Diabetes and Glucose Control
Diabetes and Glucose Control
Last Section Update: 04/2025
Contributor(s): Robert Iafelice, MS/RD/LDN; Maureen Williams, ND; Shayna Sandhaus, PhD; Stephen Tapanes, PhD; Carrie Decker, ND, MS; Chancellor Faloon, Health & Wellness Author
Table of Contents
- Overview
- Introduction
- The Difference Between Type 1 And Type 2 Diabetes
- Understanding Blood Glucose Regulation And How Diabetes Develops
- How High Blood Sugar Damages Tissues And Promotes Aging
- Diagnosis Of Type 2 Diabetes
- Type 2 Diabetes Treatment: Overview & Goals
- Dietary & Lifestyle Changes for Type 2 Diabetes
- Starting Drug Therapy for Type 2 Diabetes: What to Expect
- Overview of Type 2 Diabetes Drugs
- Type 2 Diabetes Drugs – Additional Details
- Metabolic Surgery for Longstanding Type 2 Diabetes
- Novel And Emerging Strategies
- Nutrients
- Update History
- References
1 Overview
Summary and Quick Facts for Diabetes and Glucose Control
- When diagnosed in middle age, diabetes reduces life expectancy by roughly 10 years. Worldwide, one person dies every seven seconds from diabetes-related causes.
- This protocol will explain the difference between type 1 and type 2 diabetes, and how elevated blood sugar, even within the conventional normal range, can damage tissues throughout the body. You will read about the dangers of insulin resistance and excess insulin, and how some of the drugs that mainstream medicine uses to treat diabetes increase insulin levels without regard to ambient glucose levels, potentially contributing to problems in the long term.
- A self-management and self-care program that includes an understanding of home glucose monitoring, lifestyle changes and medication is critical. The goal is to bring blood sugar under control and control blood sugar- and insulin-derived damage to the heart, small and large blood vessels and other tissues.
Diabetes mellitus is characterized by high levels of glucose in the blood. Type 2 diabetes is far more common than type 1 diabetes and is mainly caused by resistance to the effects of the hormone insulin, which facilitates removal of glucose from the blood. Type 1 diabetes is primarily caused by destruction of insulin-producing pancreatic beta cells.
Chronically elevated fasting blood glucose levels—or recurrent, excessive spikes in glucose levels after meals—can lead to devastating long-term consequences such as heart disease, blindness, kidney failure, liver disease, and cancer.
Several natural products, such as white mulberry leaf extract, brown seaweed extract, cinnamon extract, and sorghum bran extract, may promote optimal glucose metabolism and help facilitate healthy glycemic control.
Diagnosis
- Conventional diagnostic criteria for diabetes include:
- Fasting plasma glucose 126 mg/dL or greater,
- Non-fasting plasma glucose level 200 mg/dL or greater with diabetes symptoms,
- Plasma glucose level 200 mg/dL or greater 2 hours after a 75-g oral glucose tolerance test, or
- HbA1C 6.5% or greater
Note: Life Extension suggests targeting a fasting glucose level of 80 – 86 mg/dL and a HbA1C level of 5 – 5.4%.
Treatment of Type 2 Diabetes
Dietary and Lifestyle Considerations
- Blood sugar control (as assessed by the HbA1C test) is the primary goal of diabetes treatment.
- Note: The risk-benefit equation of intensive glycemic control may progressively shift in favor of less-intensive control as diabetes progresses.
- Control blood pressure and lipids (see Life Extension’s High Blood Pressure and Atherosclerosis and Cardiovascular Disease protocols).
- Eat a low-glycemic-load diet, such as the Mediterranean diet.
- In adults with diabetes, 150 minutes per week of moderate-intensity aerobic exercise is generally recommended.
Conventional Treatment
- Metformin is considered the first-line drug for type 2 diabetes. Metformin has also been shown to promote weight loss and protect against some cancers, cardiovascular disease, and Alzheimer disease.
- Acarbose is a drug that lowers glucose by blocking breakdown of starches and slowing absorption of sugar and carbohydrates.
- Other oral glucose-lowering agents or injectable drugs such as insulin may be necessary depending on individual glycemic control and diabetes severity.
Novel and Emerging Strategies
- Stem cell therapy is aimed at replacing damaged or destroyed insulin-producing pancreatic beta cells in diabetics with new beta cells derived from human stem cells.
- Glucokinase activators have been shown to lower glucose levels and stimulate proliferation of pancreatic beta cells in animal models of type 2 diabetes.
- Two anti-obesity agents, lorcaserin and the combination of phentermine and topiramate, have been shown to improve glycemic control in obese individuals with type 2 diabetes.
Integrative Interventions
- White mulberry leaf: A component of white mulberry slows carbohydrate absorption and may lessen post-meal blood sugar spikes.
- Brown seaweed: In a randomized controlled trial, brown seaweed extract caused a 48.3% decrease in post-meal blood sugar spikes. Significant reductions in post-meal insulin concentrations and improved insulin sensitivity were also observed.
- Cinnamon: Studies that supplemented type 2 diabetics and healthy individuals with cinnamon reported lower levels of fasting glucose, HbA1C, and after-meal glucose and insulin concentrations, as well as improvements in insulin sensitivity. These effects have been demonstrated even in those already taking glucose-lowering medication.
- Sorghum bran: In a randomized trial in healthy men, muffins made with sorghum were shown to reduce average after-meal glucose and insulin responses.
- Benfotiamine: In a clinical trial, type 2 diabetics consumed a high-AGE (advanced glycation end product) meal before and after a 3-day course of benfotiamine. The subjects’ vascular function was assessed after both high-AGE meals. Benfotiamine administration reduced vascular dysfunction.
Note: Under no circumstances should people suddenly stop taking antidiabetic drugs, especially insulin. Individuals with diabetes should work closely with their healthcare provider before initiating a supplement regimen due to the potential risk of hypoglycemia.
2 Introduction
Note: This protocol will focus on type 2, and to a lesser extent type 1 diabetes; readers seeking information about gestational diabetes should consult their physician.
Diabetes mellitus is characterized by high levels of glucose in the blood. Types of diabetes include type 1, type 2, and gestational. Type 2 diabetes is far more common than type 1 and is mainly caused by acquired resistance to the effects of the hormone insulin, which facilitates removal of glucose from the blood. Type 1 diabetes is primarily caused by destruction of insulin-producing pancreatic beta cells by autoimmune disease or rarely other causes such as trauma; type 1 diabetes generally necessitates lifelong insulin therapy (ADA 2015e; Kishore 2014; CDC 2015a; NIDDK 2014a; Mayo Clinic 2014; Norman 2016). Gestational diabetes is a reversible form of diabetes that occurs during pregnancy (NIDDK 2014b).
Chronically elevated fasting blood glucose levels—or recurrent, excessive spikes in glucose levels after meals—can lead to devastating long-term consequences such as heart disease and stroke, blindness, kidney failure, neuropathy, liver disease, and even cancer (Ahmadieh 2014; Kishore 2014; Szablewski 2014; Del Bene 2015; Huang 2014; Kim 2013; Fowler 2008; CDC 2015a). Abnormal glucose and insulin metabolism has been implicated in Alzheimer disease as well. In fact, this link is so compelling that many researchers have referred to Alzheimer disease as type 3 diabetes (Ahmed 2015; Halmos 2016; Mittal 2016).
When diagnosed in middle age, diabetes reduces life expectancy by roughly 10 years, and worldwide one person dies every seven seconds from diabetes-related causes (Shahbazian 2013; Gregg 2012; IDF 2014).
What is alarming, though, is that diabetes is not formally diagnosed until fasting blood glucose reaches 126 mg/dL, and levels up to 100 mg/dL are considered “normal” by mainstream medicine (NIDDK 2014c; Bjornholt 1999; Tirosh 2005).
This is regrettable—as readers of Life Extension publications have long known—because the adverse consequences of impaired glucose metabolism begin to emerge as fasting glucose surpasses about 86 mg/dL (Bjornholt 1999; Kato 2009; Muti 2002; Simons 2000; Meigs 1998).
In contrast to the conventional dogma that fasting glucose levels up to 100 mg/dL are acceptable, an upper limit for fasting glucose of 86 mg/dL is far better for longevity and health (Bjornholt 1999; Gerstein 1999). Also, maintaining a hemoglobin A1C level between 5% and 5.4% appears to be associated with several measures of health status (Cheng 2011).
Type 2 diabetes can usually be significantly improved with diet and lifestyle changes, especially in the early stages (Lagger 2015; Jain 2008; Cho 2014; Lim 2011; Steven 2013). Adopting eating habits modeled after the Mediterranean dietary pattern is a proven strategy to improve cardiometabolic risk and glucose metabolism. The Mediterranean diet is rich in fresh vegetables and fruits, whole grains, nuts and seeds, and olive oil, and contains moderate amounts of fish, dairy, and red wine or other alcoholic beverages. Sweets, highly processed foods, and meat are eaten in small amounts only (Tognon 2014). Regular exercise is also important (Inzucchi 2012b; Fonseca 2013; O'Connor 2015; Whitlatch 2015).
The antidiabetic drugs metformin and acarbose lower fasting glucose with little risk of hypoglycemia, in contrast to drugs like sulfonylureas that directly stimulate insulin secretion. They can often help control glucose levels and improve insulin sensitivity in people who cannot accomplish their blood sugar goals with diet and lifestyle alone (Delgado 2002; Meneilly 2000; Gold Standard 2015c; Gold Standard 2015a). Several natural products, such as sorghum bran extract, white mulberry leaf extract, brown seaweed extract, and cinnamon extract, may also promote optimal glucose metabolism and help users attain healthy glycemic control (Andallu 2001; Hoehn 2012; Poquette 2014; Paradis 2011).
This protocol will explain the difference between type 1 and type 2 diabetes, and how elevated blood sugar, even within the conventional “normal” range, can damage tissues throughout the body. You will read about the dangers of insulin resistance and excess insulin, and how some of the drugs that mainstream medicine uses to treat diabetes increase insulin levels without regard to ambient glucose levels, potentially contributing to problems in the long term. Several novel and emerging glucose control strategies will be described, and evidence for the benefits of many natural agents that support optimal glycemic control will be reviewed.
Since diabetes greatly increases cardiovascular risk, readers of this protocol should also review these other Life Extension protocols:
3 The Difference Between Type 1 And Type 2 Diabetes
Type 1 diabetes is usually an autoimmune disorder in which the insulin-producing beta cells of the pancreas are destroyed by the body’s immune system. As a result, little or no insulin is produced, and lifelong insulin therapy is essential (Malik 2014; CDC 2015a; Kishore 2014).
