk factors for C. difficile infection include hospitalization or institutionalization, antibiotic use, gastrointestinal surgery or procedures, serious illness, compromised immune function, and older age. Proton pump inhibitors, a class of medications used to treat gastroesophageal reflux, gastritis, and peptic ulcer disease, also increase risk (CDC 2012; Khanna 2012). The incidence and severity of C. difficile infections is rising, with hospitalizations for the infection in the United States doubling between 2000 and 2010 (Lessa 2015; Lessa 2012). Antibiotics are the standard medical treatments for C. difficile diarrhea, and recurrence is common (Al-Jashaami 2016).
Probiotic supplements have been shown to be safe and effective in preventing C. difficile-associated diarrhea. One community hospital affected by a major outbreak of C. difficile infections reported a 73% reduction in cases and a 39% reduction in recurrences after implementing a policy of giving a probiotic supplement to all patients within 12 hours of beginning any antibiotic regime. The probiotic provided 50 billion CFUs per day of a combination of L. acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2. By continuing to include probiotic therapy in their prevention strategy, the hospital was able to maintain very low occurrence and recurrence rates of C. difficile infections for the 10 years preceding the publication of their report (Maziade 2015).
In a 2017 randomized controlled trial, a four-strain probiotic containing L. acidophilus NCFM, L. paracasei Lpc-37, B. lactis BI-04, and B. lactis Bi-07 was compared with placebo as an add-on to standard medical therapy in 33 participants with their first diagnosis of mild-to-moderate C. difficile infection. After 28 days, the duration of diarrhea was found to be shorter in those treated with the probiotic combination (Barker 2017).
A 2017 systematic review and meta-analysis that included data from 19 studies, with a combined total of 6,261 hospitalized adults receiving antibiotics, found that starting probiotic therapy within the first two days of antibiotic treatment led to a 68% reduction in C. difficile infection risk, and efficacy was diminished with every day of delay in starting the probiotic therapy (Shen 2017). Other meta-analyses have drawn similar conclusions: probiotic supplements reduced antibiotic-associated C. difficile diarrhea risk by approximately 60% in adults and children, both within and outside of hospital settings, with a high degree of safety. Hospitalized patients were more likely to benefit. While most trials used supplements with one or more Lactobacillus species, Lactobacillus plus Bifidobacterium species, or Saccharomyces species, the preventive effect did not appear to be species-specific (Goldenberg 2017; Lau, Chamberlain 2016).
Constipation
The gut microbiome is one of the important factors that regulates the movement of food through the digestive system (gut motility). Beneficial intestinal microbes help support normal gut motility by inhibiting mucosal inflammation, interacting with the nervous system, modulating bile acid metabolism, and altering the intestinal environment through the production of short-chain fatty acids and other compounds (Dimidi 2017; Zhao 2016).
Numerous clinical trials have demonstrated the benefits of probiotic supplements in stimulating gut motility and relieving constipation. A review article reported on five randomized controlled trials using different strains of B. lactis, two trials using L. casei Shirota, and one trial each using L. reuteri DSM 17938 and L. paracasei IMPC 2.1 to manage chronic constipation. The results from all trials were positive, and all but one showed statistically significant improvements. Various benefits were observed, including improved stool consistency and frequency, reduced transit time, and decreased severity of constipation (Zhao 2016). A systematic review and meta-analysis of 14 studies with a combined total of 1,182 adults with constipation found significant benefits with B. lactis, but not L. casei Shirota (Dimidi 2014). The combination of L. plantarum LP01 and B. breve BR03, as well as the single probiotic B. animalis subspecies lactis BS01, have demonstrated positive effects in relieving constipation (Del Piano 2010). Other studies suggest supplements with B. lactis strains shorten transit time, especially in elderly patients with constipation (Martinez-Martinez 2017; Miller 2016; Miller 2013).
Two interesting placebo-controlled trials looked at the effects of the probiotic B. longum BB536, at a dose of 25 or 50 billion CFUs per day for 16 weeks, on bowel function in elderly patients receiving nutrition via a tube to the stomach or small intestine. When only subjects with low bowel movement frequency were considered, a significant increase in bowel movement frequency was noted, and when only those with high bowel movement frequency were considered, a significant decrease in bowel movement frequency was noted. These findings suggest a modulatory effect of this B. longum strain on bowel function (Kondo 2013).
For more information about constipation, please see the Constipation health protocol.
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a chronic digestive disorder characterized by abdominal pain, distension, and altered bowel function, and is frequently associated with depression, anxiety, and high levels of stress. Although there is no clear cause, there is mounting evidence that dysbiosis might be involved, contributing to both digestive and mood symptoms as a result of changes along the gut-brain axis that involve low-grade inflammation, increased gut permeability, and immunological perturbations (Bennet 2015; Quigley 2018; Sinagra 2017; Staudacher 2016; Canavan 2014; Ohman 2010). Although there have been mixed findings, much of the evidence indicates that probiotics reduce symptoms and improve quality of life in IBS sufferers (Didari 2015; Tiequn 2015; Zhang 2016; Sinagra 2017).
A 2016 meta-analysis pooled the results from 21 randomized controlled trials looking at the impact of probiotic therapy in IBS patients. Some of the included trials used probiotic combinations with various species of Lactobacillus and Bifidobacterium, and others used single species. Probiotics effectively improved overall symptoms and quality of life in IBS patients, but were not consistently helpful in relieving specific symptoms. Dosage, which ranged from 60 million to 450 billion CFUs per day, did not appear to influence efficacy, and single-species probiotics appeared to have an advantage over multi-species probiotics in helping improve overall symptoms and quality of life (Zhang 2016). A 2014 systematic review and meta-analysis that included 43 randomized controlled trials also identified an overall positive impact of probiotics in relieving IBS symptoms (Ford 2014).
Probiotics containing only Lactobacillus species were found in another meta-analysis to be almost 18 times more likely to be beneficial than placebo in adults with IBS (Tiequn 2015). Two studies examining the effects of S. cerevisiae CNCM I-3856 on IBS symptoms found a reduction in abdominal pain in those treated with the probiotic relative to placebo (Cayzeele-Decherf 2017). Several placebo-controlled trials have noted improvement in IBS symptoms in patients treated with probiotics containing strains of Bacillus coagulans (Majeed 2016; Hun 2009; Dolin 2009).
In a placebo-controlled crossover trial, 20 healthy adult subjects were treated for two weeks with the L. helveticus strain LAFTI® L10. Study participants reported a significant improvement in aggregate GI symptoms resembling those associated with IBS, including diarrhea, constipation, flatulence, and bloating (Welin 2005).
For more information about IBS, please see the Irritable Bowel Syndrome health protocol.
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) encompasses two major autoimmune conditions, ulcerative colitis and Crohn's disease. The gut microbial communities in IBD patients have been found to fluctuate more than those of healthy individuals, and dysbiosis together with the associated immune dysfunction and inflammation have been implicated both in the onset of this condition and in its flare-ups (Halfvarson 2017; Fakhoury 2014; DeGruttola 2016; Lane 2017).
Results from several meta-analyses of randomized controlled trials indicate that probiotic supplements in general may safely help patients with ulcerative colitis achieve and maintain remission (Derwa 2017; Ganji-Arjenaki 2018; Fujiya 2014). Although a significant benefit for probiotic use in general has not been demonstrated in Crohn's disease patients, S. boulardii, when added to standard medical treatment for Crohn's disease, was found to reduce some of the symptoms (Plein 1993) and clinical relapses (Guslandi 2000) in two small randomized controlled trials.
Two placebo-controlled trials evaluated the effect of a commercial combination probiotic called VSL#3 in patients with active mild-to-moderate ulcerative colitis being treated with standard therapies. This product contains four Lactobacillus species (L. acidophilus, L. plantarum, L. casei, and L. bulgaricus), three Bifidobacterium species (B. longum, B. breve, and B. infantis), and one Streptococcus species (S. thermophilus) (Matijasic 2016). In both trials, higher rates of clinical response and remission were seen in those treated with VSL#3 compared with placebo (Sood 2009; Tursi 2010). A similar but uncontrolled trial also noted improved response and remission rates with the addition of VSL#3 to standard treatments (Bibiloni 2005). In another clinical trial of patients with moderate-to-severe ulcerative colitis, a combination probiotic containing L. acidophilus, L. salivarius, and B. bifidus added to mesalazine, an anti-inflammatory drug used to treat IBD, showed better improvement than patients receiving only mesalazine (Palumbo 2016).
Several single-species probiotic supplements have also been useful in managing ulcerative colitis. In a placebo-controlled clinical trial, B. longum BB536, at a dose of 200–300 billion CFUs per day, increased the rate of clinical remission and improved the appearance of the colon tissue (Tamaki 2016), and Escherichia coli Nissle 1917 was as effective as mesalazine for preventing relapses in several trials of patients receiving standard medical therapy (Kruis 1997; Rembacken 1999; Kruis 2004); however, it may not be helpful for inducing remission in active cases (Petersen 2014). A clinical trial that enrolled patients with ulcerative colitis found that the probiotic L. rhamnosus GG was as effective as mesalazine or the combination of the two in preventing relapses (Zocco 2006). In addition, findings from a pilot trial suggest S. boulardii, when added to mesalazine, may help induce clinical remission in ulcerative colitis patients who experience mild-to-moderate flare-ups (Guslandi 2003).
For more information about Crohn's disease and ulcerative colitis, please see the Inflammatory Bowel Disease health protocol.
Gastroesophageal Reflux Disease
The esophagus is home to a rich microbial ecosystem and esophageal dysbiosis has been identified in those with gastroesophageal reflux disease (GERD). It is now thought that this dysbiosis may play a role in the progression of GERD to precancerous and cancerous conditions of the esophagus (Yang 2014; Di Pilato 2016). Although it is not clear yet how esophageal dysbiosis might be addressed, it is clear that the usual treatment for GERD—typically proton pump inhibitor (PPI) medications that reduce stomach acid secretion—can lead to a host of health problems in the long term, including intestinal dysbiosis and small intestinal bacterial overgrowth (SIBO) (Del Piano 2012; Lombardo 2010; Fujimori 2015).
Several studies have looked at the impact of using probiotic supplements along with PPIs. In a randomized clinical trial, children with GERD were treated with a PPI plus either a probiotic containing 100 million CFUs of L. reuteri DSM 17938 per day or a placebo. After 12 weeks, SIBO was detected in 6.2% of those who received the probiotic and 56.2% of those who received the placebo (Belei 2018). A similar trial in adults with GERD found that a probiotic containing L. paracasei F19 prevented treatment-related bloating, flatulence, and abdominal pain (Compare 2015).
For more information about GERD, please see the Gastroesophageal Reflux Disease health protocol.
Gastritis and Peptic Ulcer Disease
The most common causes of gastritis and peptic ulcer disease (PUD) are infection with the bacterium Helicobacter pylori and the use of non-steroidal anti-inflammatory medications. Other causes and contributors include certain other medications, smoking, excessive alcohol intake, older age, and stress (Mayo Clinic 2017a; Mayo Clinic 2017b; Fashner 2015; Nordenstedt 2013).
Over half of people worldwide carry gastric H. pylori (Kusters 2006). Most have no symptoms, but for others, H. pylori can trigger changes to the gastric and upper intestinal mucosa, increasing risk of gastric or duodenal ulcer and gastric cancer (Kafshdooz 2017; Ruggiero 2014). When treatment is indicated, standard medical therapy is the use of so-called triple or quadruple therapy—a proton pump inhibitor, with or without a bismuth salt, and two to three antibiotics. The development of H. pylori resistance to many commonly used antibiotics has decreased the efficacy of this approach in recent years (Goderska 2018; Mascellino 2017).
Probiotics may be helpful in gastritis and PUD due to their ability to reduce mucosal inflammation, decrease the binding of H. pylori, and promote a strong immune response against the bacteria. In addition, certain probiotic strains may produce antibacterial molecules that inhibit H. pylori (Goderska 2018; Homan 2015). A number of clinical trials using various probiotics and combinations with no other treatment have shown, while they do not appear to eradicate H. pylori, they can reduce the number of H. pylori organisms (Homan 2015).
A 2016 meta-analysis that pooled data from 30 randomized controlled trials tested probiotic therapy as an add-on to standard therapy for H. pylori infection. The authors concluded probiotics from the Lactobacillus, Bifidobacterium, or Saccharomyces species improved eradication rates and reduced antibiotic-related side effects (Lau, Ward 2016). Other meta-analyses have also found that a range of single- and multi-strain probiotics can improve eradication rates and treatment tolerance (Lu, Yu 2016; McFarland 2016; Zhang 2015).
Probiotics may also be useful in treating gastritis and PUD patients without H. pylori infection. Animal research suggests probiotic therapy may reduce the ulcer-promoting effects of stress (Konturek 2009) and non-steroidal anti-inflammatory drugs such as aspirin and ibuprofen (Senol 2011; Girard 2010). Clinical trials are needed to confirm these benefits in humans and determine the strains and doses most likely to be effective.
For more information about H. pylori, gastritis, and PUD, please see the Peptic Ulcers health protocol.
Dyspepsia and Bloating
Functional dyspepsia is a condition characterized by fullness and stomach pain, especially after eating, for which a cause cannot be identified. Gastric fluid testing has shown that patients with dyspepsia have distinctly different stomach microorganisms than healthy individuals (Igarashi 2017).
In a randomized controlled trial, patients with functional dyspepsia, and not infected with H. pylori, received either yogurt fortified with L. gasseri OLL2716 or an unfortified placebo yogurt. Major symptoms of dyspepsia were eliminated in 35.3% of those receiving the L. gasseri-enriched yogurt and in 17.3% of those receiving the placebo yogurt (Ohtsu 2017). Other researchers have noted that L. gasseri OLL2716 supplementation reversed dysbiosis of gastric fluid, and this was correlated with recovery from dyspepsia (Igarashi 2017; Nakae 2016).
Bloating is a common symptom of digestive disorders but can also occur persistently without a known cause. Probiotic strains such as B. bifidum MIMBb75 and L. plantarum 299v have been shown to relieve IBS-related bloating and distension in randomized controlled trials (Iovino 2014). In people with abdominal bloating without a clear cause, Lactobacillus GG has been reported to be more effective than placebo for reducing severity of bloating (Di Stefano 2004). In addition, Bacillus coagulans GBI-30, 6086 reduced gastrointestinal symptoms in adults with gas-related symptoms but no known digestive disorders (Kalman 2009).
Lacticaseibacillus rhamonosus IMC 501 and Lacticaseibacillus paracasei IMC 502
Lacticaseibacillus rhamonosus IMC 501 and Lacticaseibacillus paracasei IMC 502 comprise a branded probiotic blend called Synbio. Together, these probiotics have been found to reduce inflammatory markers associated with aging, improve nutritional status in elderly individuals, and alter the gut microbiome to more closely resemble that of younger individuals, which has been correlated with longevity (Pang 2023; Salvesi 2022; Salvesi 2023). In one clinical study, which was split into two separate scientific articles, 97 healthy individuals over 65 years of age (averaged 81 years old) were randomized to receive 5 billion CFUs of the probiotic blend or a placebo food product once daily for 24 weeks. The probiotic blend positively influenced microbiome diversity and significantly increased butyrate and total short-chain fatty acids compared with baseline (Salvesi 2022). An improvement in nutritional status was also observed, where the probiotic group displayed a significant reduction in the number of participants at risk of malnutrition and increased the number of participants with normal nutritional status by 28% (Salvesi 2023). In addition, the probiotic blend significantly reduced levels of the proinflammatory biomarkers interleukin (IL)-6, IL-8, TNF-alpha, and high sensitivity C-reactive protein compared with baseline (Salvesi 2022; Salvesi 2023).
Heat-treated Lactobacillus paracasei D3-5
A postbiotic is a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host. Postbiotic (heat-treated) Lactobacillus paracasei D3-5—a bacterial strain isolated from human infants—was found to extend lifespan and preserve physical function in Caenorhabditis elegans (roundworms) and ameliorate high-fat diet-induced metabolic dysfunctions in old mice (Wang 2020). A cocktail containing 10 strains of probiotics, including live L. paracasei D3-5, was also shown to positively modulate the gut microbiome and increase the production of short-chain fatty acids, particularly butyric acid and propionic acid, in mice (Nagpal 2018). In a preliminary open-label human trial, 13 subjects aged 50 years or older took four capsules of heat-treated L. paracasei D3-5 (dosage not specified) for 30 days. L. paracasei D3-5 was safe and well tolerated, and subjects reported improvements in emotional well-being and gastrointestinal symptoms. As this study was a pilot study intended to assess safety and tolerability, results regarding the improvements in gastrointestinal symptoms and emotional wellbeing should be regarded as hypothesis generating until larger trials are completed (Lloyd 2024).
