Life Extension Magazine®
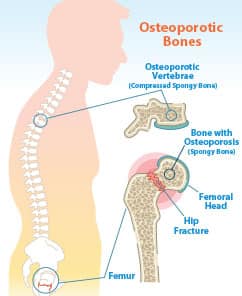
Osteoporosis affects a staggering number of men and women.
Among those 50 and older, 30% of women and 16% of men have osteoporosis.
In people over 80, those figures skyrocket to 77% of women and 46% of men.1
The disease causes bones to become weak, brittle, and prone to breaking.
But those aren’t the only dangers.
New evidence shows that having osteoporosis is associated with accelerated aging along with an increased risk of developing cardiovascular disease, cancer, and dementia.
Osteoporosis-induced age acceleration begins in the early stages of bone loss, before most people even know they have it.
Aging bones contain harmful senescent cells—cells that have stopped replicating, and release destructive signaling molecules.2,3
As these inflammatory signals travel through the body they induce a host of degenerative disorders, including dementia and cancer.4-7
Most readers of this magazine take steps to preserve their bone density.
How Bone Loss Speeds Aging
Healthy bone is constantly being remodeled. Old bone is broken down and new bone is made.
Cells that build bone are called osteoblasts. Cells that break down bone are called osteoclasts.
In young, growing bodies, osteoblast activity surpasses osteoclast activity. In healthy adulthood, the activities are roughly balanced.
But as we age, osteoclast activity begins to exceed osteoblast activity. That leads to bone mineral loss, contributing to higher risk of fractures.
In people suffering from osteoporosis, the activity of osteoclasts is especially high.
Researchers have learned that osteoclasts send out signals that can promote system-wide inflammation.8-11 Chronic inflammation increases risks for cardiovascular disease, cancer, and dementia, and the acceleration of aging.12
Aging bones are a site where senescent cells accumulate with aging.13
These senescent cells damage bones by increasing bone resorption (breakdown) while decreasing new bone formation (the definition of osteoporosis).
Senescent cells and the harmful products they release contribute not only to osteoporosis, but also Alzheimer’s, type II diabetes, cancer, and cardiovascular disease.4,13-15
Osteoporosis and Heart Disease
Osteoporosis increases the risk of cardiovascular disease in ways that go beyond inflammation.
In one study, scientists examined heavily calcified arteries, a major contributor to arterial stiffening and cardiovascular disease, and determined that actual bone was present 6% of the time.16
Osteoclast-like cells are often found in plaque deposits in the arteries.17,18 In osteoporosis patients, a marker of inflammation has been shown to be elevated, along with an increased risk for cardiovascular disease.19
Vitamin K2 helps prevent this type of arterial calcification.20
What you need to know
- Osteoporosis weakens the bones of millions of aging women and men in America, markedly increasing their risk of fractures.
- Studies now show that the process of bone breakdown releases potent pro-inflammatory molecules throughout the body.
- Increased inflammation raises the risk for disease and contributes directly to an acceleration of the aging process.
- Supplementation with eight different nutrients known to protect the bones, including calcium, magnesium, vitamin D, and vitamin K, can help combat osteoporosis, fight inflammation, and slow aging.
Osteoporosis and Dementia

People with Alzheimer’s disease frequently have low bone mineral density and a higher rate of hip fractures compared to non-Alzheimer’s patients.21
Alzheimer’s disease prevalence is also higher in postmenopausal women with severe osteoporosis, especially those with femoral fractures, than in those without osteoporosis.22 These findings suggest close connections between the two apparently different conditions.
Recent studies have identified disruption of important signaling molecules (in addition to inflammatory cytokines) that seem to drive this association.
In particular is a system called RANKL, which is involved in switching on and off certain genes involved in both osteoporosis and many dementias.23
A related system involving colony stimulating factor-1 regulates both bone resorption by osteoclasts, and also immune cells in the brain that regulate brain cell survival.24
And, the finding of large numbers of senescent cells in osteoporotic bones is likely to further accelerate body-wide aging, including in the brain.4,14
Osteoporosis and Cancer Risk
People with osteoporosis have an increased risk of cancer compared to those whose bones remain healthy with aging.25-27 While having cancer can weaken bones through malnutrition and metastases, the opposite finding is a surprise.
A recent study suggests links between osteoporosis and the risk of cancer. Bone proteins associated with osteoporosis have been identified in the molecular pathways leading to cancer. These growth proteins are normally involved in bone maintenance and healing, but when over-activated they can lead to out-of-control cell growth and replication as seen in cancers.