Although type 1 diabetes usually appears during childhood or adolescence, it can occur at any age. It accounts for roughly 5% of diagnosed cases of diabetes (CDC 2015b; Kishore 2014; Cohen 2013). Insulin therapy should begin as soon as possible after diagnosis (Whitlatch 2015; Vimalananda 2014). Type 1 diabetes may arise due to genetic predisposition, with a potential role for viral infections and other environmental or dietary factors such as inadequate vitamin D intake (Dong 2013; Cohen 2013; Inzucchi 2012a).
Type 2 diabetes accounts for up to 95% of diabetes cases; its onset is typically after age 45, and it is more common with advancing age. Unlike the relatively rapid onset of type 1 diabetes, type 2 diabetes usually develops slowly, unnoticed by the patient until routine blood testing reveals elevated glucose (Fonseca 2013; CDC 2015b; Kishore 2014; CDC 2014; UMMC 2014).
In early type 2 diabetes, the pancreas remains capable of secreting insulin. However, as muscle, fat, and liver tissues become more insulin resistant, they may eventually become unable to respond effectively to insulin’s signal. Insulin resistance contributes to poor blood glucose control and diabetes (Wilcox 2005; DeFronzo 2009). As diabetes progressively worsens, pancreatic beta cells begin to “burn out,” and exogenous insulin is often necessary in late-stage disease (Prentki 2006; White 2003).
4 Understanding Blood Glucose Regulation And How Diabetes Develops
Glucose is a chief energy source for cells. You can obtain sugars that can be broken down into glucose from food, and the liver can manufacture glucose. In order for cells to extract energy from glucose, they must first take up glucose from the bloodstream. The hormone insulin facilitates this cellular glucose uptake and utilization (Mayo Clinic 2014).
In the simplest of terms, diabetes is the result of insufficient insulin signaling. In type 1 diabetes, the pancreas does not produce enough insulin to move glucose out of the bloodstream. In early type 2 diabetes, cells become resistant to the effects of insulin; in late-stage type 2 diabetes, pancreatic beta cell dysfunction may necessitate exogenous insulin administration (Mayo Clinic 2014; White 2003; Prentki 2006). This leads to uncontrolled blood glucose in both cases.
Factors that Influence Blood Glucose Levels
Diet (especially processed carbohydrates). Dietary refined carbohydrates, such as processed sugars and starches, have the greatest impact on blood glucose, especially in the postprandial (after-meal) period (UIE 2014; Wheeler 2008). Life Extension has long recommended that 2-hour postprandial glucose levels be kept below 125 mg/dL. This is because acute surges in blood glucose and insulin that follow meals (postprandial period) are important contributors to the overall adverse effects caused by excessive glucose and insulin levels. Compelling evidence indicates that postprandial hyperglycemia—not just elevated fasting blood glucose—is an independent risk factor for cardiovascular disease in type 2 diabetes and prediabetes (glucose levels above 100 mg/dL but below 126 mg/dL) (Singh 2012; Zeymer 2006; Ceriello 2010; Cavalot 2006).
Body weight and adiposity. Being overweight or obese can impair glucose and insulin metabolism (Gossens 2017). Fat (adipose) tissue can release cytokines (cell-signaling molecules) such as tumor necrosis factor-alpha (TNF-α) that interfere with the action of insulin (in this context, these cytokines are referred to as “adipokines”). Immune cells called macrophages can infiltrate adipose tissue and drive further inflammatory processes that contribute to insulin resistance. This macrophage infiltration can be lessened through weight loss, and losing weight can even improve the expression profile of inflammatory genes in adipose tissue. Obesity also downregulates expression of adiponectin, which promotes insulin sensitivity under healthy conditions (Kawai 2021). Leptin is another key hormonal metabolic regulator that is perturbed in obesity. Leptin normally increases energy expenditure and reduces food intake. However, obesity causes resistance to the beneficial effects of leptin (Galic 2010). For more information about how excessive body weight negatively impacts health and ways to lose weight healthily, refer to Life Extension’s Weight Loss protocol.
Glycogenolysis. The liver is a critical regulator of blood glucose. One way the liver maintains normal blood glucose is by breaking down glycogen (stored carbohydrate) into glucose and releasing it into the bloodstream. This process is called glycogenolysis and provides a short-term source of glucose when blood glucose levels are low, such as during an overnight fast or a bout of intense exercise (Matsui 2012; Sprague 2011; Edgerton 2002; Roden 2008).
Normally, when blood glucose levels rise after eating, the hormone insulin suppresses glycogenolysis in the liver. However, in prediabetes and diabetes, this signal is blunted by insulin resistance in the liver. The liver then continues to release glucose in spite of already high blood levels (Edgerton 2002; Basu 2005; Nathan 2007).
Gluconeogenesis. Another way the liver regulates blood sugar is by creating new glucose from protein and other non-carbohydrate sources. This process is called gluconeogenesis. Gluconeogenesis is the cause of high fasting blood glucose after an overnight fast in type 2 diabetics (Chung 2015; Toft 2005; Magnusson 1992; Boden 2004). Insulin resistance in the liver blunts insulin’s signal to turn off gluconeogenesis (Basu 2005; Bock 2007). Metformin, the first-line drug treatment for type 2 diabetes, lowers elevated blood glucose in part by suppressing gluconeogenesis in the liver (McIntosh 2011; Nasri 2014; Gold Standard 2015c).
Increased activity of an enzyme called glucose-6-phosphatase contributes to excessive gluconeogenesis (and glycogenolysis) in diabetics. Glucose-6-phosphatase completes the final step in the creation and release of glucose in the liver (Clore 2000; Arion 1997). Chlorogenic acid, a natural compound concentrated in green coffee bean extract and specially roasted coffee, helps lower blood sugar levels by inhibiting glucose-6-phosphatase (Meng, Cao 2013).
Major Hormones Involved in Blood Glucose Regulation
Insulin. When blood glucose rises after a meal, insulin is released into the bloodstream by pancreatic beta cells. Insulin helps move glucose into cells, particularly muscle, fat, and liver cells. This process begins when insulin binds to insulin receptors embedded in cell membranes, signaling the cell to “unlock” and allow glucose to enter. Insulin also lowers high blood sugar by suppressing glucose production in the liver (NDIC 2014).
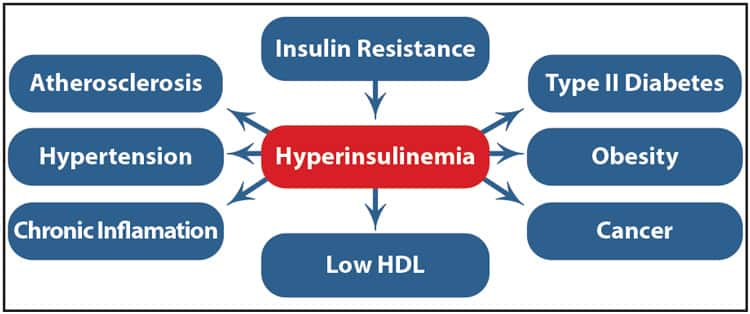
Insulin resistance is decreased responsiveness to insulin signaling. In insulin resistance, muscle, liver, and fat cells lose their sensitivity to insulin, making insulin less able to promote the movement of glucose from the bloodstream into cells. Glucose levels in the blood rise as a result. To compensate, the pancreas secretes more insulin in an effort to move sugar into the cells and maintain normal blood glucose levels. This worsens insulin resistance and increases risk of diabetes, cardiovascular disease, liver disease, and even some cancers (See “The Dangers of Excess Insulin”) (Wolpin 2013; Capasso 2013; NDIC 2014; Lopez-Alarcon 2014; DeFronzo 2009).
Excess body fat stored in and around the abdominal organs, under the muscle layer of the abdominal wall, is called visceral fat and is strongly associated with insulin resistance. Excess visceral fat and insulin resistance are strongly associated with chronic, low-grade inflammation, which may cause some of the health consequences of obesity (Hocking 2013; Hamdy 2006; Indulekha 2011; Kaess 2012; McLaughlin 2011).
Insulin resistance is the hallmark of metabolic syndrome—an increasingly common disorder diagnosed by the presence of three of five metabolic abnormalities: high blood pressure, elevated fasting triglycerides, low levels of beneficial HDL cholesterol, increased abdominal circumference, and high fasting blood glucose (Bassi 2014; Ruderman 2013; Adiels 2008). Metabolic syndrome is strongly associated with increased risk of type 2 diabetes; cardiovascular disease, including heart attack and stroke; death from any cause; non-alcoholic fatty liver disease; poor surgical outcomes; and many other conditions (Kaur 2014; Azzam 2015; Tzimas 2015; Wong 2015; Drager 2013; Kelly 2014).
Glucagon. The hormone glucagon, which is produced by alpha cells in the pancreas, partners with insulin to regulate blood glucose levels. When blood glucose falls too low, glucagon is released to raise blood sugar levels to supply energy for the body. Glucagon does this by prompting the liver to break down stored carbohydrate (glycogen) into glucose. When glycogen stores become depleted, glucagon stimulates the liver to make glucose from amino acids and other noncarbohydrate molecules (Roden 2008; NDIC 2014).
Cortisol. Cortisol is a steroid hormone made by the adrenal glands that plays a critical role in the response to stress. Among other functions, cortisol raises blood glucose by activating the enzyme glucose-6-phosphatase, which stimulates gluconeogenesis in the liver. Cortisol also makes muscle and fat cells resistant to the actions of insulin (Lehrke 2008; Nussey 2001; Smith 2006).
Over the long term, elevated cortisol from chronic physical or psychological stress compromises health, in part by promoting insulin resistance and accumulation of visceral fat (Lehrke 2008; Innes 2007).
Catecholamines. The catecholamines—epinephrine (adrenalin), norepinephrine, and dopamine—are hormones released into the blood in response to physical or emotional stress (Dimsdale 1980; Goldberg 1996; Kjaer 1987). In addition to increasing heart rate and blood pressure, catecholamines increase blood glucose levels to provide energy for the “fight-or-flight” response. Catecholamines raise blood glucose by stimulating glycogenolysis and gluconeogenesis in the liver, and by inhibiting insulin-stimulated glycogen synthesis (Barth 2007). Life Extension’s Stress Management protocol describes methods to reduce the burden of chronic stress.
Incretins. Incretins are hormones secreted by cells within the gastrointestinal tract directly into the bloodstream within minutes after food intake. They stimulate insulin secretion from the pancreas. Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are very important incretin hormones. Together, they account for 70% of after-meal glucose-stimulated insulin secretion (Kim, Egan 2008; Tasyurek 2014).