10 Probiotics For Cardiovascular Health
While the cardiovascular system does not appear to have a microbial community of its own, much attention has recently been drawn to the role of the gut microbiome on cardiovascular health and disease. Research has linked gut dysbiosis to atherosclerosis and hypertension (Lau 2017), and some evidence points to a role of gut microbiota in heart failure (Nagatomo 2015). Clinical trials have shown that probiotic and prebiotic supplements can support weight management and improve other metabolic and inflammatory markers correlated with cardiovascular health (He 2017; Upadrasta 2016).
Probiotics and Blood Pressure
Findings from numerous laboratory and animal studies suggest probiotic bacteria and their fermentation byproducts may have beneficial effects in people with high blood pressure. Possible mechanisms for this effect include reducing oxidative stress and systemic inflammation, improving cholesterol metabolism and dietary calcium absorption, and inhibiting the action of a vasoconstrictive enzyme called angiotensin-converting enzyme (ACE) (Daliri 2017; Upadrasta 2016). In a five-year observational study, intake of dairy products fermented with Lactobacillus casei strain Shirota three or more times per week was associated with a reduced risk of developing hypertension (Aoyagi 2017). In a three-week pilot trial in 40 subjects with obesity and hypertension, blood pressures and body mass indices were reduced in those who received a cheese fermented with the probiotic L. plantarum TENSIA compared to cheese without the probiotic bacteria (Sharafedtinov 2013).
Probiotics and Cholesterol Metabolism
A meta-analysis of 13 trials with a combined total of 485 participants found that probiotic therapy in general can effectively reduce total and LDL-cholesterol levels. In this analysis, L. acidophilus strains were found to have strong lipid-reducing effects (Shimizu 2015). Other Lactobacillus bacteria, such as L. reuteri and L. plantarum, have also been shown to reduce total cholesterol and LDL-cholesterol levels in clinical trials (Lau 2017; Shimizu 2015).
In a randomized trial in 127 subjects with high cholesterol levels, taking capsules with 2.9 billion CFUs of the probiotic L. reuteri NCIMB 30242 twice daily led to a 9.1% decrease in total cholesterol levels, an 11.6% decrease in LDL-cholesterol levels, and a 13.4% improvement in LDL- to HDL-cholesterol ratio compared with placebo after nine weeks. The participants receiving L. reuteri NCIMB 30242 also had lower levels of the inflammatory markers C-reactive protein and fibrinogen at the end of the trial (Jones, Martoni, Prakash 2012). In addition, a second analysis of the results from this trial showed that vitamin D levels increased in those receiving probiotic therapy (Jones 2013). Eating yogurt containing L. reuteri NCIMB 30242 for six weeks was also found to lower elevated total and LDL-cholesterol levels in a placebo-controlled trial with 114 participants (Jones, Martoni, Parent 2012).
Lactobacillus plantarum ECGC 13110402 is a probiotic strain with high bile salt hydrolase activity, which modulates cholesterol production in the body. In a randomized, double-blind, placebo-controlled trial including 16 individuals with hypercholesterolemia between the ages of 35-70 years, participants were given 4 billion CFUs of L. plantarum ECGC 13110402 or placebo once daily after lunch for six weeks. Treatment resulted in significantly improved lipid parameters compared with placebo, including a 34.6% reduction of total cholesterol, 28.4% reduction of LDL-cholesterol, 17.6% reduction of non–HDL-cholesterol, and 28.6% reduction of apolipoprotein B (ApoB) (Keleszade 2022). Another double-blind placebo-controlled trial randomized 46 individuals with normal to mildly high cholesterol levels to receive 2 billion CFUs of L. plantarum ECGC 13110402 or placebo twice daily, before breakfast and dinner, for 12 weeks. L. plantarum ECGC 13110402 resulted in significant reductions from baseline in LDL-cholesterol levels in individuals with total cholesterol levels below 193 mg/dL at 12 weeks, total cholesterol in individuals with baseline levels above 232 mg/dL at six weeks, and systolic blood pressure across all treated participants between a six- to 12-week period. Participants over 60 years old also exhibited a significant decrease in triglycerides and an increase in HDL-cholesterol in the six- to 12-week period (Costabile 2017).
11 Probiotics For Oral Health
The oral microbiome, a very important component of the human microbiome, contains hundreds to thousands of diverse species that are present in various places, such as on the teeth, gums, cheeks, palate, lips, and tonsils (Arweiler 2016; Dewhirst 2010). Dysbiosis within the oral cavity plays a role in the development of dental cavities, gingivitis, and periodontal disease. A growing body of evidence indicates that restoring healthy populations of beneficial oral bacteria through probiotic therapy can have an important role in preventing and treating these conditions (Gupta 2017; Rosier 2017; Haukioja 2010; Allaker 2017).
Periodontal Disease
Dysbiosis of the oral microbiome is associated with deepening of the periodontal pockets and periodontitis, a chronic inflammatory condition that can damage the soft tissues and bone and, eventually, lead to tooth loss (Gupta 2017; Meuric 2017; Jiao 2014; Deng 2018). Periodontitis also increases the risk of certain systemic diseases including vascular disease, diabetes, and some forms of cancer (Deng 2018). Several studies found that probiotics can reduce gum bleeding and periodontal pocket depth in patients with periodontal disease, and decrease alveolar bone loss in animal models (Allaker 2017; Maekawa 2014; Gatej 2018; Haukioja 2010; Krasse 2006). The benefits of probiotics may be related to their ability to compete with harmful microbes for nutrients, produce molecules that inhibit harmful bacteria, and cause beneficial changes in immune responses (Allaker 2017).
In a randomized controlled trial in patients with periodontal disease, taking a daily capsule containing 10 mg of heat-killed Lactobacillus plantarum L-137 enhanced the effectiveness of scaling and root planing at reducing periodontal pocket depth (Iwasaki 2016). Although the bacteria in this supplement were no longer viable, other studies have shown that the heat-killed form of L. plantarum L-137 has immune-modulating effects (Fujiki 2012), which may have been responsible for its positive impact on periodontal health.
Another promising supplement for periodontal disease is the probiotic strain L. reuteri (Martin-Cabezas 2016; Kumar 2017). Several clinical trials have found that lozenges made with this probiotic species, when added to regular scaling and root planing, reduce plaque formation, gingival bleeding, and pocket depth, and increase the attachment of the gums to the tooth root in patients with chronic periodontal disease (Ince 2015; Teughels 2013; Vivekananda 2010; Szkaradkiewicz 2014; Tekce 2015). In addition, a 2018 randomized controlled trial found that L. reuteri lozenges showed benefits in patients with mucositis and peri-implantitis, two inflammatory conditions that may involve the tissues surrounding a dental implant (Galofre 2018).
The probiotic bacterium L. salivarius WB21 has been found to decrease levels of harmful bacteria in dental plaque (Mayanagi 2009), improve gingival health (Iwamoto 2010), and reduce the risk of periodontal disease (Shimauchi 2008). In other clinical trials, a probiotic mouth rinse containing L. salivarius NK02 improved the effectiveness of scaling and root planing for periodontal disease (Sajedinejad 2017), and a lozenge containing L. rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12 decreased plaque development and enhanced gum health in healthy subjects (Toiviainen 2015).
Cavities
Streptococcus salivarius M18 is a probiotic organism that has demonstrated antibacterial effects against S. mutans, an oral bacterium implicated in the formation of dental cavities. It has also been shown to produce enzymes that decrease dental plaque accumulation and acidification (Burton 2013). In a randomized controlled trial, risk factors for cavity development improved after 90 days in children treated with a daily lozenge providing at least 1 billion CFUs of S. salivarius M18 (Di Pierro, Zanvit 2015). In another placebo-controlled trial, three months of treatment with oral S. salivarius M18, at a daily dose of 3.6 billion CFUs, led to greater improvements in plaque scores compared with placebo in children with dental cavities. In addition, bacterial analysis showed that the presence of S. mutans was diminished in children with the highest apparent oral colonization levels of the probiotic strain (Burton 2013).
Various Lactobacillus species have also been found in laboratory studies to inhibit S. mutans (Lin, Chen 2017; Wasfi 2018). Similar benefits were found in clinical studies. In a randomized controlled trial, probiotic mouth rinses containing either Bacillus coagulans or a mixture of L. acidophilus, B. longum, B. lactis, and B. bifidum, were found to both reduce plaque and improve gum health better than a placebo in children (Yousuf 2017). In another randomized controlled trial, daily use of a chewable tablet with three probiotic Streptococcus species resulted in a lower risk of cavities compared with placebo in healthy two- to three-year-old children (Hedayati-Hajikand 2015).
A broad body of research shows that xylitol, a non-digestible sugar alcohol, has prebiotic effects not only in the gut but also in the mouth (Makinen 2010; Nayak 2014). Xylitol, as a supplement and as an ingredient in toothpastes, mouth rinses, chewing gums, and candies, has been found to reduce levels of cavity-causing bacteria in the saliva and plaque, decrease plaque formation, and inhibit tooth decay (Nayak 2014). A 2017 systematic review and meta-analysis concluded that xylitol is effective for cavity prevention (Janakiram 2017). Another review found that toothpastes with both xylitol and fluoride may protect against cavities more effectively than fluoride-only toothpastes in children (Riley 2015).
For more information about periodontal disease and dental cavities, please see the Oral Health protocol.
12 Probiotics And Skin Health
The skin is home to a large and diverse microbiome that impacts skin health and has body-wide effects on health. The skin microbiome varies in the same individual across body site and also between individuals. It is shaped by both external and internal factors (Prescott 2017; Barnard 2017; SanMiguel 2015; Grice 2011). Yet, even with its exposure to constantly changing conditions, the skin microbiome appears to be largely stable within individuals over time (Oh 2016). Skin dysbiosis has been noted in an array of skin conditions, including atopic dermatitis (eczema), acne, seborrhea, psoriasis, rosacea, and dandruff (Barnard 2017; Abdallah 2017; Paulino 2017).
Probiotic bacteria can have local and systemic effects that support skin barrier function, regulate immune activity, and control microbial populations (Al-Ghazzewi 2014; Wong 2013; Lew 2013). Probiotic supplements may be beneficial in several infectious and inflammatory skin disorders, as well as sun-induced and trauma-related skin damage (Notay 2017; Roudsari 2015; Kober 2015). Most clinical trials have used oral probiotics, but emerging research suggests the topical application of probiotic bacteria may also be useful for promoting skin healing (Zoccali 2016; Lopes 2017).
Acne
Oral probiotics such as Lactobacillus acidophilus and Bifidobacterium bifidum have shown positive effects in people with acne in early clinical research (Bowe 2014). In a controlled trial in 20 subjects with acne, supplementing with L. rhamnosus SP1 at a dose of 3 billion CFUs per day led to greater improvement in skin condition than placebo after 12 weeks (Fabbrocini 2016). Other evidence suggests a combination of oral probiotics and antibiotics may be more effective than either alone for adults with mild-to-moderate acne (Jung 2013). Additional clinical studies found that a 5% L. plantarum topical solution, but not a 1% solution, reduced acne lesion size and redness (Muizzuddin 2012).
The probiotic strain Enterococcus faecalis SL-5, applied as a lotion, demonstrated antibacterial activity against bacteria associated with acne and improved the appearance of acne lesions (Kang 2009). Other probiotic bacteria, including L. reuteri, B. longum, B. adolescentis, and Streptococcus salivarius, have demonstrated similar antibacterial effects and have potential as topical agents for treating acne (Bowe 2014).
Atopic Dermatitis
Factors that shape the intestinal microbiome early in life can influence risk of childhood allergic conditions, such as atopic dermatitis, a skin disorder commonly referred to as eczema (Zheng 2016; Marrs 2016). Atopic dermatitis is associated with impaired skin barrier function, inflammation, and loss of diversity in the skin microbiome (Wollina 2017).
Probiotic supplements may help prevent atopic dermatitis, especially if used during pregnancy and given in the first years of life (Zukiewicz-Sobczak 2014; Kim 2013; Elazab 2013; Pelucchi 2012). In a systematic review and meta-analysis on preventing eczema in infants and children, most studies that were examined used supplements with Lactobacillus species, Bifidobacterium species, or both, and both strains showed protective effects (Mansfield 2014).
According to recent meta-analyses, probiotics may be beneficial for treating atopic dermatitis in adults and in children older than one year (Kim 2014; Huang 2017). Probiotics with Bifidobacterium species alone appear to be less effective than those with mixed species and with Lactobacillus species (Kim 2014; Chang 2016). Three studies showed that L. acidophilus L-92 ameliorated atopic dermatitis in three human studies (Torii 2011; Yamamoto 2016; Inoue 2014).
For more information about acne, atopic dermatitis, and other skin conditions, please see the Skin Disorders health protocol.
13 Probiotics For Vaginal And Urinary Health
Lactobacillus bacteria have long been considered a key component of a healthy vaginal ecosystem. By maintaining an acidic pH (around 3.5‒4.5), competing for energy sources and binding sites on the vaginal wall, and producing antimicrobial chemicals, Lactobacillus species in particular help control populations of potentially harmful microorganisms that may also be present in the vaginal microbiome (Huang, Fettweis 2014; Vaneechoutte 2017). One of the most common vaginal infections worldwide is bacterial vaginosis, which is often referred to not as an infection but as dysbiosis, because the mechanisms underlying the condition are not well understood, and several hypotheses were proposed (Muzny 2016). Vaginal dysbiosis is characterized by a vaginal microbial community that contains fewer Lactobacilli and the overgrowth of other, potentially harmful species, and has been associated with health problems, including increased risk of acquiring and transmitting sexually transmitted infections and preterm birth (van de Wijgert 2017; Mitra 2016). In addition, vaginal dysbiosis has been associated with an increased risk of urinary tract infections (Kirjavainen 2009).
Pilot trials in healthy women indicate that both oral and vaginal administration of Lactobacillus probiotic combinations with species such as L. casei rhamnosus, L. rhamnosus, L. paracasei, L. fermentum, L. plantarum, and L. gasseri may lead to long-term vaginal colonization by Lactobacillus bacteria (Verdenelli 2016; Bohbot 2012; Strus 2012). A 2016 systematic review of 20 studies indicate probiotics containing at least one Lactobacillus species can be an important part of prevention and treatment for bacterial vaginosis and urinary tract infections in women (Hanson 2016). More recent findings even suggest long-term Lactobacillus supplementation may help to clear infection with human papilloma virus (HPV), some strains of which cause cancerous changes in the cervix (Palma 2018).
Bacterial Vaginosis
Bacterial vaginosis is typically treated with antimicrobials, but the recurrence rate is high, at 30%–40%. Adding oral or vaginal Lactobacillus-based probiotic therapy to standard care has been shown to prevent recurrences and improve the success of treatment (Parma 2014; Kumar 2011; Homayouni 2014; Cribby 2008). A 2018 clinical trial in 34 women with bacterial vaginosis compared probiotic therapy with placebo as follow-up treatment after antibiotics. Women in the probiotic group ate a fortified yogurt providing L. crispatus LbV 88, L. gasseri LbV 150N, L. jensenii LbV 116, and L. rhamnosus LbV 96, each at 1.25 billion CFUs per day, and those in the placebo group consumed chemically acidified milk. After four weeks, none of the 17 women in the probiotic group and six of the 17 women in the placebo group had bacterial vaginosis (Laue 2018). A meta-analysis of 12 randomized clinical trials found that including probiotics in bacterial vaginosis treatments may increase cure rates by 53% or more (Huang, Song 2014).
Probiotics are also effective as a stand-alone treatment for bacterial vaginosis in some women. In 544 otherwise healthy women diagnosed with bacterial vaginosis, treatment with an oral probiotic providing 2 billion CFUs of L. rhamnosus GR-1 and L. reuteri RC-14 per day was more effective than placebo for achieving and maintaining a healthy vaginal microbial balance. In this study, almost 62% of probiotic-treated women and nearly 27% of those receiving placebo no longer had bacterial vaginosis after six weeks of treatment, and a normal balance of vaginal microflora was still present in about 51% of women in the probiotic group and about 21% of those in the placebo group six weeks after the end of the treatment (Vujic 2013).
In another clinical study, 34 pre-menopausal women with bacterial vaginosis used slow-release vaginal probiotic tablets with L. fermentum LF15 and L. plantarum LP01 or a placebo nightly for seven nights, then once every three nights for three weeks, and finally once weekly for four more weeks. At the end of the eight weeks, 20 of 24 women in the probiotic group (83%) no longer had bacterial vaginosis, and none of those in the placebo group experienced significant improvement (Vicariotto 2014).