Similarly, the master inflammation regulator NF-kB stimulates both bone resorption and cancer initiation and promotion.
Finally, many disorders that lead to bone weakness in osteoporosis, such as vitamin D deficiency and elevated parathyroid hormone, are also involved in cancer development.25
Eight Bone-Building Nutrients

Researchers have identified eight nutrients that safely help protect our bones — and can prevent osteoporosis-induced age acceleration.
Calcium
Calcium is the mineral most of us associate with building strong, healthy bones. Yet many Americans get too little calcium from their diets, with adults 50 and over at particular risk.28
Many different types of calcium supplements are available. But some, like calcium carbonate, don’t release a lot of calcium into the body to meet daily requirements.29 Calcium bisglycinate is better absorbed than calcium carbonate,30 as is calcium fructoborate.
Calcium bisglycinate is completely released into the gut in less than 150 minutes, while calcium carbonate takes four hours and still may not be entirely absorbed.29
Calcium fructoborate
is also easily absorbed and provides extra anti-inflammatory benefits that combat the age
acceleration brought on by
osteoporosis.
Magnesium
Magnesium supplementation increases bone mineral density.31
But about half of all Americans fail to consume enough of this vital mineral, and more than 40% of post-menopausal women have low magnesium levels in their blood. That puts them at high risk for bone breakdown and the problems that result.32-34
One study showed that magnesium supplementation for 30 days raises blood markers of new bone formation and reduces markers of bone breakdown.34
Manganese
Manganese plays a role in bone health and likely protects against osteoporosis. It may also protect against osteoarthritis.35
Manganese is a required cofactor, or helper molecule, for enzymes called superoxide dismutases that protect mitochondria from accumulated free-radical damage.36
Supplementation with manganese raises superoxide dismutase levels in animal models, resulting in improvements in tissue structure and function.37 Manganese supplementation has been shown to help prevent diet-induced diabetes in mice.38
Vitamin D
Vitamin D deficiency is a major contributor to osteoporosis. More than 60% of U.S. adults have either deficient (less than 20 ng/mL) or insufficient (20-30 ng/mL) vitamin D levels, and these numbers are even higher in older people.39,40
Vitamin D also influences functioning of many different organs, so deficiency can induce:41
- Muscle weakening,
- Cardiovascular disease,
- Type II diabetes, and
- Lower cognitive functioning.
That is why experts now say that year-round vitamin D supplementation is crucial in the elderly.41
Vitamin D supplementation has produced improvement in arterial stiffness and endothelial function in people at high risk for diabetes, helping reduce the risk of heart attack or stroke.42,43
A recent animal study found that vitamin D supplementation lowered blood pressure, improved heart function, and prevented liver damage in rats fed a typical Western diet, laden with fat and sugar.44
Much of this benefit comes from a reduction in pro-inflammatory signaling molecules, including those released from osteoclasts during bone breakdown.45,46
Vitamin K
Vitamin K plays a major role in balancing bone formation and destruction.47
Vitamin K supports increased calcium deposition in bones, while reducing its accumulation in blood vessel walls. This means it
reduces osteoporosis and
atherosclerosis
risk.47
Studies show that vitamin K2 supplementation helps prevent bone deterioration, decreasing the release of inflammatory cytokines that increase aging in all tissues.48,49
In those with chronic kidney disease, supplementation with K2 plus vitamin D slows arterial thickening and progress of atherosclerosis.50
Zinc
Zinc is a mineral critical for supporting healthy protein synthesis, which, when diminished, contributes to osteoporosis.51,52
Zinc deficiency exacerbates the inflammation brought on by bone breakdown, while supplementation in animal models lowers inflammation.53
Human studies show that zinc supplementation is essential for normal tissue maintenance in older adults. It decreases markers of bone resorption (when bone is broken down and the minerals are released into the blood), limiting release of inflammatory compounds from bone.51,54
Recently, zinc has also been shown to promote maturation of bone-forming osteoblasts in animal and preclinical models, supporting bone health and mitigating the age-accelerating effects of bone breakdown.54,55
Boron
A deficiency in the mineral boron is associated with poor immune function and elevated risk for osteoporosis.56,57
Animal studies have shown that boron supplementation can slow bone resorption and enhance new bone formation, fighting osteoporosis.58-61
One human study found sharp reductions in bone-released inflammatory cytokines after supplementation with boron, helping mitigate osteoporosis-induced inflammation and damage to other organ systems.62
Silicon
Higher intake of the mineral silicon correlates with healthier bones.63,64
Animal studies show that supplementation with a water-soluble form of silicon may slow the rapid bone turnover in osteoporosis, preventing the bone-breakdown-associated inflammation that causes disease and speeds aging.65,66
Lab studies of isolated cells further show that silicon stimulates formation of proteins vital to forming the protein-mineral matrix of bones. It also enhances the maturation of bone-forming osteoblasts.67
Summary

Osteoporosis, the gradual loss of bone mineral density, is not only harmful to bones, but to the entire body as well.