In addition, GLP-1 delays stomach emptying, suppresses appetite, inhibits glucagon secretion, and slows glucose production in the liver. The risk of hypoglycemia is low with GLP-1-mimetic drugs (eg, exenatide [Byetta], liraglutide [Victoza]) because their effects are dependent on high levels of glucose in the bloodstream. GLP-1 also protects insulin-producing beta cells from apoptosis (programmed cell death) and promotes their proliferation (Jellinger 2011; Kim, Egan 2008; Tasyurek 2014; Meloni 2013).
Other drugs that affect incretin function are the DPP-IV inhibitors (eg, sitagliptin [Januvia]). These compounds block the action of an enzyme, dipeptidyl peptidase-IV, which normally rapidly degrades GLP-1. As a result, more of the incretin hormones are left in circulation (Kim, Egan 2008).
Adiponectin and leptin. Adiponectin and leptin are hormones secreted from adipose tissue (fat cells) (Yadav 2013; Zuo 2013; Aleidi 2015).
Adiponectin increases glucose absorption and fat burning in muscle and liver cells, and decreases glucose production in the liver (Yadav 2013). It does this in part by activating an enzyme called AMPK—a critical cellular energy sensor—and a protein called PPAR-alpha, which turns on fat-burning genes (Coughlan 2014; Yamauchi 2003; Yamauchi 2002; Yadav 2013). Several studies show long-lived people—over the age of 100—have high concentrations of adiponectin, and suggest this may contribute to their longevity (Bik 2013; Arai 2011; Atzmon 2008; Bik 2006).
Leptin acts on the hypothalamus (control center) in the brain, causing reduced food intake and burning of fat stores for energy (Meister 2000; Zuo 2013; Zoico 2004). Leptin resistance is a condition in which the body fails to respond adequately to leptin’s satiety-inducing, fat-burning signal. Leptin resistance is a major contributor to obesity and hinders weight loss. Insulin resistant and obese individuals generally have elevated leptin levels, but are resistant to its effects (Zhou 2013; Sainz 2015; Zuo 2013; Kraegen 2008; Considine 1996). The inflammatory marker C-reactive protein (CRP) contributes to leptin resistance and weight gain by binding to leptin and reducing its signaling ability (Chen 2006; Zeki Al Hazzouri 2013).
An extract of Irvingia gabonensis, also called African mango, has been shown to produce weight loss in humans, and to lower leptin and CRP levels (Ngondi 2009; Oben, Ngondi, Blum 2008).
5 How High Blood Sugar Damages Tissues And Promotes Aging
Glucose toxicity is mediated primarily by three tissue-damaging processes: glycation, inflammation, and free radical damage. These interrelated processes contribute to complications of diabetes including endothelial dysfunction, atherosclerosis, diabetic nephropathy, and other chronic diseases (Giri 2018).
Glycation and AGEs
Glycation is a process whereby sugars such as glucose bond to proteins, fats, and nucleic acids in an uncontrolled fashion. This results in the formation of toxic compounds called advanced glycation end products, or AGEs. The accumulation of AGEs in the body irreversibly modifies the structure and function of proteins such as collagen and elastin. These damaged proteins can become joined together through a mechanism called crosslinking, which causes increased stiffness and loss of elasticity in several tissues throughout the body (Nawale 2006; Simm 2013). Glycation and AGEs contribute to the aging process and age-related diseases as well as diabetic complications (Semba 2010; van Heijst 2005; Simm 2013; Nawale 2006).
One study showed plasma levels of AGEs were significantly higher in type 2 diabetic patients compared with nondiabetics. In addition, patients with diabetic complications had significantly higher levels of AGEs compared with complication-free patients. This study also showed a strong correlation between high levels of AGEs and elevated glycated hemoglobin, or hemoglobin A1C—a blood test that reflects average blood sugar levels over a 60- to 90-day period by measuring the amount of hemoglobin that has undergone glycation (Jakus 2014; Bozkaya 2010; ADA 2014a). A study in patients with type 2 diabetes followed over 10 years found that higher levels of blood AGEs predicted cardiovascular events (Hanssen 2015).
This same glycation process that “ages” our body is responsible for the browning reaction that occurs when foods are cooked at high temperatures. When meat is seared or potatoes are fried, AGEs are created (Uribarri 2010). These dietary AGEs can be absorbed into the circulation and remain in the body long enough to cause tissue damage (Vlassara 2014; Luevano-Contreras 2013). Food preparation methods that utilize high heat, such as frying, grilling, and broiling, lead to the formation of more AGEs, while cooking at lower temperatures, shorter cooking times, steaming, boiling, and the use of acids like lemon juice or vinegar in cooking minimize AGE formation (Uribarri 2010). A detailed examination of dietary AGEs and ways to avoid them is available in the Life Extension Magazine article “Are You Cooking Yourself to Death?”.
The antidiabetic drug metformin, amino acid derivative carnosine, and B-vitamin derivatives benfotiamine and pyridoxal-5’-phosphate are examples of agents that mitigate glycation- and AGE-related tissue damage (Ceriello 2009; Schurman 2008; Miyazawa 2012; Nagai 2014; Hipkiss 2005).
Inflammation
Acute inflammation is necessary to fight infections and repair damaged tissue. However, long-term, unabated inflammation can lead to chronic disease. Chronic, low-grade inflammation is a major feature of diabetes and its complications, particularly cardiovascular disease. Persistent high blood sugar, excess abdominal fat, and insulin resistance cause chronic inflammation. Acute, excessive postprandial blood sugar surges can also induce inflammation; foods high in AGEs can aggravate postprandial inflammation (Dandona 2004; Ota 2014; Agrawal 2014; Lontchi-Yimagou 2013; Nowlin 2012; Calder 2011).
Concentrations of proinflammatory cytokines including TNF-α, C-reactive protein (CRP), and interleukin-6 (IL-6) are increased in diabetes (Nguyen 2012; Lontchi-Yimagou 2013). These cytokines impair beta cell function, lowering insulin secretion (Lontchi-Yimagou 2013; Agrawal 2014).
Proinflammatory cytokines induce negative effects throughout the body. Increased levels of these cytokines “switch on” pathways that cause muscle and bone breakdown and neuronal degeneration, accelerate atherosclerosis, and damage DNA, increasing the risk of death (Kabagambe 2011; Michaud 2013; Kiraly 2015; Xu 2015).
Numerous strategies for combatting inflammation are described in the Chronic Inflammation protocol.
Free Radical Damage
Free radicals are highly reactive, unstable molecules produced in the body as byproducts of normal metabolic processes. They can also be created by environmental factors such as X-rays, ozone, tobacco smoke, and air pollution. If excessive free radicals chronically overwhelm the body’s ability to neutralize them, free radical damage can occur within cells, triggering destructive changes that can lead to degenerative disease and aging (Guo 1999; Venkataraman 2013; Lobo 2010; Ortuno-Sahagun 2014; Masters 1995; Gilgun-Sherki 2004).
Several harmful processes induced by hyperglycemia, including AGE formation, are associated with overproduction of a free radical called superoxide. While reactive oxygen species such as superoxide are a normal byproduct of energy production in mitochondria, superoxide radicals generated by glucose overload may cause diabetic complications (Giacco 2010; Ceriello 2011; Rahman 2007; Sasaki 2012).
6 Diagnosis Of Type 2 Diabetes
Conventional Diagnostic Approach
Conventional diagnostic criteria for diabetes include:
- Fasting plasma glucose 126 mg/dL or greater (fasting glucose between 100 mg/dL and 125 mg/dL is considered “impaired fasting glucose” or “prediabetes”).
- Non-fasting plasma glucose level 200 mg/dL or greater with diabetes symptoms.
- Plasma glucose level 200 mg/dL or greater 2 hours after a 75-g oral glucose tolerance test.
- Glycated hemoglobin (HbA1C) 6.5% or greater. An HbA1C of 5.7–6.4% is considered prediabetes.
Confirmation with a repeat or second test is necessary. A repeat of the same diagnostic test is preferred, though abnormal results on two different tests are also acceptable for diagnosis (O’Connor 2015; Inzucchi 2012b; Bansal 2015).
Life Extension’s Approach
A shocking number of people unknowingly suffer from chronic high blood sugar or frequent post-meal glucose surges. The longer or more frequently your blood sugar is elevated, the more time it has to cause cellular and tissue damage and promote insulin resistance, predisposing you to degenerative disease and accelerated aging. In fact, the toxic effects of excess blood sugar may be a leading cause of premature death (Nwaneri 2013; Seshasai 2011; Ding 2014; Kalyani 2013; De Tata 2014; Huang 2015).
Life Extension believes everyone should strive for optimal glucose control, regardless of whether or not they are diabetic. This means taking action to improve your glycemic control if your fasting glucose is over 86 mg/dL or your HbA1C is higher than 5.4%. Studies have shown the incidence of age-related disease begins to increase as fasting blood sugar levels climb above 85 mg/dL—a level currently accepted as normal by conventional medicine (Kato 2009; Muti 2002; Nichols 2008; Bjornholt 1999; Simons 2000; Meigs 1998; Tirosh 2005; Gerstein 1999; Coutinho 1999).
7 Type 2 Diabetes Treatment: Overview & Goals
Overview of Type 2 Diabetes Treatment
Dietary & Lifestyle Changes. For people newly diagnosed with diabetes, as well as those with pre-diabetes, comprehensive dietary and lifestyle changes are cornerstones of the initial treatment approach. Three to six months of concerted effort to make the following changes should be initiated as soon as you become aware that you have, or are at risk for, diabetes:
- 1) lose weight (if overweight or obese),
- 2) shift to a healthy, Mediterranean-style or other plant-based diet,
- 3) increase physical activity and exercise, and
- 4) incorporate an evidence-based regimen of targeted nutrient supplementation to promote healthy glucose metabolism (see section 14 of this Protocol).
Medications. If genuine efforts to improve blood sugar control using these dietary and lifestyle strategies are not successful, medications may be indicated for those with diabetes and should be initiated under the supervision of a physician. Individuals with very high HbA1C may need to begin drug therapy immediately upon diagnosis (in addition to implementing the aforementioned dietary and lifestyle changes).
Metformin has long been a top choice for the first drug to be tried if dietary and lifestyle changes are not sufficient to regain glucose control and metabolic health. The starting dosage of metformin is typically 500 mg – 850 mg once or twice daily. The dosage is generally increased over several weeks to 1,000 mg twice daily as long as gastrointestinal tolerability does not become an issue.