Vulvovaginal Candidiasis
Probiotic Lactobacillus bacteria have been shown to exert antifungal effects and regulate the immune response against Candida yeast species in laboratory research (Chew 2015; Deidda 2016; Wagner 2012). Certain probiotic strains, such as L. rhamnosus GR-1 and L. reuteri RC-14, may alter the metabolic activity of yeast cells and even affect the function of some genes that contribute to antifungal medication resistance (Kohler 2012).
Findings from several studies demonstrate that oral and vaginal Lactobacillus probiotics enhance the effectiveness of standard antifungal treatment in women with vulvovaginal candidiasis. In a randomized placebo-controlled trial, 55 women who underwent standard medical treatment for vulvovaginal candidiasis received additional treatment with oral probiotics, providing 2 billion CFUs of L. rhamnosus GR-1 and L. reuteri RC-14, or placebo, daily for four weeks. At the end of the trial, the probiotic group had fewer symptoms and a lower presence of vaginal yeast compared with the placebo group (Martinez 2009).
In a randomized open-label study, 207 subjects with vaginal candidiasis were treated with standard antifungal medication alone and 209 were treated with medication plus 10 applications of a vaginal probiotic containing L. acidophilus, L. rhamnosus, Streptococcus thermophilus, and L. delbrueckii subspecies bulgaricus, beginning five days after completing medical treatment. At the end of the study, about 69% of those given probiotics did not have clinical complaints compared to about 20% of those treated with antifungal medication alone (Kovachev 2015). Vaginal probiotics containing single strains of L. plantarum have also been shown to decrease recurrence rates after conventional antifungal treatments (Palacios 2016; De Seta 2014).
A particular strain of L. plantarum prevalent in healthy vaginal microbiomes, L. plantarum P17630, has been shown to effectively adhere to vaginal epithelial cells and colonize the vaginal mucosa with regular use (Bonetti 2003; Montella 2013). In addition, colonization by this strain of L. plantarum interfered with the growth of some pathogenic organisms such as Candida albicans. In one clinical study, 93 women with a history of recurrent yeast infections (ie, vulvovaginal candidiasis) were given 5 billion CFUs L. plantarum P17630 orally for three 15-day cycles separated by 15-day wash out periods. By the end of the study, the probiotic improved Lactobacilli colonization on vaginal epithelial cells and was associated with improvement in redness, swelling, and discharge compared with placebo (Vladareanu 2018). A retrospective study of 89 women with yeast infections found those given L. plantarum P17630 (more than 100 million CFUs/day vaginally) once daily for six days and then once a week for an additional four weeks following treatment with clotrimazole (Lotrimin) experienced better subjective resolution of symptoms (e.g., burning or itching) compared with those given clotrimazole alone. Those given the probiotic also had an increase in Lactobacilli colonization (De Sata 2014).
For more information about vulvovaginal candidiasis, please see the Fungal Infections health protocol.
Urinary Tract Infection
Lactobacillus may inhibit colonization by infection-causing microbes near the urinary opening and enhance immune function, reducing the risk of urinary tract infection. Probiotic therapy with Lactobacillus species may be effective for preventing urinary tract infection (Foxman 2013).
In a one-year clinical trial, 252 postmenopausal women with recurrent urinary tract infections were treated preventively with either a combination oral probiotic, providing 2 billion CFUs of L. rhamnosus GR-1 and L. reuteri RC-14 per day, or trimethoprim-sulfamethoxazole, a combination of two antimicrobials. The mean number of symptomatic urinary tract infections per year dropped in both groups, but the rate of antimicrobial resistance increased substantially in the antimicrobial-treated group. The authors noted that even though the probiotic combination did not decrease the mean number of urinary tract infections more than the antimicrobial treatment, the substantially lower antimicrobial resistance in the probiotic group would make them an acceptable alternative for preventing urinary tract infections (Beerepoot 2012).
L. crispatus is one of the several beneficial Lactobacillus species found in healthy vaginal microbiomes (Lepargneur 2016; Abdelmaksoud 2016). In a pilot study, nine women with recurrent urinary tract infections were treated with a vaginal suppository containing 100 million CFUs of L. crispatus GAI 98322 every two nights for a year. The average number of infections decreased significantly from an average of 5 in the year prior to the trial to an average of 1.3 during the trial (Uehara 2006). In a randomized controlled trial, 100 women with recurrent urinary tract infections were treated with either a vaginal suppository containing 100 million CFUs of L. crispatus CTV-05 or a placebo every night for five nights and then weekly for 10 weeks. Compared with placebo, probiotic therapy reduced the incidence of urinary tract infection episodes during the trial by approximately half (Stapleton 2011).
For more information about urinary tract infection, please see the Urinary Tract Infection health protocol.
14 Probiotics For Respiratory And Immune Health
The microbial residents of the throat, nose, and sinuses make up the upper respiratory microbiome and, much like the gut microbiome, help restrict the presence of harmful microbes and regulate immune function (Schenck 2016; de Steenhuijsen Piters 2015; Gao 2014). Healthy lungs, once thought to be sterile, are now known to be home to a unique microbiome (Roux 2017; Pichon 2017; Cui 2014). Some similarities with the oral and nasal microbial communities suggest that certain bacteria may be inhaled from these locations, but the lung also contains specific resident microbes (Hauptmann 2016). The lung microbiome is suspected to play important roles in health and disease, and in regulating immunity. Understanding these links is still at a very early stage (Wang, Li 2017; Cui 2014).
Oral probiotics have been found to reduce the frequency and duration of upper respiratory tract infections and the amount of antibiotic needed for treatment (Hao 2015; Quick 2015). In addition, emerging research suggests topical probiotics in the form of nasal sprays may be effective for restoring a healthy microbial balance in the upper airways (Santagati 2015). Pilot trials indicate that nasal sprays with Streptococcus salivarius 24SMB or S. sanguinis may be helpful in children with chronic or recurrent otitis media (Skovbjerg 2009; Marchisio 2015).
Asthma and Allergic Rhinitis
Lung dysbiosis has been reported in people with asthma, where less microbial diversity and changes in the composition of the microbial communities were observed (Chung 2017). Intestinal dysbiosis also appears to play a substantial role in allergy and asthma susceptibility. Factors such as Cesarean birth, formula feeding, and antibiotic exposure early in life, which negatively impact the infant gut microbiome, are associated with higher risks of asthma and allergies (West 2016; Moya-Perez 2017; Kolokotroni 2012).
A specialized dried yeast fermentate from S. cerevisiae (Epicor) has been shown to reduce seasonal allergies in two small studies. In one study, 25 healthy participants consumed Epicor or a placebo daily for five weeks during allergy season. The placebo group experienced increased seasonal allergy frequency, but the Epicor group did not. Interestingly, allergic symptoms returned within 1–2 weeks after the participants stopped taking Epicor (Jensen 2008). In a larger study, daily doses of 500 mg of Epicor or placebo were compared in 96 healthy subjects with a history of seasonal allergies. Subjects in the Epicor group had less severe nasal congestion and runny nose, and used less allergy medication than those taking placebo (Moyad 2009).
Allergies are mediated in part by an immune-signaling protein called immunoglobulin E (IgE). If the immune system produces too much IgE in response to a stimulus, an allergic reaction can ensue. A strain of Lactobacillus acidophilus called L-92 was shown in a preclinical screening study to reduce production of IgE in mice challenged with a pro-allergenic substance (Ishida 2003). Based on these intriguing results, three clinical trials of L. acidophilus L-92 were carried out. First, L-92 was administered to subjects over two allergy seasons. Dosages were 50 billion CFUs twice daily for six weeks during the first season and 20 billion CFUs once weekly during the second season. Twenty-three people participated during season one, and 20 did so during season two. During the first season, L-92 reduced eye-related distress related to allergies by 31%. During the second, a statistically significant reduction in overall allergy-related distress among the subjects receiving L-92 was observed (Ishida, Nakamura, Kanzato, Sawada, Yamamoto 2005). In the second study, 49 participants with allergic rhinitis took either 30 billion CFUs of L-92 or placebo daily for eight weeks. Nasal mucosal swelling decreased by 24% at week eight in the L-92 group. Also, nasal symptom scores decreased 19% in the L-92 group compared with placebo at week eight (Ishida, Nakamura, Kanzato, Sawada, Hirata 2005). In the third and slightly larger study, 80 subjects who had a cedar pollen allergy and were exposed to cedar pollen at baseline took L-92 or placebo for 8 weeks before re-exposure to cedar pollen. L-92 led to a significant reduction in nasal and eye allergy symptoms compared with placebo upon re-exposure (Caplpis Co. Ltd. 2012).
In adults with allergies, the probiotic strain Bifidobacterium longum BB536 reduced some of the symptoms caused by pollen exposure significantly more than placebo (Xiao 2007). In a randomized controlled trial on children with pollen allergies, a mixture of B. longum BB536, B. infantis M-63, and B. breve M-16V significantly improved symptoms and quality of life (Miraglia Del Giudice 2017). In addition, probiotics have enhanced the effectiveness of allergen-specific immunotherapy in asthma and allergy patients (Liu 2016; Jerzynska 2016). In adults with seasonal allergic rhinitis, oral administration of B. lactis NCC2818 improved some immune parameters and allergy symptoms (Singh 2013).
For more information about allergies and asthma, please see the Allergies health protocol.
Upper Respiratory Tract Infections
Preclinical research suggests some probiotic Lactobacillus species can stimulate the antimicrobial immune response in the respiratory tract (Marranzino 2012), and clinical trials have shown their positive effects in preventing upper respiratory tract infections. Randomized controlled trials in healthy young, middle-aged, and elderly adults have shown that cultured milk products fortified with various probiotic strains of L. casei can decrease the incidence and shorten the duration of upper respiratory tract infections (Shida 2017; Fujita 2013; Jespersen 2015). Milk or yogurt products containing probiotic strains of L. paracasei have also been found to decrease upper respiratory tract infection risk in elderly subjects and children (Corsello 2017; Pu 2017). Findings from a small trial in children suggest the probiotic L. brevis KB290, found in a traditional Japanese pickle, has the potential to protect against influenza (Waki 2014).
In a randomized controlled trial, 78 healthy adults reporting high levels of psychological stress received either an oral tablet containing 10 mg of a heat-killed, non-viable form of the bacterial strain L. plantarum L-137 or a placebo daily for 12 weeks. Upper respiratory tract infections were diminished in number, severity, and duration in those receiving L. plantarum L-137. The heat-killed form of this organism has been shown to have immune-modulating actions, which may have contributed to the benefits seen in this study (Hirose 2013).
A randomized controlled trial in 198 college students found that a daily probiotic with at least 1 billion CFUs each of L. rhamnosus GG and B. animalis subspecies lactis BB-12 for 12 weeks reduced median duration and median severity scores of upper respiratory tract infections and the number of school days missed as a result (Smith 2013). B. animalis subspecies lactis BB-12 alone may also be effective in adults when used in capsules or when added to yogurt prior to fermentation (Meng 2016).
Two randomized-controlled trials found that daily administration of 20 billion CFU’s L. helveticus LAFTI L10 provided respiratory and immune support for elite athletes. In one trial, supplementing 39 elite athletes with the probiotic daily for 14 weeks in the winter resulted in a significantly shortened duration of upper respiratory tract infections compared with those who consumed placebo. The LAFTI L10 treatment group also experienced a lesser number of symptoms compared with placebo, as well as an improved feeling of vigor (Michalickova 2016). In another trial that enrolled 30 elite athletes, L. helveticus LAFTI L10 supplementation daily for 14 weeks resulted in a significant preservation of total salivary IgA concentration under demanding training conditions, while salivary IgA decreased in those receiving placebo (Michalickova 2017).
A randomized controlled trial in 465 healthy adults found that the single probiotic strain B. animalis subspecies lactis Bl-04, at a dose of 2 billion CFUs per day, prevented upper respiratory tract infections better than placebo or a probiotic combination (West 2014). Another randomized controlled trial in which undergraduate students were treated with probiotics or placebo found that B. bifidum R0071 supplementation reduced sick days due to upper respiratory tract infection (Langkamp-Henken 2015). In elderly subjects, 100 billion CFUs per day of B. longum BB536 reduced the incidence of influenza compared with placebo over a 14-week period (Namba 2010).
S. salivarius is a prominent bacterium in the healthy mouth (Burton 2006). The probiotic strain S. salivarius K12 is able to colonize the mouth and throat and may reduce the incidence of sore throat episodes caused by a common bacterium (Di Pierro 2014). Supplements with this probiotic strain have shown promise in preventing tonsillitis and pharyngitis (throat infections) and in reducing the occurrence and/or severity of otitis media (middle ear infections) (Zupancic 2017). Trials in children indicate S. salivarius K12 can prevent streptococcal throat infections, scarlet fever, and otitis media (Di Pierro, Colombo, Giuliani 2016), even in those with recent or recurrent episodes of streptococcal pharyngitis (strep throat) (Di Pierro, Colombo, Zanvit 2016; Di Pierro 2014; Di Pierro 2012) or otitis media (Di Pierro, Di Pasquale 2015). In one non-randomized trial that enrolled 40 adults diagnosed with recurrent strep throat, 20 participants received 90 days of treatment with 5 billion CFUs S. salivarius K12 daily, while the other 20 participants did not. S. salivarius K12 reduced the number of episodes of strep throat the subjects experienced during treatment and in the six months following treatment. The probiotic was well-tolerated and did not cause any side effects in the trial (Di Pierro 2013). Overall, more high-quality research is needed to establish the utility of S. salivarius K12 in the prevention of sore throat (Wilcox 2019).
In a randomized placebo-controlled trial on 721 participants and carried out over three winter cold seasons, various probiotic-prebiotic mixtures containing organisms such as L. plantarum LP02, L. rhamnosus LR04 and LR05, and B. lactis BS01 led to reduced upper respiratory tract infection frequency (Pregliasco 2008).
In a 16-week randomized, double-blind, placebo-controlled clinical trial on 100 individuals between 60 and 74 years old, the administration of 2 billion B. subtilis CU1 spores daily for 10 years, repeated 4 times, with 18-day breaks, decreased the frequency of respiratory infections in a subgroup of 44 of the participants as compared with placebo. Consumption of the probiotics also led to beneficial changes in immune response (Lefevre 2015). B. subtilis CU1 was safe and well tolerated in elderly people, and its administration was not accompanied by any adverse effects (Lefevre 2017).
For more information about upper respiratory infections and the flu, see the Common Cold and Influenza health protocols.
15 Probiotics And Mood
A randomized controlled clinical trial, which examined participants with low urinary free cortisol levels, reported that Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 improved mental well-being, and could have a preventive role against stress-related psychological changes (Messaoudi, Violle 2011).
In a double-blind placebo-controlled clinical trial on human volunteers, a probiotic formulation containing L. helveticus R0052 and B. longum R0175, taken for 30 days, led to beneficial psychological effects, including improvements in scales that measure depression, anger-hostility, and problem solving. This same probiotic combination also reduced anxiety-like behavior in rats (Messaoudi, Lalonde 2011). In another rat model, the combination of probiotics reduced depression scores after a heart attack and restored gut permeability (Arseneault-Breard 2012), and it decreased cell death in various brain regions (Girard 2009). In mice, it prevented brain changes that occur as a result of chronic psychological stress (Ait-Belgnaoui 2014).
2025
- Sep: Updated section on human milk oligosaccharides in Establishing and Shaping the Microbiome
- Jun: Added sections on Lacticaseibacillus rhamnosus IMC 501, Lacticaseibacillus paracasei IMC 502, and heat-treated Lactobacillus paracasei D3-5 to Probiotics for Digestive Health; Updated section on Lactobacillus plantarum ECGC 13110402 in Probiotics for Cardiovascular Health
2023
- Jul: Updated Overview and Probiotics for Respiratory and Immune Health
- Jun: Added section on the microbiota of centenarians to Diet and Lifestyle Strategies for Supporting a Healthy Microbiome
2022
- Jul: Updated section on vulvovaginal candidiasis in Probiotics for Vaginal and Urinary Health
- Jul: Updated section on irritable bowel syndrome in Probiotics for Digestive Health
2021
- Jan: Updated section on microbial byproducts in Background
2018
- Apr: Comprehensive update & review
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
Abdallah F, Mijouin L, Pichon C. Skin Immune Landscape: Inside and Outside the Organism. Mediators Inflamm. 2017;2017:5095293.
Abdelmaksoud AA, Koparde VN, Sheth NU, Serrano MG, Glascock AL, Fettweis JM, . . . Jefferson KK. Comparison of Lactobacillus crispatus isolates from Lactobacillus-dominated vaginal microbiomes with isolates from microbiomes containing bacterial vaginosis-associated bacteria. Microbiology. Mar 2016;162(3):466-475.
Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, . . . Tompkins T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. Apr 2014;26(4):510-520.
Al-Ghazzewi FH, Tester RF. Impact of prebiotics and probiotics on skin health. Benef Microbes. Jun 1 2014;5(2):99-107.
Al-Jashaami LS, DuPont HL. Management of Clostridium difficile Infection. Gastroenterol Hepatol (N Y). Oct 2016;12(10):609-616.
Allaker RP, Stephen AS. Use of Probiotics and Oral Health. Current oral health reports. 2017;4(4):309-318.
Amato KR, Yeoman CJ, Cerda G, Schmitt CA, Cramer JD, Miller ME, . . . Leigh SR. Variable responses of human and non-human primate gut microbiomes to a Western diet. Microbiome. Nov 16 2015;3:53.
Aoyagi Y, Park S, Matsubara S, Honda Y, Amamoto R, Kushiro A, . . . Shephard RJ. Habitual intake of fermented milk products containing Lactobacillus casei strain Shirota and a reduced risk of hypertension in older people. Benef Microbes. Feb 7 2017;8(1):23-29.
Araujo JR, Tomas J, Brenner C, Sansonetti PJ. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie. Oct 2017;141:97-106.
Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria Populations in Human Health and Aging. Front Microbiol. 2016;7:1204.
Arseneault-Breard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R, Rousseau G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. The British journal of nutrition. Jun 2012;107(12):1793-1799.
Arweiler NB, Netuschil L. The Oral Microbiota. Advances in experimental medicine and biology. 2016;902:45-60.
Barker AK, Duster M, Valentine S, Hess T, Archbald-Pannone L, Guerrant R, Safdar N. A randomized controlled trial of probiotics for Clostridium difficile infection in adults (PICO). The Journal of antimicrobial chemotherapy. Nov 1 2017;72(11):3177-3180.
Barnard E, Li H. Shaping of cutaneous function by encounters with commensals. The Journal of physiology. Jan 15 2017;595(2):437-450.
Bartosch S, Woodmansey EJ, Paterson JC, McMurdo ME, Macfarlane GT. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. Jan 1 2005;40(1):28-37.
Beerepoot MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, . . . Geerlings SE. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Archives of internal medicine. May 14 2012;172(9):704-712.
Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. Is It Useful to Administer Probiotics Together With Proton Pump Inhibitors in Children With Gastroesophageal Reflux? J Neurogastroenterol Motil. Jan 30 2018;24(1):51-57.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. Mar 27 2014;157(1):121-141.
Bennet SM, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut and liver. May 23 2015;9(3):318-331.
Biagi E, Franceschi C, Rampelli S, et al. Gut Microbiota and Extreme Longevity. Curr Biol. Jun 6 2016;26(11):1480-5. doi:10.1016/j.cub.2016.04.016. https://pubmed.ncbi.nlm.nih.gov/27185560/
Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, . . . Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. The American journal of gastroenterology. Jul 2005;100(7):1539-1546.
Blaabjerg S, Artzi DM, Aabenhus R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients-A Systematic Review and Meta-Analysis. Antibiotics (Basel, Switzerland). Oct 12 2017;6(4).
Bohbot JM, Cardot JM. Vaginal impact of the oral administration of total freeze-dried culture of LCR 35 in healthy women. Infectious diseases in obstetrics and gynecology. 2012;2012:503648.
Bonetti A, Morelli L, Campominosi E, Ganora E, Sforza F. Adherence of Lactobacillus plantarum P 17630 in soft-gel capsule formulation versus Döderlein's bacillus in tablet formulation to vaginal epithelial cells. Minerva ginecologica. Jun 2003;55(3):279-84, 284-7.
Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. Jun 1 2014;5(2):185-199.
Branton WG, Ellestad KK, Maingat F, Wheatley BM, Rud E, Warren RL, . . . Power C. Brain microbial populations in HIV/AIDS: alpha-proteobacteria predominate independent of host immune status. PloS one. 2013;8(1):e54673.
Brown RL, Clarke TB. The regulation of host defences to infection by the microbiota. Immunology. Jan 2017;150(1):1-6.
Burton JH, Johnson M, Johnson J, Hsia DS, Greenway FL, Heiman ML. Addition of a Gastrointestinal Microbiome Modulator to Metformin Improves Metformin Tolerance and Fasting Glucose Levels. Journal of Diabetes Science and Technology. 03/23 2015;9(4):808-814.
Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JD, Wescombe PA. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol. Jun 2013;62(Pt 6):875-884.
Burton JP, Wescombe PA, Moore CJ, Chilcott CN, Tagg JR. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Appl Environ Microbiol. Apr 2006;72(4):3050-3053.
Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, Satokari R, . . . Gasbarrini A. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. Apr 2017;66(4):569-580.
Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clinical epidemiology. 2014;6:71-80.
Candido FG, Valente FX, Grzeskowiak LM, Moreira APB, Rocha D, Alfenas RCG. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity. International journal of food sciences and nutrition. Jul 4 2017:1-19.
Caplpis Co. Ltd. Data on file. 2012.
Carter MM, Demis D, Perelman D, et al. A human milk oligosaccharide alters the microbiome, circulating hormones, and metabolites in a randomized controlled trial of older adults. Cell Rep Med. Aug 19 2025;6(8):102256. doi:10.1016/j.xcrm.2025.102256. https://pubmed.ncbi.nlm.nih.gov/40738103/
Cassidy-Bushrow AE, Burmeister C, Havstad S, Levin AM, Lynch SV, Ownby DR, . . . Wegienka G. Prenatal antimicrobial use and early-childhood body mass index. International journal of obesity (2005). Aug 17 2017.
Cayzeele-Decherf A, Pelerin F, Leuillet S, Douillard B, Housez B, Cazaubiel M, . . . Desreumaux P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World journal of gastroenterology: WJG. Jan 14 2017;23(2):336-344.
CDC. Centers for Disease Control and Prevention. Healthcare-Associated Infections: Frequently Asked Questions about Clostridium dificile or Healthcare Providers. https://www.cdc.gov/hai/organisms/cdiff/cdiff_faqs_hcp.html. Last updated 03/06/2012. Accessed 01/19/2018.
Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science (New York, N.Y.). Jul 28 2017;357(6349).
Cesaro S, Chinello P, Rossi L, Zanesco L. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharomyces boulardii. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. Nov 2000;8(6):504-505.
Chan YK, Estaki M, Gibson DL. Clinical consequences of diet-induced dysbiosis. Ann Nutr Metab. 2013;63 Suppl 2:28-40.
Chang YS, Trivedi MK, Jha A, Lin YF, Dimaano L, Garcia-Romero MT. Synbiotics for Prevention and Treatment of Atopic Dermatitis: A Meta-analysis of Randomized Clinical Trials. JAMA pediatrics. Mar 2016;170(3):236-242.
Chanishvili N. Phage therapy--history from Twort and d'Herelle through Soviet experience to current approaches. Advances in virus research. 2012;83:3-40.
Chedid V, Dhalla S, Clarke JO, Roland BC, Dunbar KB, Koh J, . . . Mullin GE. Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob Adv Health Med. May 2014;3(3):16-24.
Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, . . . Jia H. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nature communications. Oct 17 2017;8(1):875.
Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C, . . . Xie P. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport. Mar 21 2018;29(5):417-425.
Cherifi S, Robberecht J, Miendje Y. Saccharomyces cerevisiae fungemia in an elderly patient with Clostridium difficile colitis. Acta clinica Belgica. Jul-Aug 2004;59(4):223-224.
Chew SY, Cheah YK, Seow HF, Sandai D, Than LT. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J Appl Microbiol. May 2015;118(5):1180-1190.
Chiang JY. Recent advances in understanding bile acid homeostasis. F1000Research. 2017;6:2029.
Chung KF. Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment? The Journal of allergy and clinical immunology. Apr 2017;139(4):1071-1081.
Ciorba MA. A gastroenterologist's guide to probiotics. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. Sep 2012;10(9):960-968.
Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, . . . O'Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proceedings of the National Academy of Sciences of the United States of America. Mar 15 2011;108 Suppl 1:4586-4591.
Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, . . . O'Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. Aug 9 2012;488(7410):178-184.
Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. Journal of the International Society of Sports Nutrition. 2016;13:43.
Compare D, Rocco A, Sgamato C, Coccoli P, Campo SM, Nazionale I, . . . Nardone G. Lactobacillus paracasei F19 versus placebo for the prevention of proton pump inhibitor-induced bowel symptoms: a randomized clinical trial. Dig Liver Dis. Apr 2015;47(4):273-279.
Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Progress in food & nutrition science. Oct-Dec 1992;16(4):307-343.
Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clinical & translational immunology. Apr 2016;5(4):e73.
Corsello G, Carta M, Marinello R, Picca M, De Marco G, Micillo M, . . . Berni Canani R. Preventive Effect of Cow's Milk Fermented with Lactobacillus paracasei CBA L74 on Common Infectious Diseases in Children: A Multicenter Randomized Controlled Trial. Nutrients. Jun 27 2017;9(7).
Costabile A, Buttarazzi I, Kolida S, Quercia S, Baldini J, Swann JR, . . . Gibson GR. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PloS one. 2017;12(12):e0187964.
Cribby S, Taylor M, Reid G. Vaginal microbiota and the use of probiotics. Interdisciplinary perspectives on infectious diseases. 2008;2008:256490.
Cui L, Morris A, Huang L, Beck JM, Twigg HL, 3rd, von Mutius E, Ghedin E. The microbiome and the lung. Annals of the American Thoracic Society. Aug 2014;11 Suppl 4:S227-232.
D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clinica Chimica Acta. 2015;451(Part A):97-102.
Dahl WJ, Stewart ML. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. Journal of the Academy of Nutrition and Dietetics. Nov 2015;115(11):1861-1870.
Daliri EB, Lee BH, Oh DH. Current Perspectives on Antihypertensive Probiotics. Probiotics and antimicrobial proteins. Jun 2017;9(2):91-101.
Dalmasso M, Strain R, Neve H, Franz CM, Cousin FJ, Ross RP, Hill C. Three New Escherichia coli Phages from the Human Gut Show Promising Potential for Phage Therapy. PloS one. 2016;11(6):e0156773.
Davenport ER, Sanders JG, Song SJ, Amato KR, Clark AG, Knight R. The human microbiome in evolution. BMC Biol. Dec 27 2017;15(1):127.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, . . . Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. Jan 23 2014;505(7484):559-563.
De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. Aug 17 2010;107(33):14691-6. doi:10.1073/pnas.1005963107. https://pubmed.ncbi.nlm.nih.gov/20679230/
De Seta F, Parazzini F, De Leo R, Banco R, Maso GP, De Santo D, . . . Restaino S. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: a retrospective comparative study. European journal of obstetrics, gynecology, and reproductive biology. Nov 2014;182:136-139.
de Steenhuijsen Piters WA, Sanders EA, Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. Aug 19 2015;370(1675).
DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. May 2016;22(5):1137-1150.
Deidda F, Amoruso A, Nicola S, Graziano T, Pane M, Allesina S, . . . Mogna L. The In Vitro Effectiveness of Lactobacillus fermentum Against Different Candida Species Compared With Broadly Used Azoles. Journal of clinical gastroenterology. Nov/Dec 2016;50 Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015:S171-s174.
Del Piano M, Anderloni A, Balzarini M, Ballare M, Carmagnola S, Montino F, . . . Mogna G. The innovative potential of Lactobacillus rhamnosus LR06, Lactobacillus pentosus LPS01, Lactobacillus plantarum LP01, and Lactobacillus delbrueckii Subsp. delbrueckii LDD01 to restore the "gastric barrier effect" in patients chronically treated with PPI: a pilot study. Journal of clinical gastroenterology. Oct 2012;46 Suppl:S18-26.
Del Piano M, Carmagnola S, Anderloni A, Andorno S, Ballare M, Balzarini M, . . . Capurso L. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: a double-blind, randomized, placebo-controlled study. Journal of clinical gastroenterology. Sep 2010;44 Suppl 1:S30-34.
Deng ZL, Sztajer H, Jarek M, Bhuju S, Wagner-Dobler I. Worlds Apart - Transcriptome Profiles of Key Oral Microbes in the Periodontal Pocket Compared to Single Laboratory Culture Reflect Synergistic Interactions. Front Microbiol. 2018;9:124.
Derrien M, van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends in microbiology. Jun 2015;23(6):354-366.
Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Alimentary pharmacology & therapeutics. Aug 2017;46(4):389-400.
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, . . . Wade WG. The human oral microbiome. J Bacteriol. Oct 2010;192(19):5002-5017.
Di Pierro F, Adami T, Rapacioli G, Giardini N, Streitberger C. Clinical evaluation of the oral probiotic Streptococcus salivarius K12 in the prevention of recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes in adults. Expert Opin Biol Ther. Mar 2013;13(3):339-43. doi:10.1517/14712598.2013.758711. https://pubmed.ncbi.nlm.nih.gov/23286823/
Di Pierro F, Colombo M, Giuliani MG, Danza ML, Basile I, Bollani T, . . . Rottoli AS. Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children. European review for medical and pharmacological sciences. Nov 2016;20(21):4601-4606.
Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc Patient Saf. 2014;6:15-20.
Di Pierro F, Colombo M, Zanvit A, Rottoli AS. Positive clinical outcomes derived from using Streptococcus salivarius K12 to prevent streptococcal pharyngotonsillitis in children: a pilot investigation. Drug Healthc Patient Saf. 2016;8:77-81.
Di Pierro F, Di Pasquale D, Di Cicco M. Oral use of Streptococcus salivarius K12 in children with secretory otitis media: preliminary results of a pilot, uncontrolled study. International journal of general medicine. 2015;8:303-308.
Di Pierro F, Donato G, Fomia F, Adami T, Careddu D, Cassandro C, Albera R. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. International journal of general medicine. 2012;5:991-997.
Di Pierro F, Zanvit A, Nobili P, Risso P, Fornaini C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clinical, cosmetic and investigational dentistry. 2015;7:107-113.
Di Pilato V, Freschi G, Ringressi MN, Pallecchi L, Rossolini GM, Bechi P. The esophageal microbiota in health and disease. Annals of the New York Academy of Sciences. Oct 2016;1381(1):21-33.
Di Stefano M, Miceli E, Armellini E, Missanelli A, Corazza GR. Probiotics and functional abdominal bloating. Journal of clinical gastroenterology. Jul 2004;38(6 Suppl):S102-103.
Dibb WL. The normal microbial flora of the outer ear canal in healthy Norwegian individuals. NIPH annals. Jun 1990;13(1):11-16.
Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World journal of gastroenterology: WJG. Mar 14 2015;21(10):3072-3084.
Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. The American journal of clinical nutrition. Oct 2014;100(4):1075-1084.
Dimidi E, Christodoulides S, Scott SM, Whelan K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr. May 2017;8(3):484-494.
Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World journal of gastroenterology. Feb 21 2016;22(7):2219-2241.
Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods and findings in experimental and clinical pharmacology. Dec 2009;31(10):655-659.
Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, . . . Clemente JC. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. Mar 2016;22(3):250-253.
Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. May 15 2015;60 Suppl 2:S129-134.
Doss J, Culbertson K, Hahn D, Camacho J, Barekzi N. A Review of Phage Therapy against Bacterial Pathogens of Aquatic and Terrestrial Organisms. Viruses. Mar 18 2017;9(3).
Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. Sep 2013;132(3):e666-676.
Etienne-Mesmin L, Chassaing B, Gewirtz AT. Tryptophan: A gut microbiota-derived metabolites regulating inflammation. World J Gastrointest Pharmacol Ther. Feb 6 2017;8(1):7-9.
Evivie SE, Huo GC, Igene JO, Bian X. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. Food Nutr Res. 2017;61(1):1318034.
Fabbiano S, Suarez-Zamorano N, Trajkovski M. Host-Microbiota Mutualism in Metabolic Diseases. Frontiers in endocrinology. 2017;8:267.
Fabbrocini G, Bertona M, Picazo O, Pareja-Galeano H, Monfrecola G, Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. Nov 30 2016;7(5):625-630.
Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120.
Fakruddin M, Hossain MN, Ahmed MM. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC complementary and alternative medicine. Jan 21 2017;17(1):64.
Falana K, Knight R, Martin CR, Goldszmid R, Greathouse KL, Gere J, . . . Kuo WP. Short Course in the Microbiome. Journal of circulating biomarkers. Jan-Dec 2015;4:8.
Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. Oct 2012;10(10):1096-1100.
Fashner J, Gitu AC. Diagnosis and Treatment of Peptic Ulcer Disease and H. pylori Infection. American family physician. Feb 15 2015;91(4):236-242.