Bone-resorbing cells eat their way into mineralized bone, releasing it into the bloodstream, where it can wind up in arteries and other tissues, impeding their function. Those cells also release powerful inflammatory compounds that fuel harmful inflammation throughout the body.
This type of chronic inflammation raises risks for cardiovascular disease, cancer, and dementia, and accelerates certain aging processes.
Scientists now recognize at least eight different, essential nutrients with powerful bone-protecting properties: calcium, magnesium, manganese, vitamin D, vitamin K, zinc, boron, and silicon.
These nutrients also help temper inflammation and lessen the impact of bone degeneration on the body.
Anyone interested in supporting skeletal health and preventing osteoporosis-induced aging should explore supplementation with these low-cost nutrients.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
References
- Wright NC, Saag KG, Dawson-Hughes B, et al. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporos Int. 2017 Apr;28(4):1225-32.
- Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017 Sep;23(9):1072-9.
- Chung PL, Zhou S, Eslami B, et al. Effect of age on regulation of human osteoclast differentiation. J Cell Biochem. 2014 Aug;115(8):1412-9.
- Ovadya Y, Krizhanovsky V. Senescent cells: SASPected drivers of age-related pathologies. Biogerontology. 2014 Dec;15(6):627-42.
- Walters HE, Cox LS. mTORC Inhibitors as Broad-Spectrum Therapeutics for Age-Related Diseases. Int J Mol Sci. 2018 Aug 8;19(8).
- Farr JN, Rowsey JL, Eckhardt BA, et al. Independent Roles of Estrogen Deficiency and Cellular Senescence in the Pathogenesis of Osteoporosis: Evidence in Young Adult Mice and Older Humans. J Bone Miner Res. 2019 Mar 26.
- Kritsilis M, V Rizou S, Koutsoudaki PN, et al. Ageing, Cellular Senescence and Neurodegenerative Disease. International journal of molecular sciences. 2018;19(10):2937.
- Bussard KM, Venzon DJ, Mastro AM. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J Cell Biochem. 2010 Dec 1;111(5):1138-48.
- Ibanez L, Abou-Ezzi G, Ciucci T, et al. Inflammatory Osteoclasts Prime TNFalpha-Producing CD4(+) T Cells and Express CX3 CR1. J Bone Miner Res. 2016 Oct;31(10):1899-908.
- Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005 Nov 4;2:14.
- Hadjidakis DJ, Androulakis, II. Bone remodeling. Ann N Y Acad Sci. 2006 Dec;1092:385-96.
- Chang KH, Chung CJ, Lin CL, et al. Increased risk of dementia in patients with osteoporosis: a population-based retrospective cohort analysis. Age (Dordr). 2014 Apr;36(2):967-75.
- Khosla S, Farr JN, Kirkland JL. Inhibiting Cellular Senescence: A New Therapeutic Paradigm for Age-Related Osteoporosis. J Clin Endocrinol Metab. 2018 Apr 1;103(4):1282-90.
- Malaquin N, Tu V, Rodier F. Assessing Functional Roles of the Senescence-Associated Secretory Phenotype (SASP). Methods Mol Biol . 2019;1896:45-55.
- Zhu Y, Armstrong JL, Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014 Jul;17(4):324-8.
- Han KH, Hennigar RA, O’Neill WC. The association of bone and osteoclasts with vascular calcification. Vasc Med. 2015 Dec;20(6):527-33.
- Qiao JH, Mishra V, Fishbein MC, et al. Multinucleated giant cells in atherosclerotic plaques of human carotid arteries: Identification of osteoclast-like cells and their specific proteins in artery wall. Exp Mol Pathol. 2015 Dec;99(3):654-62.
- Pal SN, Clancy P, Golledge J. Circulating concentrations of stem-cell-mobilizing cytokines are associated with levels of osteoprogenitor cells and aortic calcification severity. Circ J. 2011;75(5):1227-34.
- Yu XY, Li XS, Li Y, et al. Neutrophil-lymphocyte ratio is associated with arterial stiffness in postmenopausal women with osteoporosis. Arch Gerontol Geriatr. 2015 Jul-Aug;61(1):76-80.