Other, newer drugs, such as SGLT2 inhibitors and GLP-1 receptor agonists are now also favored as first-line therapies in some cases, particularly for people who have cardiovascular and/or kidney health concerns in addition to diabetes. In some cases, drug therapy may begin with a combination of medications, such as metformin plus an SGLT2 inhibitor (which is available in a single pill for improved convenience).
Your drug regimen may be adjusted over time, possibly to include a third drug and/or insulin, depending on how you respond to initial therapy.
Determining your ideal drug treatment regimen is your physician’s responsibility. Many variables such as baseline glycemic control, comorbidities, liver and kidney function, and hypoglycemia risk influence the selection of drug therapy. A successful treatment regimen for one person with diabetes may not be appropriate for another, so working closely with your physician to plan and initiate a drug regimen is essential.
Goals of Treatment
Achieve blood sugar goals. Blood sugar control (as assessed by the HbA1C test) is a primary goal of diabetes treatment (Delamater 2006; ADA 2014c; Inzucchi 2012b; Whitlatch 2015; Fonseca 2013). The HbA1C test measures glycated hemoglobin and reflects average blood sugar over two to three months. Hemoglobin is the oxygen-carrying component of red blood cells; glycated hemoglobin is irreversibly bound to glucose, forming an advanced glycation end product (AGE). Once you are diagnosed with diabetes and begin treatment, HbA1C testing should be performed routinely to monitor how well you blood glucose is being controlled (Bozkaya 2010; ADA 2014a).
- Target A1C Levels
- The American Diabetes Association (ADA) recommends most diabetic patients attempt to achieve an HbA1C level of about 7% or less (ADA 2021a).
- The American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) recommend an A1C level of 6.5% or less for diabetics without concurrent serious illness and low hypoglycemia risk, and a level of about 6.5% - 7% for those with concurrent illnesses or increased hypoglycemia risk (Garber 2020).
- A less stringent target of 8% or less may be appropriate for diabetics in whom the harms of treatment are expected to outweigh the benefits of further A1C lowering.
- On the other hand, more aggressive treatment to goal A1C levels closer to 6% may be beneficial in diabetics with long life expectancy and in whom the benefits of more intensive treatment are expected to outweigh the risks.
- Target fasting glucose levels
- The ADA suggests a target fasting glucose level of 80 – 130 mg/dL for many (non-pregnant) adults with diabetes (ADA 2021a).
- The AACE/ACE suggest a fasting glucose target of less than 110 mg/dL (Garber 2020).
- Fasting glucose levels are not generally relied upon to independently monitor blood sugar control and should be interpreted in conjunction with A1C.
- Target two-hour after-meal (postprandial) glucose levels
- For people with diabetes whose A1C levels are not at goal despite fasting glucose levels being within the targeted range (80 – 130 mg/dL), the ADA suggests assessment of two-hour after-meal (postprandial) glucose levels, with a targeted level of less than 180 mg/dL (ADA 2021a).
- The AACE/ACE suggests intensifying treatment for people with diabetes whose postprandial glucose levels are not at the target level of less than 140 mg/dL.
- Life Extension’s suggested optimal goals for health and longevity. The following are suggested target levels for variables related to glycemic control. Note: it may be very difficult for patients with long-standing type 2 diabetes to achieve these goals. Risks of hypoglycemia should be discussed with a healthcare provider, especially among individuals who attempt intensive glucose control using drugs that stimulate insulin secretion (eg, sulfonylureas) or insulin itself.
- Hemoglobin A1C: 5 – 5.4% (Adams 2009; Jorgensen 2004)
- Fasting glucose: 80 ‒ 86 mg/dL (Bjornholt 1999; Kato 2009; Muti 2002; Nichols 2008; Simons 2000; Meigs 1998; Tirosh 2005; Gerstein 1999; Coutinho 1999)
- Glucose: 125 mg/dL or less 2 hours after a meal (or no more than 40 mg/dL above baseline fasting level) (Gerstein 1999)
Reduce cardiovascular complications. Controlling blood pressure and lipids is an important part of diabetes treatment (Fonseca 2013; Whitlatch 2015; Inzucchi 2012b; O'Connor 2015). Recommendations for blood pressure targets in diabetes range from less than 130/80 mm Hg to less than 140/90 mm Hg. Statin medications are typically used as first-line cholesterol-lowering treatment (unless contraindicated) based on individualized cardiovascular risk assessment and LDL cholesterol levels (Whitlatch 2015; O'Connor 2015; Garber 2020).
Strategies for reducing cardiovascular risk are reviewed in the Atherosclerosis and Cardiovascular Disease protocol.
8 Dietary & Lifestyle Changes for Type 2 Diabetes
Dietary changes are an essential aspect of type 2 diabetes treatment. Increasing consumption of vegetables, nuts and seeds, whole grains, legumes, and fruit, as reflected in the Mediterranean dietary pattern, is an effective approach. Avoiding highly processed foods, fast food, and junk food can help reduce “empty calories” in the diet. Avoiding high-heat cooking methods can reduce exposure to dietary AGEs. Sugar-sweetened beverages, including sodas and drinks made with high-fructose corn syrup, should be eliminated. Also, eating a balanced diet can help prevent blood sugar surges, as protein, fat, and fiber may help slow glucose absorption (AHA 2014; ADA 2015a; JDC 2011; Moghaddam 2006). Typical recommendations are that low-glycemic, high-fiber carbohydrates should comprise about 45–60% of total caloric intake, fats should provide about 20–35%, and protein about 15–20% (Dworatzek 2013; Hung 1989; Bazzano 2008; Vlassara 2014; Xi 2014; ADA 2015a; Esposito 2014; Takahashi 2012; Carter 2013; Odegaard 2012). Some of the most thoroughly studied dietary considerations in the context of type 2 diabetes are briefly described below.
- Mediterranean diet. Mediterranean-type diets are primarily characterized by abundant quantities of legumes, whole grains, vegetables, fruits, and nuts, and light-to-moderate alcohol consumption, with olive oil as a primary dietary fat (Tognon 2014). The Mediterranean diet includes moderate amounts of fish and dairy products, and low amounts of poultry, meat, highly processed foods, and refined sugars. A rigorous analysis of 10 studies involving over 136 000 participants found highest (versus lowest) compliance with the Mediterranean dietary pattern was associated with a 23% reduction in type 2 diabetes risk (Koloverou 2014). The Mediterranean diet is associated with improved glycemic control and lower risk of multiple degenerative conditions associated with advancing age and diabetes, including cardiovascular disease, cancer, obesity, lung disease, and cognitive decline (Gotsis 2015; Milenkovic 2021).
- Caloric restriction. Caloric restriction, which has multiple health benefits, is an effective strategy in diabetes prevention and treatment. Calorie restriction has been shown to significantly improve several measures of glucose metabolism and diabetic complication risk (Colman 2009; Wing 1994; Soare 2014; Lefevre 2009; Bales 2013; Rizza 2014). The benefits of caloric restriction, and how to approach this dietary regimen, are presented in the Caloric Restriction protocol.
- Low-glycemic-index and low-glycemic-load diet. Glycemic index and glycemic load are estimates of the impact foods have on postprandial blood sugar. Glycemic index is a numerical value that reflects the blood sugar response to a given food in comparison with the response to a specific amount of a pure glucose solution and of white bread (Sheard 2004; Barclay 2008; Juanola-Falgarona 2014; Derdemezis 2014; Afaghi 2012; Higdon 2009). Glycemic load is determined by both glycemic index as well as the total amount of carbohydrate in a food. A low glycemic load denotes a smaller expected postprandial glucose elevation than a high glycemic load (Bhupathiraju 2014; Venn 2007).
Beans, fruits, vegetables, and unsweetened dairy products are generally low-glycemic-index foods, while rice and grains (whole and refined), breads, and breakfast cereals can be high- or low-glycemic index foods, depending in part on how they are prepared. Fiber-containing foods usually have a lower glycemic index and load (Atkinson 2008; Sheard 2004; ADA 2014b; AHA 2014).
A comprehensive analysis of observational studies found that, compared with the lowest glycemic index and glycemic load diets, the highest glycemic index and glycemic load diets were associated with significantly higher risk of developing type 2 diabetes, heart disease, gallbladder disease, and other diseases. Intervention trials using low glycemic index and glycemic load diets have shown improved metabolic parameters in diabetics and overweight or obese subjects. The American Diabetes Association endorses the use of glycemic index and glycemic load as an adjunct to counting carbohydrates for controlling blood glucose (ADA 2014b; Sheard 2004; Atkinson 2008; Barclay 2008; Juanola-Falgarona 2014; Afaghi 2012).
- Diet rich in prebiotic fibers. Consuming a diet high in fiber (especially cereal-derived whole-grain fiber) is perhaps the most well-documented dietary strategy for reducing risk of developing diabetes and helping control existing diabetes (McRae 2018; Silva, Kramer 2013; Weickert 2018). Certain kinds of dietary fiber, often referred to as “prebiotics,” support healthy microbiota growth in the gut. There are many sources and types of prebiotic fibers, but some more common examples include psyllium, whole grain wheat, bananas, acacia gum, and various oligosaccharides. Upon metabolizing prebiotic fibers, the gut bacteria generate short-chain fatty acids (SCFAs) that can be used as an energy source by both bacteria and people, and which may help mitigate inflammation (Slavin 2013; Davani-Davari 2019). Type 2 diabetes is associated with deficiency in SCFA production. In a 12-week clinical study, 43 patients with type 2 diabetes were randomized to receive either standard dietary recommendations or a high-fiber diet that consisted of whole grains, traditional Chinese medicinal foods, and prebiotics. Both groups were also given acarbose. While both groups experienced improvements in HbA1c, fasting glucose, and postprandial glucose, participants in the high-fiber group experienced better glycemic control (89% achieved HbA1c < 7% vs. only 50% in the control group) and faster improvements in glucose levels. Part of this improvement was attributed to increased GLP-1 production. Those in the high-fiber group also had better reductions in body weight and blood lipid profiles. The researchers determined that the high-fiber diet promoted SCFA-producing bacterial strains, which helped improve glycemic control (Zhao 2018). Probiotic supplementation, which may also promote SCFA-producing strains, has been shown to be beneficial in type 2 diabetes as well (Perraudeau 2020; Kocsis 2020).
Self-monitoring. Self-monitoring of blood glucose levels with a glucose meter is an important part of diabetes management (Benjamin 2002). Keeping a log of blood glucose readings can help patients recognize patterns and fluctuations in their blood sugar levels in relation to diet, lifestyle, and medications (Kishore 2014; Benjamin 2002; ADA 2015b). Effective self-monitoring, as part of a comprehensive treatment plan, has been shown to reduce complications of diabetes; and greater frequency of self-monitoring is associated with lower HbA1C levels (ADA 2015f).