Ferrario C, Statello R, Carnevali L, Mancabelli L, Milani C, Mangifesta M, . . . Turroni F. How to Feed the Mammalian Gut Microbiota: Bacterial and Metabolic Modulation by Dietary Fibers. Front Microbiol. 2017;8:1749.
Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. Jul-Aug 2012;3(4):289-306.
Flowers SA, Ellingrod VL. The Microbiome in Mental Health: Potential Contribution of Gut Microbiota in Disease and Pharmacotherapy Management. Pharmacotherapy. Oct 2015;35(10):910-916.
Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, . . . Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. The American journal of gastroenterology. Oct 2014;109(10):1547-1561; quiz 1546, 1562.
Foxman B, Buxton M. Alternative approaches to conventional treatment of acute uncomplicated urinary tract infection in women. Current infectious disease reports. Apr 2013;15(2):124-129.
Foxman B, Goldberg D. Why the human microbiome project should motivate epidemiologists to learn ecology. Epidemiology (Cambridge, Mass.). Nov 2010;21(6):757-759.
Francino MP. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front Microbiol. 2015;6:1543.
Fujiki T, Hirose Y, Yamamoto Y, Murosaki S. Enhanced immunomodulatory activity and stability in simulated digestive juices of Lactobacillus plantarum L-137 by heat treatment. Bioscience, biotechnology, and biochemistry. 2012;76(5):918-922.
Fujimori S. What are the effects of proton pump inhibitors on the small intestine? World journal of gastroenterology. Jun 14 2015;21(22):6817-6819.
Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. May 13 2015;17(5):592-602.
Fujita R, Iimuro S, Shinozaki T, Sakamaki K, Uemura Y, Takeuchi A, . . . Ohashi Y. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. American journal of infection control. Dec 2013;41(12):1231-1235.
Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clinical journal of gastroenterology. Feb 2014;7(1):1-13.
Galland L. The gut microbiome and the brain. Journal of medicinal food. Dec 2014;17(12):1261-1272.
Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World journal of gastroenterology: WJG. Aug 28 2016;22(32):7186-7202.
Galofre M, Palao D, Vicario M, Nart J, Violant D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. Journal of periodontal research. Jan 19 2018.
Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J Cell Physiol. Mar 2018;233(3):2091-2103.
Gao Z, Kang Y, Yu J, Ren L. Human pharyngeal microbiome may play a protective role in respiratory tract infections. Genomics, proteomics & bioinformatics. Jun 2014;12(3):144-150.
Gatej SM, Marino V, Bright R, Fitzsimmons TR, Gully N, Zilm P, . . . Bartold PM. Probiotic Lactobacillus rhamnosus GG prevents alveolar bone loss in a mouse model of experimental periodontitis. J Clin Periodontol. Feb 2018;45(2):204-212.
Gaya P, Medina M, Sanchez-Jimenez A, Landete JM. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules. Aug 9 2016;21(8).
Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, . . . Huttenhower C. The Human Microbiome Project: a community resource for the healthy human microbiome. PLoS biology. 2012;10(8):e1001377.
Ghoshal UC, Shukla R, Ghoshal U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut and liver. Mar 15 2017;11(2):196-208.
Giddings SL, Stevens AM, Leung DT. Traveler's Diarrhea. The Medical clinics of North America. Mar 2016;100(2):317-330.
Girard P, Coppe MC, Pansart Y, Gillardin JM. Gastroprotective effect of Saccharomyces boulardii in a rat model of ibuprofen-induced gastric ulcer. Pharmacology. 2010;85(3):188-193.
Girard SA, Bah TM, Kaloustian S, Lada-Moldovan L, Rondeau I, Tompkins TA, . . . Rousseau G. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. The British journal of nutrition. Nov 2009;102(10):1420-1425.
Goderska K, Agudo Pena S, Alarcon T. Helicobacter pylori treatment: antibiotics or probiotics. Appl Microbiol Biotechnol. Jan 2018;102(1):1-7.
Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, Johnston BC. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. The Cochrane database of systematic reviews. Dec 19 2017;12:Cd006095.
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, . . . Ley RE. Human genetics shape the gut microbiome. Cell. Nov 6 2014;159(4):789-799.
Graf D, Di Cagno R, Fak F, Flint HJ, Nyman M, Saarela M, Watzl B. Contribution of diet to the composition of the human gut microbiota. Microbial ecology in health and disease. 2015;26:26164.
Greenway F, Wang S, Heiman M. A novel cobiotic containing a prebiotic and an antioxidant augments the glucose control and gastrointestinal tolerability of metformin: a case report. Benef Microbes. Mar 2014;5(1):29-32.
Grice EA, Segre JA. The skin microbiome. Nature reviews. Microbiology. Apr 2011;9(4):244-253.
Gulden E, Vudattu NK, Deng S, Preston-Hurlburt P, Mamula M, Reed JC, . . . Herold KC. Microbiota control immune regulation in humanized mice. JCI insight. Nov 2 2017;2(21).
Gupta ND, Sharma S, Sharma VK. Probiotic - An emerging therapy in recolonizing periodontal pocket. Journal of oral biology and craniofacial research. Jan-Apr 2017;7(1):72-73.
Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. European journal of gastroenterology & hepatology. Jun 2003;15(6):697-698.
Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Digestive diseases and sciences. Jul 2000;45(7):1462-1464.
Halfvarson J, Brislawn CJ, Lamendella R, Vazquez-Baeza Y, Walters WA, Bramer LM, . . . Jansson JK. Dynamics of the human gut microbiome in inflammatory bowel disease. Nature microbiology. Feb 13 2017;2:17004.
Hanson L, VandeVusse L, Jerme M, Abad CL, Safdar N. Probiotics for Treatment and Prevention of Urogenital Infections in Women: A Systematic Review. Journal of midwifery & women's health. May 2016;61(3):339-355.
Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. The Cochrane database of systematic reviews. Feb 3 2015(2):Cd006895.
Happel AU, Barnabas SL, Froissart R, Passmore JS. Weighing in on the risks and benefits of probiotic use in HIV-infected and immunocompromised populations. Benef Microbes. Feb 27 2018;9(2):239-246.
Haro C, Garcia-Carpintero S, Rangel-Zuniga OA, Alcala-Diaz JF, Landa BB, Clemente JC, . . . Camargo A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol Nutr Food Res. Dec 2017;61(12).
Haukioja A. Probiotics and oral health. European journal of dentistry. Jul 2010;4(3):348-355.
Hauptmann M, Schaible UE. Linking microbiota and respiratory disease. FEBS letters. Nov 2016;590(21):3721-3738.
He M, Shi B. Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell & bioscience. 2017;7:54.
Hedayati-Hajikand T, Lundberg U, Eldh C, Twetman S. Effect of probiotic chewing tablets on early childhood caries--a randomized controlled trial. BMC oral health. 2015;15(1):112.
Heinlen L, Ballard JD. Clostridium difficile infection. The American journal of the medical sciences. Sep 2010;340(3):247-252.
Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014/08/01 2014;11(8):506-514. doi:10.1038/nrgastro.2014.66. https://doi.org/10.1038/nrgastro.2014.66
Hirose Y, Yamamoto Y, Yoshikai Y, Murosaki S. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. Journal of nutritional science. 2013;2:e39.
Ho JT, Chan GC, Li JC. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC immunology. Mar 26 2015;16:21.
Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World journal of gastroenterology. Oct 7 2015;21(37):10644-10653.
Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, Mehrabany EV. Effects of probiotics on the recurrence of bacterial vaginosis: a review. Journal of lower genital tract disease. Jan 2014;18(1):79-86.
Huang B, Fettweis JM, Brooks JP, Jefferson KK, Buck GA. The Changing Landscape of the Vaginal Microbiome. Clinics in laboratory medicine. Dec 2014;34(4):747-761.
Huang H, Song L, Zhao W. Effects of probiotics for the treatment of bacterial vaginosis in adult women: a meta-analysis of randomized clinical trials. Archives of gynecology and obstetrics. Jun 2014;289(6):1225-1234.
Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the Treatment of Atopic Dermatitis in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Frontiers in cellular and infection microbiology. 2017;7:392.
Huang YJ, Lynch SV. The emerging relationship between the airway microbiota and chronic respiratory disease: clinical implications. Expert review of respiratory medicine. Dec 2011;5(6):809-821.
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. Jun 13 2012;486(7402):207-214.
Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med. Mar 2009;121(2):119-124.
Huse SM, Ye Y, Zhou Y, Fodor AA. A core human microbiome as viewed through 16S rRNA sequence clusters. PloS one. 2012;7(6):e34242.
Igarashi M, Nakae H, Matsuoka T, Takahashi S, Hisada T, Tomita J, Koga Y. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ open gastroenterology. 2017;4(1):e000144.
Ince G, Gursoy H, Ipci SD, Cakar G, Emekli-Alturfan E, Yilmaz S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J Periodontol. Jun 2015;86(6):746-754.
Inoue Y, Kambara T, Murata N, Komori-Yamaguchi J, Matsukura S, Takahashi Y, . . . Aihara M. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum cytokines of atopic dermatitis in Japanese adults: a double-blind, randomized, clinical trial. Int Arch Allergy Immunol. 2014;165(4):247-254.
Iovino P, Bucci C, Tremolaterra F, Santonicola A, Chiarioni G. Bloating and functional gastro-intestinal disorders: where are we and where are we going? World journal of gastroenterology: WJG. Oct 21 2014;20(39):14407-14419.
Ishida Y, Bandou I, Kanzato H, Yamamoto N. Decrease in ovalbumin specific IgE of mice serum after oral uptake of lactic acid bacteria. Bioscience, biotechnology, and biochemistry. May 2003;67(5):951-957.
Ishida Y, Nakamura F, Kanzato H, Sawada D, Hirata H, Nishimura A, . . . Fujiwara S. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: a double-blind, placebo-controlled study. J Dairy Sci. Feb 2005;88(2):527-533.
Ishida Y, Nakamura F, Kanzato H, Sawada D, Yamamoto N, Kagata H, . . . Fujiwara S. Effect of milk fermented with Lactobacillus acidophilus strain L-92 on symptoms of Japanese cedar pollen allergy: a randomized placebo-controlled trial. Bioscience, biotechnology, and biochemistry. Sep 2005;69(9):1652-1660.
Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, . . . Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. Nov 2011;141(5):1773-1781.
Islam SU. Clinical Uses of Probiotics. Medicine. Feb 2016;95(5):e2658.
Iwamoto T, Suzuki N, Tanabe K, Takeshita T, Hirofuji T. Effects of probiotic Lactobacillus salivarius WB21 on halitosis and oral health: an open-label pilot trial. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. Aug 2010;110(2):201-208.
Iwasaki K, Maeda K, Hidaka K, Nemoto K, Hirose Y, Deguchi S. Daily Intake of Heat-killed Lactobacillus plantarum L-137 Decreases the Probing Depth in Patients Undergoing Supportive Periodontal Therapy. Oral health & preventive dentistry. 2016;14(3):207-214.
Janakiram C, Deepan Kumar CV, Joseph J. Xylitol in preventing dental caries: A systematic review and meta-analyses. Journal of natural science, biology, and medicine. Jan-Jun 2017;8(1):16-21.
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World journal of gastroenterology: WJG. Aug 7 2015;21(29):8787-8803.
Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, McDonald SD. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth. Jan 8 2018;18(1):14.
Jasarevic E, Howard CD, Misic AM, Beiting DP, Bale TL. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. Mar 7 2017;7:44182.
Jasarevic E, Howerton CL, Howard CD, Bale TL. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology. Sep 2015;156(9):3265-3276.
Jasarevic E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiology of stress. Jan 1 2015;1:81-88.
Jenkins TA, Nguyen JC, Polglaze KE, Bertrand PP. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. Jan 20 2016;8(1).
Jensen GS, Patterson KM, Barnes J, Schauss AG, Beaman R, Reeves SG, Robinson LE. A double-blind placebo-controlled, randomized pilot study: consumption of a high-metabolite immunogen from yeast culture has beneficial effects on erythrocyte health and mucosal immune protection in healthy subjects. The Open Nutrition Journal. 2008;2(1).
Jerzynska J, Stelmach W, Balcerak J, Woicka-Kolejwa K, Rychlik B, Blauz A, . . . Stelmach I. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy and asthma proceedings: the official journal of regional and state allergy societies. Jul 2016;37(4):324-334.
Jespersen L, Tarnow I, Eskesen D, Morberg CM, Michelsen B, Bugel S, . . . Calder PC. Effect of Lactobacillus paracasei subsp. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. The American journal of clinical nutrition. Jun 2015;101(6):1188-1196.
Jiao Y, Hasegawa M, Inohara N. The Role of Oral Pathobionts in Dysbiosis during Periodontitis Development. Journal of dental research. Jun 2014;93(6):539-546.
Johnson KV, Burnet PW. Microbiome: Should we diversify from diversity? Gut Microbes. Nov 2016;7(6):455-458.
Jones ML, Martoni CJ, Parent M, Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. The British journal of nutrition. May 2012;107(10):1505-1513.
Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. European journal of clinical nutrition. Nov 2012;66(11):1234-1241.
Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. The Journal of clinical endocrinology and metabolism. Jul 2013;98(7):2944-2951.
Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. Journal of cutaneous medicine and surgery. Mar-Apr 2013;17(2):114-122.
Kafshdooz T, Akbarzadeh A, Majdi Seghinsara A, Pourhassan M, Nasrabadi HT, Milani M. Role of Probiotics in Managing of Helicobacter Pylori Infection: A Review. Drug research. Feb 2017;67(2):88-93.
Kalman DS, Schwartz HI, Alvarez P, Feldman S, Pezzullo JC, Krieger DR. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. Nov 18 2009;9:85.
Kang BS, Seo JG, Lee GS, Kim JH, Kim SY, Han YW, . . . Park YM. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. Journal of microbiology (Seoul, Korea). Feb 2009;47(1):101-109.
Kang C, Wang B, Kaliannan K, Wang X, Lang H, Hui S, . . . Mi M. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. MBio. May 23 2017;8(3).
Kang C, Zhang Y, Zhu X, Liu K, Wang X, Chen M, . . . Mi M. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. The Journal of clinical endocrinology and metabolism. Dec 2016;101(12):4681-4689.
Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, . . . Krajmalnik-Brown R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. Jan 23 2017;5(1):10.
Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, . . . Louie T. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. Nov 28 2017;318(20):1985-1993.
Kato S. Role of serotonin 5-HT(3) receptors in intestinal inflammation. Biological & pharmaceutical bulletin. 2013;36(9):1406-1409.
Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. Jun 15 2011;474(7351):327-336.
Keen EC. A century of phage research: bacteriophages and the shaping of modern biology. BioEssays: news and reviews in molecular, cellular and developmental biology. Jan 2015;37(1):6-9.
Keleszade E, Kolida S, Costabile A. The cholesterol lowering efficacy of Lactobacillus plantarum ECGC 13110402 in hypercholesterolemic adults: a double-blind, randomized, placebo controlled, pilot human intervention study. Journal of Functional Foods. 2022/02/01/ 2022;89:104939. doi:10.1016/j.jff.2022.104939. https://www.sciencedirect.com/science/article/pii/S1756464622000093
Kelly G. Inulin-type prebiotics--a review: part 1. Alternative medicine review: a journal of clinical therapeutic. Dec 2008;13(4):315-329.
Khalighi A, Behdani R, Kouhestani S. Probiotics: A Comprehensive Review of Their Classification, Mode of Action and Role in Human Nutrition. In: Rao V, Rao LG, eds. Probiotics and Prebiotics in Human Nutrition and Health. Rijeka: InTech; 2016:Ch. 02.
Khanna S, Pardi DS. Clostridium difficile infection: new insights into management. Mayo Clinic proceedings. Nov 2012;87(11):1106-1117.
Kim HJ, Kim HY, Lee SY, Seo JH, Lee E, Hong SJ. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean journal of pediatrics. Sep 2013;56(9):369-376.
Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. Aug 2014;113(2):217-226.
Kirjavainen PV, Pautler S, Baroja ML, Anukam K, Crowley K, Carter K, Reid G. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clinical and vaccine immunology: CVI. Jan 2009;16(1):29-36.
Knaus UG, Hertzberger R, Pircalabioru GG, Yousefi SP, Branco Dos Santos F. Pathogen control at the intestinal mucosa - H2O2 to the rescue. Gut Microbes. Jan 2 2017;8(1):67-74.
Knight R, Callewaert C, Marotz C, Hyde ER, Debelius JW, McDonald D, Sogin ML. The Microbiome and Human Biology. Annual review of genomics and human genetics. Aug 31 2017;18:65-86.