- Maresz K. Proper Calcium Use: Vitamin K2 as a Promoter of Bone and Cardiovascular Health. Integr Med (Encinitas). 2015 Feb;14(1):34-9.
- Guo JP, Pan JX, Xiong L, et al. Iron Chelation Inhibits Osteoclastic Differentiation In Vitro and in Tg2576 Mouse Model of Alzheimer’s Disease. PLoS One. 2015;10(11):e0139395.
- Amouzougan A, Lafaie L, Marotte H, et al. High prevalence of dementia in women with osteoporosis. Joint Bone Spine. 2017 Oct;84(5):611-4.
- Luckhaus C, Mahabadi B, Grass-Kapanke B, et al. Blood biomarkers of osteoporosis in mild cognitive impairment and Alzheimer’s disease. J Neural Transm (Vienna). 2009 Jul;116(7):905-11.
- Chitu V, Stanley ER. Regulation of Embryonic and Postnatal Development by the CSF-1 Receptor. Curr Top Dev Biol. 2017;123:229-75.
- Lee HF, Wu CE, Lin YS, et al. Low bone mineral density may be associated with long-term risk of cancer in the middle-aged population: A retrospective observational study from a single center. J Formos Med Assoc. 2018 Apr;117(4):339-45.
- Ji J, Sundquist K, Sundquist J. Cancer risk after hospitalization for osteoporosis in Sweden. Eur J Cancer Prev. 2012 Jul;21(4):395-9.
- McGlynn KA, Gridley G, Mellemkjaer L, et al. Risks of cancer among a cohort of 23,935 men and women with osteoporosis. Int J Cancer. 2008 Apr 15;122(8):1879-84.
- Available at: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/calcium-supplements/art-20047097. Accessed 16 April, 2019.
- Siemiradzka W, Dolinska B, Ryszka F. New Sources of Calcium (Chicken Eggshells, Chelates) - Preparation of Raw Material and Tablets. Curr Pharm Biotechnol. 2018;19(7):566-72.
- Pawlos M, Znamirowska A, Szajnar K, et al. The influence of the dose of calcium bisglycinate on physicochemical properties, sensory analysis and texture profile of kefirs during 21 days of cold storage. Acta Sci Pol Technol Aliment. 2016 Jan-Mar;15(1):37-45.
- Castiglioni S, Cazzaniga A, Albisetti W, et al. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013 Jul 31;5(8):3022-33.
- Mahdavi-Roshan M, Ebrahimi M, Ebrahimi A. Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin Cases Miner Bone Metab. 2015 Jan-Apr;12(1):18-21.
- Costello RB, Elin RJ, Rosanoff A, et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv Nutr. 2016 Nov;7(6):977-93.
- Aydin H, Deyneli O, Yavuz D, et al. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol Trace Elem Res. 2010 Feb;133(2):136-43.
- Samsel A, Seneff S. Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surg Neurol Int . 2015;6:45.
- Holley AK, Bakthavatchalu V, Velez-Roman JM, et al. Manganese superoxide dismutase: guardian of the powerhouse. Int J Mol Sci. 2011;12(10):7114-62.
- Welsh JJ, Narbaitz R, Begin-Heick N. Metabolic effects of dietary manganese supplementation in ob/ob mice. J Nutr. 1985 Jul;115(7):919-28.
- Lee SH, Jouihan HA, Cooksey RC, et al. Manganese supplementation protects against diet-induced diabetes in wild type mice by enhancing insulin secretion. Endocrinology. 2013 Mar;154(3):1029-38.
- Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011 Jan;31(1):48-54.
- Liu X, Baylin A, Levy PD. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Br J Nutr. 2018 Apr;119(8):928-36.
- Belz A, Stolecki M, Kudla M, et al. [Vitamin D - a geriatric point of view]. Wiad Lek. 2018;71(8):1628-31.
- Forouhi NG, Menon RK, Sharp SJ, et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab. 2016 Apr;18(4):392-400.
- Kumar V, Yadav AK, Lal A, et al. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J Am Soc Nephrol. 2017 Oct;28(10):3100-8.
- Mazzone G, Morisco C, Lembo V, et al. Dietary supplementation of vitamin D prevents the development of western diet-induced metabolic, hepatic and cardiovascular abnormalities in rats. United European Gastroenterol J. 2018 Aug;6(7):1056-64.
- Salekzamani S, Bavil AS, Mehralizadeh H, et al. The effects of vitamin D supplementation on proatherogenic inflammatory markers and carotid intima media thickness in subjects with metabolic syndrome: a randomized double-blind placebo-controlled clinical trial. Endocrine. 2017 Jul;57(1):51-9.