Finger-stick glucose meters are available for purchase over the counter, and even people who are not diabetic should consider obtaining a glucose meter and periodically monitoring their fasting as well as postprandial glucose levels.
Weight loss. For individuals with diabetes who are overweight, even relatively modest weight loss has been shown to improve glycemic control and cardiovascular risk markers (Wing 1987; Wing 2011). Potential benefits include improvements in HbA1C, increased HDL cholesterol, decreased triglycerides, and lower blood pressure (ADA 2015a). Moreover, for those who are not diabetic but are overweight, losing 7% of body weight, or 15 lbs. for individuals weighing 200 lbs. or more, reduces the risk of developing type 2 diabetes by 58% (Diabetes Prevention Program (DPP) Research Group 2002; NIDDK 2012). In obese individuals with diabetes, weight loss of about 15 kg (33 lbs.), achieved by a regimen involving calorie restriction, can lead to remission of type 2 diabetes in about 80% of individuals (Magkos 2020). Several strategies to promote healthy weight loss are reviewed in the Weight Loss protocol.
Physical activity. Regular exercise improves blood glucose control, lowers risk of cardiovascular disease, aids in weight loss, and improves overall health. It may also prevent development of diabetes in those at high risk. In adults with diabetes, a minimum of 150 minutes per week of moderate-intensity aerobic exercise (to 50‒70% max heart rate) or vigorous aerobic exercise (to >70% max heart rate) is generally recommended. Seventy-five minutes of vigorous-intensity activity may suffice for younger and physically fit individuals. Additional strength training is also beneficial at least twice weekly. Adults over 65 or those with disabilities for whom this level of activity is not possible should be as physically active as possible. Sedentary time should be minimized and prolonged sitting should be interrupted every 30 minutes for blood glucose benefits (ADA 2021b). Moderate exercise, such as rapid walking for 30 minutes per day, five days per week, has been shown to substantially lower the risk of type 2 diabetes (Colberg 2010). Methods to maximize the benefits of exercise are described in Life Extension’s Exercise Enhancement protocol.
9 Starting Drug Therapy for Type 2 Diabetes: What to Expect
When you are newly diagnosed with diabetes, your doctor will assess your current health status (particularly your cardiovascular and kidney health), blood sugar control, and overall risk for further health problems before selecting the treatment strategy best suited to your needs. Depending on your baseline assessment, your doctor may start you on a single drug (monotherapy) or more than one drug with different mechanisms of action (combination therapy).
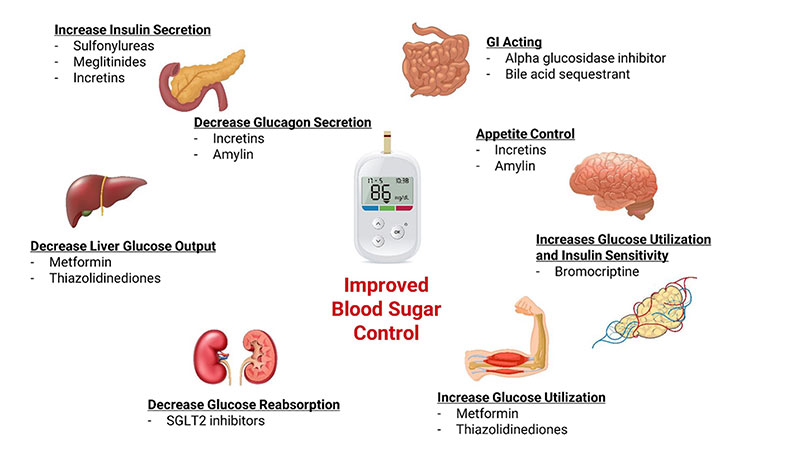
What follows is a general overview of some common approaches to drug treatment of type 2 diabetes based upon initial blood sugar control and health status. These approaches will not be suitable for everyone and are presented here only to help you generally understand what to expect when beginning drug therapy.
Monotherapy versus Combination Therapy
The decision to initiate drug therapy with metformin alone or in combination with another agent is influenced by HbA1C level and health status when diabetes is newly diagnosed. The presence and severity of symptoms as well as health status and comorbidities will influence the selection drugs for combination therapy.
First-line medication (if not contraindicated) is typically metformin; additional medication selection is based on age, life expectancy, and comorbidities such as heart or kidney disease (Feingold 2000; Wexler 2021a). The dosage of most hypoglycemic medications is gradually increased, if needed, according to a prescribed dosing schedule and therapeutic glycemic targets or until maximal dosage is reached. However, dosage may be limited by kidney (or liver) function, as well as side effects of treatment.
For patients with cardiovascular or kidney disease, SGLT2 inhibitors or GLP-1 receptor agonists (oral or injectable) may be considered as part of first-line therapy for their cardiorenal benefits. A review and analysis of trials of GLP-1 receptor agonists found that they reduce the risk of cardiovascular and all-cause mortality and stroke; this review also found that these drugs appear to reduce the risk of decline in kidney function. The same analysis found that SGLT2 inhibitors also reduce the risk of cardiovascular and all-cause mortality, protect kidney function, and reduce the risk of hospitalization for heart failure (Htike 2017; Kanie 2021; Lou, Zhang 2015; Rehman 2017).
Although weight loss is an important consideration for a considerable proportion of type 2 diabetics, medication is not first-line therapy for weight loss, at least in part due to medication side effects (Wexler 2021a). However, among the glycemia-lowering agents used in diabetes care, liraglutide 3 mg (a GLP-1 receptor agonist) is the most clearly indicated for weight loss (Garber 2020). The treatment selected must consider the additional potential benefits, contraindications, and side effects (some of which are described above) on a case-by-case basis. Beyond this, selection may be guided by cost and the availability of generic alternatives, although safety and efficacy should be higher priorities.
Your doctor will follow-up with you regularly to evaluate your progress and watch for medication-associated adverse events. If target goals are not being achieved at maximum medication dosage, a medication from another category may be added. However, keep in mind that if a medication is added as a third agent, it is typically less effective in lowering glucose than if it was used as a first- or second-line agent.5
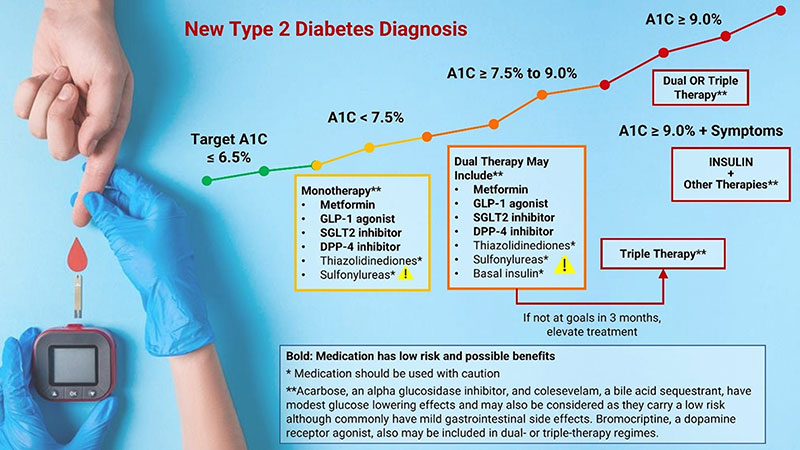
Initial HbA1C Levels and Therapeutic Selection
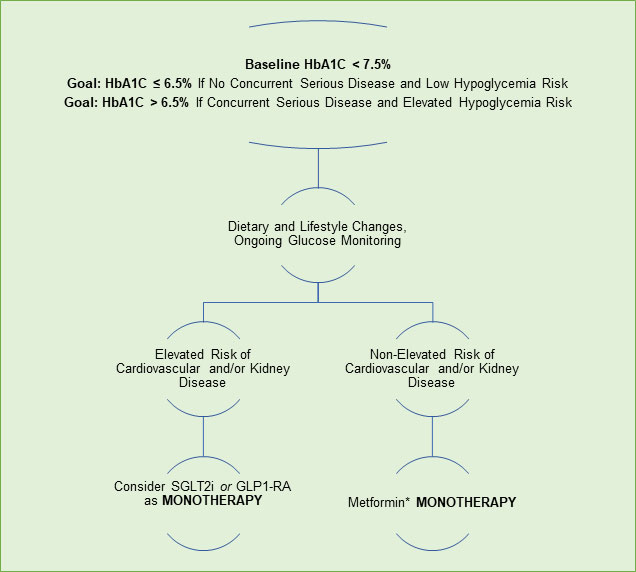
HbA1C < 7.5%. Metformin is the preferred first-line pharmacologic agent for the treatment of type 2 diabetes in those for whom it is not contraindicated due to its efficacy, favorable side effect profile, and affordability (Garber 2020). In the absence of cardiovascular or renal disease, newly diagnosed diabetics with HbA1C levels below 7.5% are typically started on metformin plus dietary and lifestyle changes. An SGLT2 inhibitor or GLP-1 receptor agonist may be started as an alternate first-line agent in people with pre-existing cardiovascular or renal disease. Diet and lifestyle changes, including weight loss for those who are overweight, are key parts of treating diabetes at all stages, and are a very important cornerstone of treatment.
HbA1C ≥ 7.5% and < 9%. For newly diagnosed diabetics with an HbA1C of ≥ 7.5% to < 9%, a combination of metformin and one or two additional pharmacologic agents, in addition to intensive and comprehensive diet and lifestyle changes, is indicated to most effectively control blood sugar. Data support the use of combination therapies at the time of diagnosis for more rapid glucose lowering effects, particularly if HbA1C levels are 1.5–2.0% above the target goal (Garber 2020; ADA 2021c). Therapies used in combination should have complementary mechanisms of action (and not be within the same category) so they act simultaneously by different pathways to achieve treatment goals (Garber 2020). Because antidiabetic medications are often used in combination, single-pill options with fixed doses are available, which may improve compliance. Available combinations of oral medications include metformin and/or SGLT2 inhibitors, DPP4 inhibitors, thiazolidinediones, and sulfonylureas.