Kober MM, Bowe WP. The effect of probiotics on immune regulation, acne, and photoaging. International journal of women's dermatology. Jun 2015;1(2):85-89.
Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free radical biology & medicine. Mar 2014;68:122-133.
Kohler GA, Assefa S, Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infectious diseases in obstetrics and gynecology. 2012;2012:636474.
Kolokotroni O, Middleton N, Gavatha M, Lamnisos D, Priftis KN, Yiallouros PK. Asthma and atopy in children born by caesarean section: effect modification by family history of allergies - a population based cross-sectional study. BMC Pediatr. Nov 16 2012;12:179.
Kondo J, Xiao JZ, Shirahata A, Baba M, Abe A, Ogawa K, Shimoda T. Modulatory effects of Bifidobacterium longum BB536 on defecation in elderly patients receiving enteral feeding. World journal of gastroenterology: WJG. 2013;19(14):2162-2170.
Konieczna M, Koryszewska-Bagińska A, Bzikowska-Jura A, Chmielewska-Jeznach M, Jarzynka S, Olędzka G. Modifiable and Non-Modifiable Factors That Affect Human Milk Oligosaccharides Composition. Nutrients. Aug 28 2024;16(17)doi:10.3390/nu16172887. https://pubmed.ncbi.nlm.nih.gov/39275203/
Konturek PC, Sliwowski Z, Koziel J, Ptak-Belowska A, Burnat G, Brzozowski T, Konturek SJ. Probiotic bacteria Escherichia coli strain Nissle 1917 attenuates acute gastric lesions induced by stress. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. Dec 2009;60 Suppl 6:41-48.
Korpela K, Salonen A, Virta LJ, Kekkonen RA, de Vos WM. Association of Early-Life Antibiotic Use and Protective Effects of Breastfeeding: Role of the Intestinal Microbiota. JAMA pediatrics. Aug 1 2016;170(8):750-757.
Kovachev SM, Vatcheva-Dobrevska RS. Local Probiotic Therapy for Vaginal Candida albicans Infections. Probiotics and antimicrobial proteins. Mar 2015;7(1):38-44.
Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. Apr 2012;27(2):201-214.
Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swedish dental journal. 2006;30(2):55-60.
Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, . . . Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. Nov 2004;53(11):1617-1623.
Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Alimentary pharmacology & therapeutics. Oct 1997;11(5):853-858.
Kumar N, Behera B, Sagiri SS, Pal K, Ray SS, Roy S. Bacterial vaginosis: Etiology and modalities of treatment-A brief note. Journal of pharmacy & bioallied sciences. Oct 2011;3(4):496-503.
Kumar S, Madurantakam P. Limited evidence shows short-term benefit of probiotics when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis. Evidence-based dentistry. Dec 22 2017;18(4):109-110.
Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical microbiology reviews. Jul 2006;19(3):449-490.
Lamuel-Raventos RM, Onge MS. Prebiotic nut compounds and human microbiota. Critical reviews in food science and nutrition. Sep 22 2017;57(14):3154-3163.
Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 2017;10:63-73.
Langkamp-Henken B, Rowe CC, Ford AL, Christman MC, Nieves C, Jr., Khouri L, . . . Dahl WJ. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: a randomised, double-blind, placebo-controlled study. The British journal of nutrition. Feb 14 2015;113(3):426-434.
Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. International journal of general medicine. 2016;9:27-37.
Lau CS, Ward A, Chamberlain RS. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta-analysis. Infection and drug resistance. 2016;9:275-289.
Lau K, Srivatsav V, Rizwan A, Nashed A, Liu R, Shen R, Akhtar M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients. Aug 10 2017;9(8).
Laue C, Papazova E, Liesegang A, Pannenbeckers A, Arendarski P, Linnerth B, . . . Schrezenmeir J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women - a double-blind, randomised, controlled clinical pilot trial. Benef Microbes. Jan 29 2018;9(1):35-50.
Ledoux D, Labombardi VJ, Karter D. Lactobacillus acidophilus bacteraemia after use of a probiotic in a patient with AIDS and Hodgkin's disease. International journal of STD & AIDS. Apr 2006;17(4):280-282.
Lefevre M, Racedo SM, Denayrolles M, Ripert G, Desfougeres T, Lobach AR, . . . Urdaci MC. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regulatory toxicology and pharmacology: RTP. Feb 2017;83:54-65.
Lefevre M, Racedo SM, Ripert G, Housez B, Cazaubiel M, Maudet C, . . . Urdaci MC. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: a randomized, double-blind placebo-controlled study. Immunity & ageing: I & A. 2015;12:24.
Lemas DJ, Yee S, Cacho N, Miller D, Cardel M, Gurka M, . . . Shenkman E. Exploring the contribution of maternal antibiotics and breastfeeding to development of the infant microbiome and pediatric obesity. Seminars in fetal & neonatal medicine. Dec 2016;21(6):406-409.
Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, . . . Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell host & microbe. May 13 2015;17(5):681-689.
Lepargneur JP. Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann Biol Clin (Paris). Aug 1 2016;74(4):421-427.
Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. Aug 2012;55 Suppl 2:S65-70.
Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, . . . McDonald LC. Burden of Clostridium difficile Infection in the United States. New England Journal of Medicine. 2015;372(9):825-834.
Lew LC, Liong MT. Bioactives from probiotics for dermal health: functions and benefits. J Appl Microbiol. May 2013;114(5):1241-1253.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. Dec 21 2006;444(7122):1022-1023.
Lherm T, Monet C, Nougiere B, Soulier M, Larbi D, Le Gall C, . . . Malbrunot C. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive care medicine. Jun 2002;28(6):797-801.
Li DK, Chen H, Ferber J, Odouli R. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. The lancet. Diabetes & endocrinology. Jan 2017;5(1):18-25.
Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. Aug 6 2017;8(3):162-173.
Lin X, Chen X, Tu Y, Wang S, Chen H. Effect of Probiotic Lactobacilli on the Growth of Streptococcus Mutans and Multispecies Biofilms Isolated from Children with Active Caries. Med Sci Monit. Aug 30 2017;23:4175-4181.
Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, . . . Hackl E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. Aug 24 2017;8(4):545-556.
Liu J, Chen FH, Qiu SQ, Yang LT, Zhang HP, Liu JQ, . . . Yang PC. Probiotics enhance the effect of allergy immunotherapy on regulating antigen specific B cell activity in asthma patients. Am J Transl Res. 2016;8(12):5256-5270.
Liubakka A, Vaughn BP. Clostridium difficile Infection and Fecal Microbiota Transplant. AACN advanced critical care. Jul 2016;27(3):324-337.
Lloyd A, Warren-Walker A, Holt N, et al. SAFETY AND EFFECTS OF HEAT-INACTIVATED LACTOBACILLUS PARACASEI D3.5 (LPD3.5) ON MENTAL WELLBEING IN OLDER ADULTS. Innovation in Aging. 2024;8(Supplement_1):1273-1273. doi:10.1093/geroni/igae098.4069. https://doi.org/10.1093/geroni/igae098.4069
Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome medicine. Apr 27 2016;8(1):51.
Lokossou GAG, Kouakanou L, Schumacher A, Zenclussen AC. Human Breast Milk: From Food to Active Immune Response With Disease Protection in Infants and Mothers. Front Immunol. 2022;13:849012. doi:10.3389/fimmu.2022.849012. https://pmc.ncbi.nlm.nih.gov/articles/PMC9016618/
Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. Jun 2010;8(6):504-508.
Lopes EG, Moreira DA, Gullon P, Gullon B, Cardelle-Cobas A, Tavaria FK. Topical application of probiotics in skin: adhesion, antimicrobial and antibiofilm in vitro assays. J Appl Microbiol. Feb 2017;122(2):450-461.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. Sep 13 2012;489(7415):220-230.
Lu LJ, Liu J. Human Microbiota and Ophthalmic Disease. Yale J Biol Med. Sep 2016;89(3):325-330.
Łubiech K, Twarużek M, Sinkiewicz-Darol E, Martysiak-Żurowska D, Kusznierewicz B. Breast Milk as a Source of Prebiotic Human Milk Oligosaccharides and Bacteria from the Lactobacillaceae Family. Folia Biol (Praha). 2025;71(1):44-53. doi:10.14712/fb2025071010044. https://pubmed.ncbi.nlm.nih.gov/40308202/
Ma J, Zhou Q, Li H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients. Oct 16 2017;9(10).
Maekawa T, Hajishengallis G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. Journal of periodontal research. Dec 2014;49(6):785-791.
Mai V, Ukhanova M, Reinhard MK, Li M, Sulakvelidze A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage. Oct-Dec 2015;5(4):e1088124.
Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, . . . Karri SK. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J. Feb 27 2016;15:21.
Makinen KK. Sugar alcohols, caries incidence, and remineralization of caries lesions: a literature review. International journal of dentistry. 2010;2010:981072.
Mansfield JA, Bergin SW, Cooper JR, Olsen CH. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Military medicine. Jun 2014;179(6):580-592.
Marasco G, Di Biase AR, Schiumerini R, Eusebi LH, Iughetti L, Ravaioli F, . . . Festi D. Gut Microbiota and Celiac Disease. Digestive diseases and sciences. Jun 2016;61(6):1461-1472.
Marchisio P, Santagati M, Scillato M, Baggi E, Fattizzo M, Rosazza C, . . . Principi N. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. Dec 2015;34(12):2377-2383.
Marotz CA, Zarrinpar A. Treating Obesity and Metabolic Syndrome with Fecal Microbiota Transplantation. Yale J Biol Med. Sep 2016;89(3):383-388.
Marranzino G, Villena J, Salva S, Alvarez S. Stimulation of macrophages by immunobiotic Lactobacillus strains: influence beyond the intestinal tract. Microbiology and immunology. Nov 2012;56(11):771-781.
Marrs T, Flohr C. The role of skin and gut microbiota in the development of atopic eczema. Br J Dermatol. Oct 2016;175 Suppl 2:13-18.
Martin-Cabezas R, Davideau JL, Tenenbaum H, Huck O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. Jun 2016;43(6):520-530.
Martin DH. The microbiota of the vagina and its influence on women's health and disease. The American journal of the medical sciences. Jan 2012;343(1):2-9.
Martinez-Martinez MI, Calabuig-Tolsa R, Cauli O. The effect of probiotics as a treatment for constipation in elderly people: A systematic review. Arch Gerontol Geriatr. Jul 2017;71:142-149.
Martinez RC, Franceschini SA, Patta MC, Quintana SM, Candido RC, Ferreira JC, . . . Reid G. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett Appl Microbiol. Mar 2009;48(3):269-274.
Mascellino MT, Porowska B, De Angelis M, Oliva A. Antibiotic susceptibility, heteroresistance, and updated treatment strategies in Helicobacter pylori infection. Drug design, development and therapy. 2017;11:2209-2220.
Matijasic M, Mestrovic T, Peric M, Cipcic Paljetak H, Panek M, Vranesic Bender D, . . . Verbanac D. Modulating Composition and Metabolic Activity of the Gut Microbiota in IBD Patients. International journal of molecular sciences. Apr 19 2016;17(4).
Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nature reviews. Gastroenterology & hepatology. Aug 2013;10(8):473-486.
Mayanagi G, Kimura M, Nakaya S, Hirata H, Sakamoto M, Benno Y, Shimauchi H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: a double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol. Jun 2009;36(6):506-513.
Mayor S. Genome sequence of one individual is published for first time. BMJ (Clinical research ed.). Sep 15 2007;335(7619):530-531.
Mayo Clinic. Diseases and Conditions: Gastritis. https://www.mayoclinic.org/diseases-conditions/gastritis/symptoms-causes/syc-20355807. Last updated 05/18/2017a. Accessed 01/23/2018.
Mayo Clinic. Diseases and Conditions: Peptic Ulcer. https://www.mayoclinic.org/diseases-conditions/peptic-ulcer/symptoms-causes/syc-20354223. Last updated 08/12/2017b. Accessed 01/23/2018.
Maziade PJ, Pereira P, Goldstein EJ. A Decade of Experience in Primary Prevention of Clostridium difficile Infection at a Community Hospital Using the Probiotic Combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. May 15 2015;60 Suppl 2:S144-147.
McCarville JL, Caminero A, Verdu EF. Novel perspectives on therapeutic modulation of the gut microbiota. Therapeutic advances in gastroenterology. Jul 2016;9(4):580-593.
McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. Aug 2016;4(4):546-561.
Meng H, Lee Y, Ba Z, Peng J, Lin J, Boyer AS, . . . Rogers CJ. Consumption of Bifidobacterium animalis subsp. lactis BB-12 impacts upper respiratory tract infection and the function of NK and T cells in healthy adults. Molecular nutrition & food research. May 2016;60(5):1161-1171.
Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, . . . Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition. Mar 2011;105(5):755-764.
Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut microbes. Jul-Aug 2011;2(4):256-261.
Meuric V, Le Gall-David S, Boyer E, Acuna-Amador L, Martin B, Fong SB, . . . Bonnaure-Mallet M. Signature of Microbial Dysbiosis in Periodontitis. Appl Environ Microbiol. Jul 15 2017;83(14).
Michalickova D, Minic R, Dikic N, et al. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. Jul 2016;41(7):782-9. doi:10.1139/apnm-2015-0541. https://www.ncbi.nlm.nih.gov/pubmed/27363733
Michalickova DM, Kostic-Vucicevic MM, Vukasinovic-Vesic MD, et al. Lactobacillus helveticus Lafti L10 Supplementation Modulates Mucosal and Humoral Immunity in Elite Athletes: A Randomized, Double-Blind, Placebo-Controlled Trial. J Strength Cond Res. Jan 2017;31(1):62-70. doi:10.1519/JSC.0000000000001456. https://www.ncbi.nlm.nih.gov/pubmed/27100317
Miller LE, Ouwehand AC. Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World journal of gastroenterology: WJG. Aug 7 2013;19(29):4718-4725.
Miller LE, Zimmermann AK, Ouwehand AC. Contemporary meta-analysis of short-term probiotic consumption on gastrointestinal transit. World journal of gastroenterology: WJG. Jun 7 2016;22(21):5122-5131.
Miraglia Del Giudice M, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Italian journal of pediatrics. Mar 7 2017;43(1):25.
Mirzaei MK, Maurice CF. Menage a trois in the human gut: interactions between host, bacteria and phages. Nature reviews. Microbiology. Jul 2017;15(7):397-408.
Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. Nov 1 2016;4(1):58.
Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, . . . Messina G. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative medicine and cellular longevity. 2017;2017:3831972.
Montella R, Malfa P, Giuliano A, Brustia G, Coïsson JD, Arlorio M. Vaginal adhesion of Lactobacillus plantarum P17630 after probiotic food supplement oral administration: a preliminary in vivo study. Nutrafoods. 2013/06/01 2013;12(2):35-42. doi:10.1007/s13749-013-0030-x
Montemurno E, Cosola C, Dalfino G, Daidone G, De Angelis M, Gobbetti M, Gesualdo L. What Would You Like to Eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney & blood pressure research. Jul 29 2014;39(2-3):114-123.
Moon Y. Microbiome-Linked Crosstalk in the Gastrointestinal Exposome towards Host Health and Disease. Pediatric gastroenterology, hepatology & nutrition. Dec 2016;19(4):221-228.
Mor A, Antonsen S, Kahlert J, Holsteen V, Jorgensen S, Holm-Pedersen J, . . . Ehrenstein V. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. International journal of obesity (2005). Oct 2015;39(10):1450-1455.
Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PloS one. 2012;7(5):e37160.
Morita E, Yokoyama H, Imai D, et al. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients. Apr 17 2019;11(4)doi:10.3390/nu11040868. https://pubmed.ncbi.nlm.nih.gov/30999699/
Morowitz MJ, Carlisle EM, Alverdy JC. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. The Surgical clinics of North America. Aug 2011;91(4):771-785, viii.
Morris G, Berk M, Carvalho AF, Caso JR, Sanz Y, Maes M. The Role of Microbiota and Intestinal Permeability in the Pathophysiology of Autoimmune and Neuroimmune Processes with an Emphasis on Inflammatory Bowel Disease Type 1 Diabetes and Chronic Fatigue Syndrome. Curr Pharm Des. 2016;22(40):6058-6075.
Moya-Perez A, Luczynski P, Renes IB, Wang S, Borre Y, Anthony Ryan C, . . . Cryan JF. Intervention strategies for cesarean section-induced alterations in the microbiota-gut-brain axis. Nutrition reviews. Apr 1 2017;75(4):225-240.
Moyad MA, Robinson LE, Kittelsrud JM, Reeves SG, Weaver SE, Guzman AI, Bubak ME. Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: a randomized, double-blind, placebo-controlled trial. Advances in therapy. 2009;26(8):795-804.
Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends in molecular medicine. Feb 2015;21(2):109-117.
Muizzuddin N, Maher W, Sullivan M, Schnittger S, Mammone T. Physiological effect of a probiotic on skin. Journal of cosmetic science. Nov-Dec 2012;63(6):385-395.
Muzny CA, Schwebke JR. Pathogenesis of Bacterial Vaginosis: Discussion of Current Hypotheses. The Journal of infectious diseases. Aug 15 2016;214 Suppl 1:S1-5.
Nagatomo Y, Tang WH. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J Card Fail. Dec 2015;21(12):973-980.
Nagpal R, Wang S, Ahmadi S, et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep. Aug 23 2018;8(1):12649. doi:10.1038/s41598-018-30114-4. https://www.ncbi.nlm.nih.gov/pubmed/30139941
Nagpal R, Yadav H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann Nutr Metab. 2017;71 Suppl 1:11-16.
Naik NC, Holzhausen EA, Chalifour BN, et al. Air pollution exposure may impact the composition of human milk oligosaccharides. Sci Rep. Mar 20 2024;14(1):6730. doi:10.1038/s41598-024-57158-z. https://pubmed.ncbi.nlm.nih.gov/38509153/
Nakae H, Tsuda A, Matsuoka T, Mine T, Koga Y. Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ open gastroenterology. 2016;3(1):e000109.
Nale JY, Spencer J, Hargreaves KR, Buckley AM, Trzepinski P, Douce GR, Clokie MR. Bacteriophage Combinations Significantly Reduce Clostridium difficile Growth In Vitro and Proliferation In Vivo. Antimicrob Agents Chemother. Feb 2016;60(2):968-981.
Namba K, Hatano M, Yaeshima T, Takase M, Suzuki K. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Bioscience, biotechnology, and biochemistry. 2010;74(5):939-945.
Nayak PA, Nayak UA, Khandelwal V. The effect of xylitol on dental caries and oral flora. Clinical, cosmetic and investigational dentistry. 2014;6:89-94.
Nie YF, Hu J, Yan XH. Cross-talk between bile acids and intestinal microbiota in host metabolism and health. Journal of Zhejiang University. Science. B. Jun 2015;16(6):436-446.
Noh K, Kang YR, Nepal MR, Shakya R, Kang MJ, Kang W, . . . Jeong TC. Impact of gut microbiota on drug metabolism: an update for safe and effective use of drugs. Archives of pharmacal research. Dec 2017;40(12):1345-1355.
Nordenstedt H, Graham DY, Kramer JR, Rugge M, Verstovsek G, Fitzgerald S, . . . El-Serag HB. Helicobacter pylori-negative gastritis: prevalence and risk factors. The American journal of gastroenterology. Jan 2013;108(1):65-71.
Notay M, Foolad N, Vaughn AR, Sivamani RK. Probiotics, Prebiotics, and Synbiotics for the Treatment and Prevention of Adult Dermatological Diseases. American journal of clinical dermatology. Dec 2017;18(6):721-732.
O'Callaghan A, van Sinderen D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front Microbiol. 2016;7:925.
O'Dwyer DN, Dickson RP, Moore BB. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J Immunol. Jun 15 2016;196(12):4839-4847.
Ofosu A. Clostridium difficile infection: a review of current and emerging therapies. Annals of Gastroenterology: Quarterly Publication of the Hellenic Society of Gastroenterology. Apr-Jun 2016;29(2):147-154.
Oh J, Byrd AL, Park M, Kong HH, Segre JA. Temporal Stability of the Human Skin Microbiome. Cell. May 5 2016;165(4):854-866.
Ohlsson B, Orho-Melander M, Nilsson PM. Higher Levels of Serum Zonulin May Rather Be Associated with Increased Risk of Obesity and Hyperlipidemia, Than with Gastrointestinal Symptoms or Disease Manifestations. International journal of molecular sciences. Mar 8 2017;18(3).
Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. Mar 2010;7(3):163-173.
Ohtsu T, Takagi A, Uemura N, Inoue K, Sekino H, Kawashima A, . . . Koga Y. The Ameliorating Effect of Lactobacillus gasseri OLL2716 on Functional Dyspepsia in Helicobacter pylori-Uninfected Individuals: A Randomized Controlled Study. Digestion. 2017;96(2):92-102.
Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, . . . Shimazu T. Metagenomic Analysis Reveals Dynamic Changes of Whole Gut Microbiota in the Acute Phase of Intensive Care Unit Patients. Digestive diseases and sciences. Jun 2016;61(6):1628-1634.
Ouwehand AC. A review of dose-responses of probiotics in human studies. Benef Microbes. Apr 26 2017;8(2):143-151.
Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients. Feb 6 2016;8(2):78.
Paiva SA, Sepe TE, Booth SL, Camilo ME, O'Brien ME, Davidson KW, . . . Russell RM. Interaction between vitamin K nutriture and bacterial overgrowth in hypochlorhydria induced by omeprazole. The American journal of clinical nutrition. Sep 1998;68(3):699-704.
Palacios S, Espadaler J, Fernandez-Moya JM, Prieto C, Salas N. Is it possible to prevent recurrent vulvovaginitis? The role of Lactobacillus plantarum I1001 (CECT7504). European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. Oct 2016;35(10):1701-1708.
Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A, Panici PB. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC infectious diseases. Jan 5 2018;18(1):13.
Palumbo VD, Romeo M, Marino Gammazza A, Carini F, Damiani P, Damiano G, . . . Tomasello G. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. Sep 2016;160(3):372-377.
Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science (New York, N.Y.). Apr 29 2016;352(6285):535-538.
Pang S, Chen X, Lu Z, et al. Longevity of centenarians is reflected by the gut microbiome with youth-associated signatures. Nat Aging. Apr 2023;3(4):436-449. doi:10.1038/s43587-023-00389-y. https://pubmed.ncbi.nlm.nih.gov/37117794/
Parma M, Stella Vanni V, Bertini M, Candiani M. Probiotics in the prevention of recurrences of bacterial vaginosis. Altern Ther Health Med. Winter 2014;20 Suppl 1:52-57.
Paulino LC. New perspectives on dandruff and seborrheic dermatitis: lessons we learned from bacterial and fungal skin microbiota. European journal of dermatology : EJD. Jun 1 2017;27(S1):4-7.
Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach JF, La Vecchia C. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology (Cambridge, Mass.). May 2012;23(3):402-414.
Perapoch J, Planes AM, Querol A, Lopez V, Martinez-Bendayan I, Tormo R, . . . Salcedo S. Fungemia with Saccharomyces cerevisiae in two newborns, only one of whom had been treated with ultra-levura. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. Jun 2000;19(6):468-470.
Petersen AM, Mirsepasi H, Halkjaer SI, Mortensen EM, Nordgaard-Lassen I, Krogfelt KA. Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: a double-blind randomized placebo controlled clinical trial. Journal of Crohn's & colitis. Nov 2014;8(11):1498-1505.
Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, . . . Guyer M. The NIH Human Microbiome Project. Genome Res. Dec 2009;19(12):2317-2323.
Pichon M, Lina B, Josset L. Impact of the Respiratory Microbiome on Host Responses to Respiratory Viral Infection. Vaccines. Nov 3 2017;5(4).
Plein K, Hotz J. Therapeutic effects of Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn's disease with special respect to chronic diarrhea--a pilot study. Z Gastroenterol. Feb 1993;31(2):129-134.
Ponziani FR, Gerardi V, Gasbarrini A. Diagnosis and treatment of small intestinal bacterial overgrowth. Expert review of gastroenterology & hepatology. 2016;10(2):215-227.
Potgieter M, Bester J, Kell DB, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev. Jul 2015;39(4):567-591.
Pregliasco F, Anselmi G, Fonte L, Giussani F, Schieppati S, Soletti L. A new chance of preventing winter diseases by the administration of synbiotic formulations. Journal of clinical gastroenterology. Sep 2008;42 Suppl 3 Pt 2:S224-233.
Prescott SL, Larcombe DL, Logan AC, West C, Burks W, Caraballo L, . . . Campbell DE. The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. The World Allergy Organization journal. 2017;10(1):29.
Proctor DM, Relman DA. The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat. Cell Host Microbe. Apr 12 2017;21(4):421-432.
Pu F, Guo Y, Li M, Zhu H, Wang S, Shen X, . . . He F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin Interv Aging. 2017;12:1223-1231.
Quick M. Cochrane Commentary: Probiotics For Prevention of Acute Upper Respiratory Infection. Explore (New York, N.Y.). Sep-Oct 2015;11(5):418-420.
Quigley EMM. The Gut-Brain Axis and the Microbiome: Clues to Pathophysiology and Opportunities for Novel Management Strategies in Irritable Bowel Syndrome (IBS). J Clin Med. Jan 3 2018;7(1).
Ramirez-Perez O, Cruz-Ramon V, Chinchilla-Lopez P, Mendez-Sanchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann Hepatol. Oct 28 2017;16(0):15-20.
Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. Aug 21 1999;354(9179):635-639.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577-1585.
Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile Acids and the Gut Microbiome. Current opinion in gastroenterology. May 2014;30(3):332-338.
Riley P, Moore D, Ahmed F, Sharif MO, Worthington HV. Xylitol-containing products for preventing dental caries in children and adults. The Cochrane database of systematic reviews. Mar 26 2015(3):Cd010743.
Robson MJ, Quinlan MA, Blakely RD. Immune System Activation and Depression: Roles of Serotonin in the Central Nervous System and Periphery. ACS chemical neuroscience. May 17 2017;8(5):932-942.
Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, . . . Collado MC. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050.
Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends in endocrinology and metabolism: TEM. Sep 2015;26(9):493-501.
Rosier BT, Marsh PD, Mira A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. Journal of dental research. Nov 1 2017:22034517742139.
Roudsari MR, Karimi R, Sohrabvandi S, Mortazavian AM. Health effects of probiotics on the skin. Critical reviews in food science and nutrition. 2015;55(9):1219-1240.
Roux D, van Oort PM, Ricard JD, Bos LDJ. Airway microbiome research: a modern perspective on surveillance cultures? Annals of translational medicine. Nov 2017;5(22):445.
Ruggiero P. Use of probiotics in the fight against Helicobacter pylori. World journal of gastrointestinal pathophysiology. Nov 15 2014;5(4):384-391.
Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. Jul 30 2016;16(1):86.
Ryan PM, Stanton C, Caplice NM. Bile acids at the cross-roads of gut microbiome-host cardiometabolic interactions. Diabetol Metab Syndr. 2017;9:102.
Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Therapeutic advances in chronic disease. Sep 2013;4(5):223-231.
Saini R, Saini S, Sharma S. Potential of probiotics in controlling cardiovascular diseases. Journal of cardiovascular disease research. Oct 2010;1(4):213-214.
Sajedinejad N, Paknejad M, Houshmand B, Sharafi H, Jelodar R, Shahbani Zahiri H, Noghabi KA. Lactobacillus salivarius NK02: a Potent Probiotic for Clinical Application in Mouthwash. Probiotics and antimicrobial proteins. Jun 19 2017.
Salvesi C, Silvi S, Fiorini D, et al. Impact of a probiotic diet on well-being of healthy senior: THE PROBIOSENIOR PROJECT. J Appl Microbiol. Nov 2022;133(5):2941-2953. doi:10.1111/jam.15747. https://www.ncbi.nlm.nih.gov/pubmed/35938351
Salvesi C, Silvi S, Fiorini D, et al. Six-Month Synbio((R)) Administration Affects Nutritional and Inflammatory Parameters of Older Adults Included in the PROBIOSENIOR Project. Microorganisms. Mar 21 2023;11(3)doi:10.3390/microorganisms11030801. https://www.ncbi.nlm.nih.gov/pubmed/36985374
Salvetti E, Torriani S, Felis GE. The Genus Lactobacillus: A Taxonomic Update. Probiotics and antimicrobial proteins. Dec 2012;4(4):217-226.
Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hormannsperger G, . . . Vaughan E. Safety assessment of probiotics for human use. Gut Microbes. May-Jun 2010;1(3):164-185.
SanMiguel A, Grice EA. Interactions between host factors and the skin microbiome. Cellular and molecular life sciences: CMLS. Apr 2015;72(8):1499-1515.
Santagati M, Scillato M, Muscaridola N, Metoldo V, La Mantia I, Stefani S. Colonization, safety, and tolerability study of the Streptococcus salivarius 24SMBc nasal spray for its application in upper respiratory tract infections. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. Oct 2015;34(10):2075-2080.
Schenck LP, Surette MG, Bowdish DM. Composition and immunological significance of the upper respiratory tract microbiota. FEBS letters. Nov 2016;590(21):3705-3720.
Schenk S, Bode L, Jensen SR, Schönknecht YB, Simon MC. Systemic Availability of Human Milk Oligosaccharides in Infants and Adults: A Narrative Review. Adv Nutr. Aug 6 2025:100488. doi:10.1016/j.advnut.2025.100488. https://pubmed.ncbi.nlm.nih.gov/40780447/
Schönknecht YB, Moreno Tovar MV, Jensen SR, Parschat K. Clinical Studies on the Supplementation of Manufactured Human Milk Oligosaccharides: A Systematic Review. Nutrients. Aug 17 2023;15(16)doi:10.3390/nu15163622. https://pubmed.ncbi.nlm.nih.gov/37630811/
Sedighi M, Razavi S, Navab-Moghadam F, Khamseh ME, Alaei-Shahmiri F, Mehrtash A, Amirmozafari N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microbial pathogenesis. Oct 2017;111:362-369.
Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. Jan 28 2016;164(3):337-340.
Senol A, Isler M, Karahan AG, Kilic GB, Kuleasan H, Goren I, . . . Cakmakci LM. Effect of probiotics on aspirin-induced gastric mucosal lesions. The Turkish journal of gastroenterology: the official journal of Turkish Society of Gastroenterology. Feb 2011;22(1):18-26.
Sghir A, Gramet G, Suau A, Rochet V, Pochart P, Dore J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl Environ Microbiol. May 2000;66(5):2263-2266.
Sharafedtinov KK, Plotnikova OA, Alexeeva RI, Sentsova TB, Songisepp E, Stsepetova J, . . . Mikelsaar M. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients--a randomized double-blind placebo-controlled pilot study. Nutr J. Oct 12 2013;12:138.
Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell metabolism. Nov 4 2014;20(5):719-730.
Shen NT, Maw A, Tmanova LL, Pino A, Ancy K, Crawford CV, . . . Evans AT. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology. Jun 2017;152(8):1889-1900.e1889.
Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS. Beneficial Properties of Probiotics. Tropical life sciences research. Aug 2016;27(2):73-90.
Shi Y, Mu L. An expanding stage for commensal microbes in host immune regulation. Cellular & molecular immunology. Apr 2017;14(4):339-348.
Shibata N, Kunisawa J, Kiyono H. Dietary and Microbial Metabolites in the Regulation of Host Immunity. Front Microbiol. 2017;8:2171.
Shida K, Sato T, Iizuka R, Hoshi R, Watanabe O, Igarashi T, . . . Ishikawa F. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur J Nutr. Feb 2017;56(1):45-53.
Shimauchi H, Mayanagi G, Nakaya S, Minamibuchi M, Ito Y, Yamaki K, Hirata H. Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: a randomized, double-blind, placebo-controlled study. J Clin Periodontol. Oct 2008;35(10):897-905.
Shimizu M, Hashiguchi M, Shiga T, Tamura HO, Mochizuki M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PloS one. 2015;10(10):e0139795.
Silva Figueiredo P, Carla Inada A, Marcelino G, Maiara Lopes Cardozo C, de Cassia Freitas K, de Cassia Avellaneda Guimaraes R, . . . Aiko Hiane P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients. Oct 22 2017;9(10).
Sinagra E, Morreale GC, Mohammadian G, Fusco G, Guarnotta V, Tomasello G, . . . Raimondo D. New therapeutic perspectives in irritable bowel syndrome: Targeting low-grade inflammation, immuno-neuroendocrine axis, motility, secretion and beyond. World journal of gastroenterology: WJG. Sep 28 2017;23(36):6593-6627.
Singh A, Hacini-Rachinel F, Gosoniu ML, Bourdeau T, Holvoet S, Doucet-Ladeveze R, . . . Nutten S. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: an exploratory, randomized, placebo-controlled clinical trial. European journal of clinical nutrition. Feb 2013;67(2):161-167.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, . . . Liao W. Influence of diet on the gut microbiome and implications for human health. J Transl Med. Apr 8 2017;15(1):73.