- Tabatabaeizadeh SA, Avan A, Bahrami A, et al. High Dose Supplementation of Vitamin D Affects Measures of Systemic Inflammation: Reductions in High Sensitivity C-Reactive Protein Level and Neutrophil to Lymphocyte Ratio (NLR) Distribution. J Cell Biochem. 2017 Dec;118(12):4317-22.
- Grober U, Reichrath J, Holick MF, et al. Vitamin K: an old vitamin in a new perspective. Dermatoendocrinol. 2014 Jan-Dec;6(1):e968490.
- Knapen MH, Drummen NE, Smit E, et al. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. 2013 Sep;24(9):2499-507.
- Ronn SH, Harslof T, Pedersen SB, et al. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Eur J Endocrinol. 2016 Dec;175(6):541-9.
- Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, et al. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol Arch Med Wewn. 2015;125(9):631-40.
- Rodondi A, Ammann P, Ghilardi-Beuret S, et al. Zinc increases the effects of essential amino acids-whey protein supplements in frail elderly. J Nutr Health Aging. 2009 Jun;13(6):491-7.
- Yamaguchi M. Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem. 2010 May;338(1-2):241-54.
- Chen J, Wang S, Luo M, et al. From the Cover: Zinc Deficiency Worsens and Supplementation Prevents High-Fat Diet Induced Vascular Inflammation, Oxidative Stress, and Pathological Remodeling. Toxicol Sci. 2016 Sep;153(1):124-36.
- Bhardwaj P, Rai DV, Garg ML. Zinc as a nutritional approach to bone loss prevention in an ovariectomized rat model. Menopause. 2013 Nov;20(11):1184-93.
- Park KH, Choi Y, Yoon DS, et al. Zinc Promotes Osteoblast Differentiation in Human Mesenchymal Stem Cells Via Activation of the cAMP-PKA-CREB Signaling Pathway. Stem Cells Dev. 2018 Aug 15;27(16):1125-35.
- Abdelnour SA, Abd El-Hack ME, Swelum AA, et al. The vital roles of boron in animal health and production: A comprehensive review. J Trace Elem Med Biol. 2018 Dec;50:296-304.
- Khaliq H, Juming Z, Ke-Mei P. The Physiological Role of Boron on Health. Biol Trace Elem Res. 2018 Nov;186(1):31-51.
- Movahedi Najafabadi BA, Abnosi MH. Boron Induces Early Matrix Mineralization via Calcium Deposition and Elevation of Alkaline Phosphatase Activity in Differentiated Rat Bone Marrow Mesenchymal Stem Cells. Cell J. 2016 Spring;18(1):62-73.
- Dessordi R, Spirlandeli AL, Zamarioli A, et al. Boron supplementation improves bone health of non-obese diabetic mice. J Trace Elem Med Biol. 2017 Jan;39:169-75.
- Xu P, Hu WB, Guo X, et al. [Therapeutic effect of dietary boron supplement on retinoic acid-induced osteoporosis in rats]. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Dec;26(12):1785-8.
- Toker H, Ozdemir H, Balci Yuce H, et al. The effect of boron on alveolar bone loss in osteoporotic rats. J Dent Sci. 2016 Sep;11(3):331-7.
- Naghii MR, Mofid M, Asgari AR, et al. Comparative effects of daily and weekly boron supplementation on plasma steroid hormones and proinflammatory cytokines. J Trace Elem Med Biol. 2011 Jan;25(1):54-8.
- Jugdaohsingh R, Tucker KL, Qiao N, et al. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort. J Bone Miner Res. 2004 Feb;19(2):297-307.
- Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging. 2007 Mar-Apr;11(2):99-110.
- Bae YJ, Kim JY, Choi MK, et al. Short-term administration of water-soluble silicon improves mineral density of the femur and tibia in ovariectomized rats. Biol Trace Elem Res. 2008 Aug;124(2):157-63.
- Price CT, Koval KJ, Langford JR. Silicon: a review of its potential role in the prevention and treatment of postmenopausal osteoporosis. Int J Endocrinol. 2013;2013:316783.
- Dong M, Jiao G, Liu H, et al. Biological Silicon Stimulates Collagen Type 1 and Osteocalcin Synthesis in Human Osteoblast-Like Cells Through the BMP-2/Smad/RUNX2 Signaling Pathway. Biol Trace Elem Res. 2016 Oct;173(2):306-15.