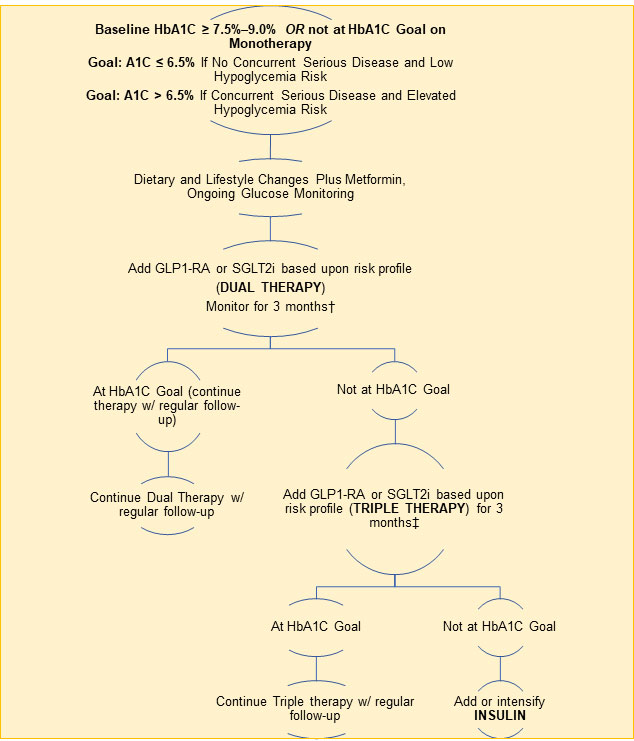
A1C > 9%. If HbA1C levels are 9.0% or higher, initial treatment may comprise two or three medications at maximal dosages. Insulin may be added if there are symptoms of hyperglycemia (excessive thirst, hunger, or urination or unintentional weight loss). A 2021 analysis of three-year follow-up data from the EDICT (Efficacy and Durability of Initial Combination Therapy) randomized controlled trial found that initiation of triple therapy with metformin, pioglitazone, and exenatide at the time of diagnosis not only led to greater improvements in HbA1C values at six months, it also significantly reduced HbA1C levels by 0.5% more than sequential initiation of metformin followed by glipizide and insulin in diabetics with a HbA1C value of approximately 9% (Abdul-Ghani 2021). Furthermore, triple therapy increased insulin sensitivity three-fold and beta-cell function 30-fold while sequential treatment did not improve insulin sensitivity and led to a more modest 34% increase in beta-cell function.
10 Overview of Type 2 Diabetes Drugs
Oral Type 2 Diabetes Drugs
There are 10 classes of oral drugs used to treat type 2 diabetes (Feingold 2000; Lexicomp 2022). These drugs may be used alone (monotherapy) or in combinations of two or more medications from different classes. Also, several branded products that combine two drugs from different classes into a single pill are available.
- Biguanide (metformin)
- Primary mechanism(s) of action
- Reduces glucose production in the liver and improves liver insulin sensitivity. Metformin also alters the intestinal microbiome (Vallianou 2019), increases intestinal glucose utilization, and stimulates GLP-1 secretion.
- Examples
- Metformin
-
Dosage
- Immediate release
- Initial: 500 mg once or twice daily OR 850 mg once daily
- Maximum: 2,550 mg daily (dosages above 2,000 mg daily are typically administered in three divided doses)
-
Extended release
- Initial: 500–1,000 mg once daily
- Maximum: 2,000 mg daily
- Immediate release
-
Dosage
-
Possible side effects include:
- Gastrointestinal: diarrhea, nausea, and/or abdominal discomfort
- Vitamin B12 deficiency (and elevated homocysteine)
- Testosterone reduction (blood tests can measure this and natural testosterone prescription creams may be considered)
- Primary mechanism(s) of action
- Oral glucagon-like peptide-1 (GLP-1) receptor agonist
- Primary mechanism(s) of action
- Enhance incretin signaling to increase glucose-dependent insulin secretion, reduce post-prandial glucagon, and delay gastric emptying (resulting in reduced after-meal glucose levels and increased satiety)
-
Examples
-
Semaglutide (Rybelsus) is the only approved medication in this class as
of early 2022.
-
Dosage:
- Initial: 3 mg once daily for 30 days. It may then be increased, if indicated, to 7 mg once daily.
- Maximum: 14 mg once daily, if 7 mg dose is insufficient
-
Dosage:
-
Semaglutide (Rybelsus) is the only approved medication in this class as
of early 2022.
- Possible side effects include:
- Gastrointestinal: nausea, vomiting, diarrhea
- The long-term safety of GLP-1 receptor agonists has not yet been established.
- Primary mechanism(s) of action
- Sodium-glucose transport protein 2 (SGLT2) inhibitors
-
Primary mechanism(s) of action
- Reduce reabsorption of glucose in the kidneys (renal tubules) by inhibiting sodium-glucose transport protein 2 (SGLT2). This leads to increased urinary glucose excretion.
- Examples
- Canagliflozin (Invokana)
-
Dosage
- Initial: 100 mg once daily before the first meal of the day
- Maximum: 300 mg once daily
-
Dosage
- Dapagliflozin (Farxiga)
-
Dosage
- Initial: 5‒10 mg once daily
- Maximum: 10 mg once daily
-
Dosage
- Empagliflozin (Jardiance)
-
Dosage
- Initial: 10 mg once daily
- Maximum: 25 mg once daily
-
Dosage
- Canagliflozin (Invokana)
- Possible side effects include:
- Urinary tract infections
- Genital fungal (mycotic) infections (ie, Candida infection)
- Hypovolemia (low extracellular fluid) and hypotension (low blood pressure)
- Acute kidney injury, possibly as a result of volume depletion (limited and contradictory evidence)
- Diabetic ketoacidosis (rare)
- Osteoporosis and bone fractures
-
Primary mechanism(s) of action
- Primary mechanism(s) of action
- Enhance incretin signaling via inhibition of the enzyme dipeptidyl peptidase-IV (DPP-4), which breaks down GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). This results in increased concentrations of GLP-1 and GIP (incretin hormones), both of which stimulate insulin secretion, while GLP-1 also promotes satiety.
-
Examples
-
Sitagliptin (Januvia)
-
Dosage
- Initial: 25–100 mg once daily (depending on kidney function)
- Maximum: 100 mg once daily
-
Dosage
-
Saxagliptin (Onglyza)
-
Dosage
- Initial: 2.5–5 mg once daily
- Maximum: 5 mg once daily
-
Dosage
- Linagliptin (Tradjenta)
-
Dosage
- Initial: 5 mg once daily
- Maximum: 5 mg once daily
-
Dosage
-
Alogliptin (Nesina)
-
Dosage
- Initial: 6.25–25 mg once daily (depending on kidney function)
- Maximum: 25 mg once daily
-
Dosage
-
Sitagliptin (Januvia)
-
Possible side effects include:
- Acute pancreatitis (this appears to be rare)
- Severe joint pain
- Primary mechanism(s) of action
- Modulation of glucose and lipid metabolism via activation of the PPAR-gamma nuclear hormone receptor. This leads to reduction of insulin resistance/improved insulin sensitivity and decreased fat accumulation in the liver, muscle, and pancreas.
- Examples
- Pioglitazone (Actos)
-
Dosage
- Initial: 15–30 mg once daily
- Maximum: 45 mg daily
-
Dosage
- Rosiglitazone (Avandia)
-
Dosage
- Initial: 4 mg daily as a single dose or in two divided doses
- Maximum: 8 mg daily as a single dose or in two divided doses
-
Dosage
- Pioglitazone (Actos)
- Possible side effects include:
- Weight gain
- Fluid retention (edema), including macular edema
- Increased risk of heart failure (related to fluid retention)
- Osteoporosis and bone fracture
- Possible increased risk of bladder cancer with pioglitazone
-
Primary mechanism(s) of action
- Inhibition of intestinal α-glucosidase and pancreatic α-amylase, which reduces carbohydrate absorption.
- Examples
- Acarbose (Precose)
-
Dosage
- Initial: 25 mg three times daily with first bite of each meal
- Maximum: Generally 300 mg, with meals, per day
-
Dosage
- Miglitol (Glyset)
-
Dosage
- Initial: 25 mg three times daily at the start of each meal
- Maximum: 50 mg three times daily
-
Dosage
- Possible side effects include:
-
Gastrointestinal: flatulence (gas), abdominal discomfort, diarrhea
- High-carbohydrate diet may worsen these side effects
- Gastrointestinal side effects usually become less pronounced with continued use
- Liver enzyme (transaminases) elevation
-
Gastrointestinal: flatulence (gas), abdominal discomfort, diarrhea
- Acarbose (Precose)
- Primary mechanism(s) of action
- Sulfonylureas stimulate insulin release by pancreatic beta cells. Their effect is independent of blood glucose levels.
-
Examples
- Glyburide [glybenclamide] (Glynase)
-
Dosage
- Micronized
- Initial: 0.75–3 mg once daily with the first main meal
- Maximum: 12 mg daily
- Conventional:
- Initial: 1.25–5 mg once daily with the first main meal
- Maximum: 20 mg daily
- Micronized
-
Dosage
-
Glipizide (Glucotrol, Glucotrol XL)
-
Dosage
- Immediate release
- Initial: 2.5–5 mg daily 30 minutes before first meal. Higher doses may necessitate divided dose (twice/day) administration.
- Maximum: 20 mg daily
-
Extended release
- Initial: 2.5–5 mg once daily with first main meal
- Maximum: 20 mg daily
- Immediate release
-
Dosage
- Glimepiride (Amaryl)
-
Dosage
- Initial: 1–2 mg once daily with first main meal
- Maximum: 8 mg daily
-
Dosage
- Glyburide [glybenclamide] (Glynase)
- Possible side effects include:
- Hypoglycemia
- Weight gain
-
Primary mechanism(s) of action
- Meglitinides stimulate insulin release by pancreatic beta cells. Their effect is dependent on glucose levels. Their effects have a rapid onset but do not last long.
- Examples
-
Repaglinide (Prandin)
-
Dosage
- Initial: 0.5–2 mg before each meal, up to four times daily
- Maximum: 16 mg daily, 4 mg per dose
-
Dosage
- Nateglinide (Starlix)
-
Dosage
- Initial: 60–120 mg before meals, up to three times daily
- Maximum: 360 mg daily, 120 mg per dose
-
Dosage
-
Repaglinide (Prandin)
- Possible side effects include:
- Hypoglycemia (lower risk than sulfonylureas)
- Weight gain
-
Primary mechanism(s) of action
- Bile acid sequestrants were originally developed and approved to lower LDL-cholesterol. The mechanism(s) by which they lower glucose are unclear, although an increase in GLP-1 and GIP may be involved.