Skovbjerg S, Roos K, Holm SE, Grahn Hakansson E, Nowrouzian F, Ivarsson M, . . . Wold AE. Spray bacteriotherapy decreases middle ear fluid in children with secretory otitis media. Archives of disease in childhood. Feb 2009;94(2):92-98.
Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. Apr 22 2013;5(4):1417-1435.
Slyepchenko A, Maes M, Jacka FN, Kohler CA, Barichello T, McIntyre RS, . . . Carvalho AF. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychotherapy and psychosomatics. 2017;86(1):31-46.
Smith TJ, Rigassio-Radler D, Denmark R, Haley T, Touger-Decker R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12(R) on health-related quality of life in college students affected by upper respiratory infections. The British journal of nutrition. Jun 2013;109(11):1999-2007.
Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, . . . Knight R. Cohabiting family members share microbiota with one another and with their dogs. Elife. Apr 16 2013;2:e00458.
Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. Nov 2009;7(11):1202-1209, 1209.e1201.
Soto A, Martín V, Jiménez E, Mader I, Rodríguez JM, Fernández L. Lactobacilli and Bifidobacteria in Human Breast Milk: Influence of Antibiotherapy and Other Host and Clinical Factors. Journal of pediatric gastroenterology and nutrition. Jul 2014;59(1):78-88.
Spreckels JE, Kurilshikov A, Fernández-Pato A, et al. Host and environmental determinants of human milk oligosaccharides and microbiota in the Lifelines NEXT cohort. Cell reports. Aug 8 2025;44(8):116124. doi:10.1016/j.celrep.2025.116124. https://pubmed.ncbi.nlm.nih.gov/40783943/
Stadlbauer V. Immunosuppression and probiotics: are they effective and safe? Benef Microbes. 2015;6(6):823-828.
Stahl B, Barrangou R. Complete Genome Sequence of Probiotic Strain Lactobacillus acidophilus La-14. Genome announcements. Jun 20 2013;1(3).
Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, . . . Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. May 2011;52(10):1212-1217.
Staudacher HM, Whelan K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: probiotics, prebiotics and the low FODMAP diet. The Proceedings of the Nutrition Society. Aug 2016;75(3):306-318.
Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, . . . De Filippo C. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. Feb 22 2017;5(1):24.
Strus M, Chmielarczyk A, Kochan P, Adamski P, Chelmicki Z, Chelmicki A, . . . Heczko PB. Studies on the effects of probiotic Lactobacillus mixture given orally on vaginal and rectal colonization and on parameters of vaginal health in women with intermediate vaginal flora. European journal of obstetrics, gynecology, and reproductive biology. Aug 2012;163(2):210-215.
Sun W, Tao L, Qian C, Xue PP, Du SS, Tao YN. Human milk oligosaccharides: bridging the gap in intestinal microbiota between mothers and infants. Frontiers in cellular and infection microbiology. 2024;14:1386421. doi:10.3389/fcimb.2024.1386421. https://pubmed.ncbi.nlm.nih.gov/39835278/
Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence Properties of Probiotics in Combating Microbial Pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
Swanson HI. Drug Metabolism by the Host and Gut Microbiota: A Partnership or Rivalry? Drug Metab Dispos. Oct 2015;43(10):1499-1504.
Szafranski SP, Winkel A, Stiesch M. The use of bacteriophages to biocontrol oral biofilms. Journal of biotechnology. May 20 2017;250:29-44.
Szajewska H, Kolodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Alimentary pharmacology & therapeutics. Nov 2015a;42(10):1149-1157.
Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Alimentary pharmacology & therapeutics. Oct 2015b;42(7):793-801.
Szkaradkiewicz AK, Stopa J, Karpinski TM. Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on pro-inflammatory cytokine response in patients with chronic periodontitis. Arch Immunol Ther Exp (Warsz). Dec 2014;62(6):495-500.
Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, . . . Shibatouge M. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. Jan 2016;28(1):67-74.
Taoka H, Yokoyama Y, Morimoto K, Kitamura N, Tanigaki T, Takashina Y, . . . Watanabe M. Role of bile acids in the regulation of the metabolic pathways. World J Diabetes. Jul 10 2016;7(13):260-270.
Tasnim N, Abulizi N, Pither J, Hart MM, Gibson DL. Linking the Gut Microbial Ecosystem with the Environment: Does Gut Health Depend on Where We Live? Front Microbiol. 2017;8:1935.
Tekce M, Ince G, Gursoy H, Dirikan Ipci S, Cakar G, Kadir T, Yilmaz S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: a 1-year follow-up study. J Clin Periodontol. Apr 2015;42(4):363-372.
Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. Nov 2013;40(11):1025-1035.
Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, . . . Elinav E. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. Oct 23 2014;159(3):514-529.
Theilmann MC, Goh YJ, Nielsen KF, Klaenhammer TR, Barrangou R, Abou Hachem M. Lactobacillus acidophilus Metabolizes Dietary Plant Glucosides and Externalizes Their Bioactive Phytochemicals. MBio. Nov 21 2017;8(6).
Thomas-White K, Brady M, Wolfe AJ, Mueller ER. The bladder is not sterile: History and current discoveries on the urinary microbiome. Current bladder dysfunction reports. Mar 2016;11(1):18-24.
Thompson JR. Is irritable bowel syndrome an infectious disease? World journal of gastroenterology: WJG. Jan 28 2016;22(4):1331-1334.
Thursby E, Juge N. Introduction to the human gut microbiota. The Biochemical journal. May 16 2017;474(11):1823-1836.
Tiequn B, Guanqun C, Shuo Z. Therapeutic effects of Lactobacillus in treating irritable bowel syndrome: a meta-analysis. Intern Med. 2015;54(3):243-249.
Toiviainen A, Jalasvuori H, Lahti E, Gursoy U, Salminen S, Fontana M, . . . Soderling E. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin Oral Investig. Jan 2015;19(1):77-83.
Tomas-Barberan FA, Selma MV, Espin JC. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Current opinion in clinical nutrition and metabolic care. Nov 2016;19(6):471-476.
Torii S, Torii A, Itoh K, Urisu A, Terada A, Fujisawa T, . . . Fujiwara S. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum markers of atopic dermatitis in children. Int Arch Allergy Immunol. 2011;154(3):236-245.
Tosh PK, McDonald LC. Infection Control in the Multidrug-Resistant Era: Tending the Human Microbiome. Clinical Infectious Diseases. 2012;54(5):707-713.
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. Oct 18 2007;449(7164):804-810.
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Science translational medicine. 2009;1(6):6ra14-16ra14.
Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, . . . Gasbarrini A. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. The American journal of gastroenterology. Oct 2010;105(10):2218-2227.
Uehara S, Monden K, Nomoto K, Seno Y, Kariyama R, Kumon H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. International journal of antimicrobial agents. Aug 2006;28 Suppl 1:S30-34.
Upadrasta A, Madempudi RS. Probiotics and blood pressure: current insights. Integrated blood pressure control. 2016;9:33-42.
Urashima T, Ajisaka K, Ujihara T, Nakazaki E. Recent advances in the science of human milk oligosaccharides. BBA Adv. 2025;7:100136. doi:10.1016/j.bbadva.2024.100136. https://pubmed.ncbi.nlm.nih.gov/39991261/
Urbaniak C, Burton JP, Reid G. Breast, milk and microbes: a complex relationship that does not end with lactation. Womens Health (Lond). Jul 2012;8(4):385-398.
Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Alimentary pharmacology & therapeutics. May 2016;43(10):1025-1034.
Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the Human Microbiome. Nutrition reviews. Aug 2012;70(Suppl 1):S38-44.
Vahabnezhad E, Mochon AB, Wozniak LJ, Ziring DA. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. Journal of clinical gastroenterology. May-Jun 2013;47(5):437-439.
van de Wijgert J, Jespers V. The global health impact of vaginal dysbiosis. Res Microbiol. Nov - Dec 2017;168(9-10):859-864.
van der Meulen TA, Harmsen H, Bootsma H, Spijkervet F, Kroese F, Vissink A. The microbiome-systemic diseases connection. Oral diseases. Nov 2016;22(8):719-734.
Vanamala JK, Knight R, Spector TD. Can Your Microbiome Tell You What to Eat? Cell metabolism. Dec 1 2015;22(6):960-961.
Vaneechoutte M. The human vaginal microbial community. Res Microbiol. Nov - Dec 2017;168(9-10):811-825.
Verdenelli MC, Cecchini C, Coman MM, Silvi S, Orpianesi C, Coata G, . . . Di Renzo GC. Impact of Probiotic SYNBIO((R)) Administered by Vaginal Suppositories in Promoting Vaginal Health of Apparently Healthy Women. Curr Microbiol. Oct 2016;73(4):483-490.
Vicariotto F, Mogna L, Del Piano M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: a pilot study. Journal of clinical gastroenterology. Nov-Dec 2014;48 Suppl 1:S106-112.
Vinke PC, El Aidy S, van Dijk G. The Role of Supplemental Complex Dietary Carbohydrates and Gut Microbiota in Promoting Cardiometabolic and Immunological Health in Obesity: Lessons from Healthy Non-Obese Individuals. Frontiers in nutrition. 2017;4:34.
Vivekananda MR, Vandana KL, Bhat KG. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical trial. Journal of oral microbiology. Nov 2 2010;2.
Vladareanu R, Mihu D, Mitran M, et al. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): a randomized double-blind placebo-controlled study. European review for medical and pharmacological sciences. Jan 2018;22(1):262-267. doi:10.26355/eurrev_201801_14128
Vujic G, Jajac Knez A, Despot Stefanovic V, Kuzmic Vrbanovic V. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. European journal of obstetrics, gynecology, and reproductive biology. May 2013;168(1):75-79.
Wagner RD, Johnson SJ. Probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J Biomed Sci. Jun 20 2012;19:58.
Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell metabolism. Jul 12 2016;24(1):41-50.
Waki N, Matsumoto M, Fukui Y, Suganuma H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: an open-label pilot study. Lett Appl Microbiol. Dec 2014;59(6):565-571.
Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Annals of general psychiatry. 2017;16:14.
Wan XQ, Tang DS, Liu AP, Tan SY, Li WK, Kuang J, Li HM. [Isolation of a wild-type virulent phage of Helicobacter pylori and its simulated treatments of gastrointestinal Hp in vitro]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. Feb 2011;31(2):304-307.
Wang J, Li F, Tian Z. Role of microbiota on lung homeostasis and diseases. Science China. Life sciences. Dec 2017;60(12):1407-1415.
Wang JZ, Du WT, Xu YL, Cheng SZ, Liu ZJ. Gut microbiome-based medical methodologies for early-stage disease prevention. Microbial pathogenesis. Apr 2017;105:122-130.
Wang S, Ahmadi S, Nagpal R, et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: from C. elegans to mice. GeroScience. Feb 2020;42(1):333-352. doi:10.1007/s11357-019-00137-4. https://www.ncbi.nlm.nih.gov/pubmed/31814084
Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, Hooper LV. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science (New York, N.Y.). Sep 1 2017;357(6354):912-916.
Wasfi R, Abd El-Rahman OA, Zafer MM, Ashour HM. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. Journal of cellular and molecular medicine. Mar 2018;22(3):1972-1983.
Weese JS, Martin H. Assessment of commercial probiotic bacterial contents and label accuracy. The Canadian veterinary journal = La revue veterinaire canadienne. Jan 2011;52(1):43-46.
Welin A. Survival of L. acidophilus and L. casei in the Human GI Tract. Perceived Effects on Health. Nutrafoods. Vol. 4(2/3)2005:9-14
West CE, Jenmalm MC, Kozyrskyj AL, Prescott SL. Probiotics for treatment and primary prevention of allergic diseases and asthma: looking back and moving forward. Expert review of clinical immunology. Jun 2016;12(6):625-639.
West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PA, Cripps AW. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clinical nutrition (Edinburgh, Scotland). Aug 2014;33(4):581-587.
Whisner CM, Castillo LF. Prebiotics, Bone and Mineral Metabolism. Calcified tissue international. Oct 27 2017.
Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract--a role beyond infection. Nature reviews. Urology. Feb 2015;12(2):81-90.
Wilcox CR, Stuart B, Leaver H, et al. Effectiveness of the probiotic Streptococcus salivarius K12 for the treatment and/or prevention of sore throat: a systematic review. Clin Microbiol Infect. Jun 2019;25(6):673-680. doi:10.1016/j.cmi.2018.12.031. https://pubmed.ncbi.nlm.nih.gov/30616011/
Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. American family physician. Aug 1 2017;96(3):170-178.
Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and 'dysbiosis therapy' in critical illness. Current opinion in critical care. Aug 2016;22(4):347-353.
Wollina U. Microbiome in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:51-56.
Wong VW, Martindale RG, Longaker MT, Gurtner GC. From germ theory to germ therapy: skin microbiota, chronic wounds, and probiotics. Plastic and reconstructive surgery. Nov 2013;132(5):854e-861e.
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, . . . Backhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. Jul 2017;23(7):850-858.
Xiao JZ, Kondo S, Yanagisawa N, Miyaji K, Enomoto K, Sakoda T, . . . Enomoto T. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergology international: official journal of the Japanese Society of Allergology. Mar 2007;56(1):67-75.
Xu Z, Knight R. Dietary effects on human gut microbiome diversity. The British journal of nutrition. Jan 2015;113 Suppl:S1-5.
Yamamoto K, Yokoyama K, Matsukawa T, Kato S, Kato S, Yamada K, Hirota T. Efficacy of prolonged ingestion of Lactobacillus acidophilus L-92 in adult patients with atopic dermatitis. J Dairy Sci. Jul 2016;99(7):5039-5046.
Yamazaki Y, Nakamura Y, Nunez G. Role of the microbiota in skin immunity and atopic dermatitis. Allergology international: official journal of the Japanese Society of Allergology. Oct 2017;66(4):539-544.
Yang BG, Hur KY, Lee MS. Alterations in Gut Microbiota and Immunity by Dietary Fat. Yonsei medical journal. Nov 2017;58(6):1083-1091.
Yang L, Chaudhary N, Baghdadi J, Pei Z. Microbiome in reflux disorders and esophageal adenocarcinoma. Cancer J. May-Jun 2014;20(3):207-210.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, . . . Gordon JI. Human gut microbiome viewed across age and geography. Nature. May 9 2012;486(7402):222-227.
Yen M, Cairns LS, Camilli A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nature communications. Feb 1 2017;8:14187.
Yi DY, Kim SY. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients. Sep 2 2021;13(9)doi:10.3390/nu13093094. https://pubmed.ncbi.nlm.nih.gov/34578971/
Yousuf A, Sidiq M, Ganta S, Nagaraj A, Vishnani P, Jan I. Effect of Freeze Dried Powdered Probiotics on Gingival Status and Plaque Inhibition: A Randomized, Double-blind, Parallel Study. Contemporary clinical dentistry. Jan-Mar 2017;8(1):116-121.
Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. Apr 2015;63(4):776-781.
Zarrinpar A, Chaix A, Panda S. Daily Eating Patterns and Their Impact on Health and Disease. Trends in endocrinology and metabolism: TEM. Feb 2016;27(2):69-83.
Zhang MM, Qian W, Qin YY, He J, Zhou YH. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World journal of gastroenterology: WJG. Apr 14 2015;21(14):4345-4357.
Zhang Y, Li L, Guo C, Mu D, Feng B, Zuo X, Li Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol. Jun 13 2016;16(1):62.
Zhao Y, Yu YB. Intestinal microbiota and chronic constipation. Springerplus. 2016;5(1):1130.
Zheng H, Liang H, Wang Y, Miao M, Shi T, Yang F, . . . Li DK. Altered Gut Microbiota Composition Associated with Eczema in Infants. PloS one. 2016;11(11):e0166026.
Zoccali G, Cinque B, La Torre C, Lombardi F, Palumbo P, Romano L, . . . Giuliani M. Improving the outcome of fractional CO2 laser resurfacing using a probiotic skin cream: Preliminary clinical evaluation. Lasers in medical science. Nov 2016;31(8):1607-1611.
Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, . . . Gasbarrini A. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Alimentary pharmacology & therapeutics. Jun 1 2006;23(11):1567-1574.
Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients. 2020;12(8):2189. doi:10.3390/nu12082189
Zukiewicz-Sobczak W, Wroblewska P, Adamczuk P, Silny W. Probiotic lactic acid bacteria and their potential in the prevention and treatment of allergic diseases. Central-European journal of immunology / Polish Society for Immunology and eleven other Central-European immunological societies. 2014;39(1):104-108.
Zupancic K, Kriksic V, Kovacevic I, Kovacevic D. Influence of Oral Probiotic Streptococcus salivarius K12 on Ear and Oral Cavity Health in Humans: Systematic Review. Probiotics and antimicrobial proteins. Jun 2017;9(2):102-110.