- Example
- Colesevelam (Welchol)
-
Dosage
- Initial: 3,750 mg daily, either in one dose or divided into two doses (available as pills or packets of powder)
- Maximum: 3,750 mg in one or two doses
-
Dosage
- Colesevelam (Welchol)
-
Primary mechanism(s) of action
- Acts on the central nervous system, particularly the hypothalamus, as a dopamine D2 receptor agonist, increasing insulin sensitivity in liver, muscle, and fat (adipose tissue)
- Dosage
- Initial: 0.8 mg once daily, within two hours of waking
- Maximum: 4.8 mg daily
- Possible side effects include:
- Nausea and vomiting (usually improves with continued use)
- Low blood pressure (hypotension)
Injectable (Non-Insulin) Type 2 Diabetes Drugs
There are three classes of injectable drugs approved to treat type 2 diabetes. Insulin also may be included in combination with other therapies if glucose or HbA1C levels are particularly high for a faster glucose lowering effect. If the individual is experiencing symptoms of hyperglycemia such as increased urination or thirst or unintentional weight loss along with very high blood sugar, insulin is typically indicated (Feingold 2000; ADA 2021c; Lexicomp 2022).
- GLP-1 receptor agonists
- Primary mechanism(s) of action
- Increase glucose-dependent insulin secretion, reduce post-prandial glucagon, and delay gastric emptying (resulting in reduced after meal glucose levels and increased satiety)
- Examples
- Exenatide (Byetta 10 mcg Pen, Byetta 5 mcg Pen, Bydureon BCise 2 mcg Pen)
- Dosage
- Immediate release (Byetta)
- Initial: 5 mcg subcutaneous injection twice daily a maximum of 60 minutes before morning and evening meals, or before the two main meals of the day
- Maximum: 10 mcg subcutaneous injection twice daily a maximum of 60 minutes before morning and evening meals, or before the two main meals of the day
- Extended release (Bydureon BCise)
- Initial: 2 mg subcutaneous injection once weekly
- Maximum: 2 mg subcutaneous injection weekly
- Lixisenatide (Adlyxin) – Short-acting
- Dosage
- Initial: 10 mcg subcutaneous injection once daily, up to one hour before the first meal of the day, for 14 days; then increase to 20 mcg once daily on day 15
- Maximum: 20 mcg subcutaneous injection once daily
- Liraglutide (Victoza) – Long-acting
- Dosage
- Initial: 0.6 mg subcutaneous injection once daily for one week, then increase to 1.2 mg once daily
- Maximum: 1.8 mg subcutaneous injection once daily
- Dulaglutide (Trulicity) – Long-acting
- Dosage
- Initial: 0.75 mg subcutaneous injection once weekly, then may increase to 1.5 mg once weekly after four to eight weeks if needed to achieve blood sugar goals
- Maximum: 4.5 mg subcutaneous injection once weekly. Dosage increased only after a minimum of four weeks at the prior dose.
- Semaglutide (Ozempic) – Long-acting
- Dosage
- Initial: 0.25 mg subcutaneous injection once weekly for four weeks. This dosage is intended for treatment initiation only, not for glycemic control. Then, increase to 0.5 mg once weekly.
- Maximum: 1 mg subcutaneous injection once weekly, only after a minimum of four weeks at the 0.5 mg dose does not sufficiently control glycemia.
- Possible side effects include:
- The long-term safety of GLP-1 receptor agonists has not yet been established.
- Gastrointestinal: nausea, vomiting, diarrhea. Nausea may decrease over time and may respond to dose titration.
- Gall bladder/bile duct disease
- Acute pancreatitis and other pancreatic disorders have been reported, though the relationship to treatment is unclear
- Injection-site reactions
- Dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist
- Primary mechanism(s) of action
- Selectively binds and activates both GIP and GLP-1 receptors
- Example
- Tirzepatide (Mounjaro)
- Dosage
- Initial: 2.5 mg subcutaneous injection once weekly, titrated up to 5 mg weekly after 4 weeks
- If additional glycemic control is needed, dosage can be increased in 2.5 mg increments after at least 4 weeks on the current dosage.
- Maximum: 15 mg subcutaneous injection once weekly
- Possible side effects include:
- Gastrointestinal (eg, nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, abdominal pain)
- Pancreatitis
- Acute gallbladder disease
- Hypoglycemia (when used with insulin or insulin secretagogues)
- Changes in vision (people with a history of diabetic retinopathy should be monitored closely)
- Acute kidney injury (primarily in people with pre-existing kidney problems who experience gastrointestinal side effects)
- Allergic reactions (eg, swelling, rash, dizziness)
- Amylin mimetic
- Primary mechanism(s) of action
- Reduces glucagon secretion, slows gastric emptying, suppresses appetite
- Example
- Pramlintide (SymlinPen 60, SymlinPen 120)
- Dosage
- Initial: 60 mcg subcutaneous injection immediately before each major meal, then increase to 120 mcg subcutaneous injection before each major meal if no significant nausea occurs after three days
- Maximum: 120 mcg subcutaneous injection before each major meal
- Possible side effects include:
- Nausea
11 Type 2 Diabetes Drugs – Additional Details
Oral Drugs
Metformin has long been the preferred first-line pharmacologic agent for the treatment of type 2 diabetes in those for whom it is not contraindicated (namely those with significant kidney dysfunction, alcohol abuse, liver disease, unstable or acute heart failure, or elevated risk of lactic acidosis) (ADA 2021c; Wexler 2021a). It is well-tolerated, inexpensive, and does not increase risk of hypoglycemia or weight gain. However, with the more recently introduced SGLT2 inhibitors and GLP-1 receptor agonists having shown impressive cardiovascular risk reduction in addition to glycemic control efficacy, these newer agents may also be considered as first-line therapies in patients with pre-existing cardiovascular or renal disease (Garber 2020). Nevertheless, metformin remains the typical first-line choice because of its well-established safety profile, efficacy, low cost, and wide accessibility (Wexler 2021b).
Life Extension has reported on the myriad potential health and longevity benefits of metformin for many years. In addition to its glucose-lowering benefits, metformin has been clinically demonstrated to have anti-cancer effects, especially as regards pancreatic, colorectal, and liver cancers (Yu 2019; Kasznicki 2014). There has also been considerable interest in metformin’s ability to protect cardiovascular health, and it has been shown to protect the heart and prolong life in patients with heart failure (Monami 2021; Halabi 2020). The potential of metformin as a more general anti-aging drug is being actively investigated (Glossmann 2019).
Gastrointestinal side effects are common upon initiation of metformin therapy but can also begin anew in patients who have taken metformin for many years. These side effects can often be managed by taking the medication with food, taking a drug holiday, switching to an extended-release formulation, or starting with a low dosage and gradually increasing over time (Wexler 2021b). However, if metformin is still not tolerated, the presence (or absence) of comorbidities helps inform the choice of alternative medications.
Chemically, metformin resembles naturally occurring biguanide molecules in the French lilac, a plant used in traditional medicine for hundreds of years (Witters 2001). Metformin, the only biguanide medication currently available for the treatment of diabetes, is considered the first-line drug for type 2 diabetes (Misbin 2004; Kajbaf 2016). The primary reason is that the glucose-lowering action of metformin, when used alone, is at least as effective as that of any other oral glucose-lowering drug, without promoting weight gain and only rarely causing excessively low blood sugar. Also, metformin can be safely used with other drugs, including insulin. According to the American Diabetes Association and the European Association for the Study of Diabetes, metformin therapy, together with lifestyle intervention, should be initiated at the time of diagnosis of type 2 diabetes. Metformin was selected as first-line treatment because of its record of safety and efficacy, as well as its low cost (Gold Standard 2015c). Worldwide, metformin is prescribed to over 100 million patients annually (Inzucchi 2012c; Rena 2013; Scheen 2013).
Metformin lowers blood glucose by several different mechanisms (Viollet 2012; LaMoia 2021). Its main modes of action are suppressing glucose production in the liver, mostly through inhibition of gluconeogenesis, and opposing the activity of the hormone glucagon (Kishore 2014; Pernicova 2014; LaMoia 2021). Metformin also functions as an insulin sensitizer, and it decreases intestinal glucose absorption (Gold Standard 2015c). Unlike other antidiabetic drugs that cause the pancreas to secrete more insulin, metformin makes the body’s tissues more sensitive to insulin, thus reducing insulin resistance (Nasri 2014). Side effects of metformin may include gastrointestinal distress or a slight taste disturbance, usually a metallic taste. Rarely, metformin may cause potentially serious lactic acidosis, a buildup of lactic acid in the blood (Diabetes.co.uk 2016).
The health benefits of metformin extend beyond diabetes. In particular, abundant evidence indicates metformin has significant anti-cancer activity. In a large, rigorous review of over 50 studies involving more than one million patients, metformin use in patients with type 2 diabetes was associated with a 35% reduction in risk of death from cancer, and a 27% reduction in risk of any cancer (Franciosi 2013). In a study of over 1000 breast cancer patients with diabetes, those taking metformin had an almost 24% lower mortality risk compared with controls, whereas those not taking metformin had an almost 71% higher mortality risk (Hou 2013). Numerous epidemiologic and observational studies have also found metformin use is associated with dramatically reduced risk of developing cancer (Morales 2015). Clinical trials are investigating metformin as an anti-cancer therapy in breast, prostate, endometrial, and pancreatic cancers (Dowling 2011). In addition to its benefits in diabetes and cancer, metformin has been shown to promote weight loss and protect against cardiovascular disease, Alzheimer disease, and non-alcoholic fatty liver disease (Forouzandeh 2014; Blagosklonny 2009; Gupta 2011; Mazza 2012; Berstein 2012; Miles 2014; LaMoia 2021).
In fact, metformin shows such great promise as an anti-aging drug (Martin-Montalvo 2013; Anisimov 2013; Violett 2012; Blagosklonny 2009; Greer 2007) that the US Food and Drug Administration (FDA) approved a large-scale clinical trial—the Targeting Aging with Metformin (TAME) trial—to test its effects on biological aging (AFAR 2016).
One underlying reason for metformin’s many benefits is thought to be its ability to activate AMPK—a critical enzyme that functions as a key regulator of energy balance in the body (Coughlan 2014; Boyle 2010; Trefts 2021). AMPK (adenosine monophosphate-activated protein kinase) is a major hub for a network of cell signaling that, when activated, keeps the metabolism running smoothly (Ruderman 2013). Activation of AMPK, for example, signals cells to burn glucose and fatty acids for energy rather than store them as body fat. Exercise and calorie restriction are proven methods to boost AMPK activity. However, the body’s response to AMPK signaling becomes blunted with age. Disturbances in AMPK signaling and overall energy balance lead to chronic inflammation, obesity, and the development of age-related diseases (Lee 2013; Lee 2006; Rana 2015). AMPK even helps control the aging process itself by inducing several known longevity factors (such as SIRT1) that have been shown to extend lifespan in many organisms (Salminen 2011).
Since metformin is a prescription medication, people without diabetes may have difficulty accessing it. Fortunately, medicinal plants, such as Gynostemma pentaphyllum, may activate AMPK and provide some of the same metabolic benefits as metformin (Yoo 2016).
Another explanation for metformin’s wide-ranging benefits has more recently gained recognition: its ability to impact the gut and the intestinal microbial community (microbiome) (McCreight 2016; Pryor 2015). The intestinal microbiome is increasingly thought to be a crucial factor in the development of obesity and diabetes (Palau-Rodriguez 2015; Hur 2015), and a growing body of laboratory, animal, and human research indicates metformin may normalize the disturbed microbial balance associated with abnormal glucose metabolism (Pryor 2015; Forslund 2015; McCreight 2016).
In a pilot study, 12 diabetic subjects being treated with metformin were examined as they discontinued and then re-started their metformin treatment. Tests showed that, within one week of stopping metformin, bile acid metabolism was altered such that total bile acid levels were increased, and levels of GLP-1, which is derived from intestinal cells, were decreased. Both of these changes point to alterations in digestive system functioning. In addition, significant individual changes in the composition of the intestinal microbiome were seen. These effects were reversed when metformin was reintroduced (Napolitano 2014).
Findings from a two-part study add to evidence that the gut is an important site of metformin’s action. In the first phase, 20 overweight but otherwise healthy individuals were treated with four distinct metformin preparations, each for one day. Metformin (immediate release, extended release, and two doses of delayed release) were used. The delayed-release preparations, designed to deliver metformin to the lower small intestine, were found to be poorly absorbed, resulting in lower metformin blood levels than the other preparations. In the second phase, 240 subjects with type 2 diabetes were treated with a placebo; delayed-release metformin with breakfast at doses of 600 mg, 800 mg, or 1000 mg; or extended-release metformin with evening meals at doses of 1000 or 2000 mg for 12 weeks. At all doses, the delayed-release metformin preparation had a significant effect on blood glucose and HbA1C levels versus placebo. Delayed-release metformin was about half as bioavailable as immediate-release or extended-release metformin, but was about 40% more potent than extended-release metformin in reducing fasting plasma glucose. Based on this research, it appears a significant proportion of metformin’s action could be attributed to its effect on the lower digestive tract (Buse 2016).
Acarbose. Acarbose belongs to a class of drugs called alpha-glucosidase inhibitors, commonly referred to as “starch blockers.” These drugs lower glucose by blocking breakdown of starches and slowing absorption of sugar and carbohydrates (ADA 2015d).
Acarbose is the most researched and widely used of the alpha-glucosidase inhibitors (Josse 2006). In China, the country with the largest diabetic population, acarbose is one of the most commonly prescribed oral glucose-lowering medications (Standl 2014; Gu 2015). Like metformin, acarbose is effective as a first-line drug for type 2 diabetes insufficiently controlled by diet alone, and does not stimulate insulin secretion or cause hypoglycemia or weight gain. Acarbose blocks the intestinal enzyme alpha-glucosidase needed to digest complex carbohydrates (eg, starches) into simple sugars such as glucose that can be easily absorbed into the bloodstream. As a result, acarbose helps reduce after-meal blood sugar levels (Campbell 1996; Hanefeld 1998; Gu 2015; Joshi 2014; Derosa 2012).
In a rigorous review of 19 randomized controlled trials that enrolled type 2 diabetics, acarbose was found to significantly reduce HbA1C levels when given alone or as add-on treatment with other antidiabetic drugs (Derosa 2012). In an international trial involving over 500 patients with impaired glucose tolerance, treatment with acarbose significantly improved glucose tolerance, and reduced the risk of progression to diabetes by 25% over 3.3 years (Chiasson 2002). The Acarbose Cardiovascular Evaluation (ACE) trial, which enrolled 7,500 Chinese subjects (of which 6,522 completed the trial) with coronary heart disease and impaired glucose tolerance, found acarbose treatment for four months reduced the incidence of diabetes and promoted regression to normoglycemia (Gerstein 2020).
The mechanism of action of acarbose is complementary to that of metformin. While metformin primarily controls fasting blood sugar, acarbose regulates postprandial glucose levels. Combination therapy with acarbose and metformin has been demonstrated, in multiple studies, to be more effective for the control of blood glucose than treatment with either drug alone (Mooradian 1999; Johnson 1993; Joshi 2014; Jayaram 2010; Wang 2013).
In a study in diet-treated diabetic patients, even a single mixed meal of 450 Calories caused immediate and significant impairment in endothelial function, a key early step in the development of atherosclerosis. Prior treatment with acarbose markedly reduced the postprandial rise in blood glucose and associated endothelial dysfunction (Steyers 2014; Shimabukuro 2006).
In a review of seven long-term placebo-controlled studies in type 2 diabetics, acarbose reduced the risk of any cardiovascular event by 35% and the incidence of heart attack by 64% (Hanefeld 2004). The aforementioned ACE trial tested whether acarbose treatment can prevent heart attacks, strokes, or death from these events. Results suggest acarbose did not reduce the risk of major adverse cardiovascular events, but it did reduce the incidence of diabetes (Wamil 2020).
Side effects of acarbose treatment are generally mild, and tend to be short-lived. Gastrointestinal effects such as flatulence and diarrhea are most common (Coniff 1995; Joshi 2014; Derosa 2012; Standl 2014).
Since acarbose is a prescription medication, some individuals who wish to use it to control post-meal glucose elevations but are not diabetic may have difficulty obtaining the drug. Fortunately, natural products such as white mulberry leaf extract, green tea extract, and brown seaweed extract also inhibit the alpha-glucosidase enzyme and help reduce postprandial hyperglycemia (Lordan 2013; Zhang 2007; Yatsunami 2008; Hansawasdi 2006). Other bioactive compounds from plant-based foods such as mangos and jackfruit have displayed inhibitory effects against alpha-glucosidase (Assefa 2019).
SGLT2 inhibitors. The sodium-glucose cotransporter 2 (SGLT2) is responsible for reabsorption of glucose from the kidneys’ filtration system back into the blood (Sano 2020). SGLT2 inhibitors are a class of prescription drugs that modestly lower blood sugar levels by preventing glucose reabsorption in the kidneys. This results in increased urinary glucose excertion (Tat 2018). SGLT2 inhibitors are generally combined with other treatments in type 2 diabetes. For instance, SGLT2 inhibitors may be added if first-line therapies such as diet and lifestyle changes plus metformin are insufficient. These drugs may also be used to more specifically target and prevent kidney and cardiac complications in certain patient populations (Garber 2020; ADA 2019). SGLT2 inhibitors such as dapagliflozin (Farxiga), canagliflozin (Invokana), and empagliflozin (Jardiance) have been shown to reduce HbA1C levels, promote weight loss, and lower blood glucose levels. A growing body of research is establishing that SGLT2 inhibitors improve cardiovascular and renal outcomes in both diabetics and non-diabetics (Tat 2018).
A systematic review and meta-analysis of 58 randomized controlled trials involving over 16,400 participants with diabetes found that SGLT2 inhibitors reduced Hb1AC levels, promoted reductions in body weight, and decreased systolic blood pressure (Vasilakou 2013). Several meta-analyses in various diabetic populations have found that SGLT2 inhibitor treatment is associated with reduced risk of dying from any cause (Garber 2020).
In a meta-analysis of 10 placebo-controlled trials including over 71,000 subjects with or without diabetes at recruitment, treatment with SGLT2 inhibitors reduced the risk of heart failure hospitalization or cardiovascular death by 24% and lowered the risk of poor renal outcomes by 32% (Barbarawi 2021). Another meta-analysis, published in 2021, examined the results of six SGLT2 inhibitor trials that included nearly 47,000 subjects with type 2 diabetes, about 31,000 of whom had atherosclerotic cardiovascular disease. The meta-analysis found that SGLT2 inhibitors were associated with a 10% reduced risk of major adverse cardiovascular events, a 22% reduced risk of heart failure or cardiovascular death, and a 38% risk reduction in a composite of adverse kidney outcomes (Inzucchi 2021). Results of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial, which compared dapagliflozin (10 mg once daily) to placebo, found that dapagliflozin was associated with a 44% reduction in a composite of adverse kidney outcomes and a 29% reduction in death from cardiovascular causes or hospitalization for heart failure; this trial was ended early due to overwhelming efficacy (Heerspink 2020). The SGLT2 inhibitor dapagliflozin has been shown to decrease the risk of developing type 2 diabetes. In a clinical trial including 2,605 people with heart failure but no prior history of diabetes, dapagliflozin (10 mg daily) led to a 32% reduction in new diabetes incidence over a median follow-up of 18 months (Barbarawi 2021; Silverii 2021; Neuen 2021; Koh 2021).
Blood pressure medications are also commonly prescribed to people who have diabetes, since diabetes and high blood pressure tend to co-occur. A meta-analysis of seven studies including 1,757 participants with type 2 diabetes found that SGLT2 inhibitor therapy in combination with blood pressure medications (known as renin-angiotensin system blockers) was well-tolerated and resulted in greater improvement in blood pressure, glucose control, body weight, and indices of kidney function compared with the blood pressure drugs alone (Tian 2021).
Although typically considered second-line therapy, SGLT2 inhibitors may have a role as initial pharmacological treatment in type 2 diabetes in conjunction with metformin in appropriately selected patients (Donnan 2019; Chen 2020). In a systematic review and meta-analysis of four randomized controlled trials including over 3,700 subjects, combination therapy with an SGLT2 inhibitor and metformin beginning at diabetes diagnosis resulted in greater weight loss and reduction of HbA1C levels compared with either drug alone. Adverse effects of combination therapy included an increased risk of genital infections and diarrhea (Milder 2019). As of late 2021, available evidence suggests first-line therapy with SGLT2 inhibitors may improve some outcomes, particularly in individuals with newly diagnosed diabetes and comorbid heart and kidney health concerns; however, increased ischemic stroke risk in this context has been observed as well (Chen 2020; Milder 2019). Additional randomized controlled trials are needed to clarify the efficacy and safety of SGLT2 inhibitors in the first-line treatment of type 2 diabetes.
SGLT2 inhibitors have limited glucose-lowering efficacy in individuals with an estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2 because these drugs’ ability to lower glucose is a function of kidney filtration rate. Nevertheless, individuals with diabetes and eGFR in the range of 30–90 mL/min/1.73 m2 have been shown to derive kidney health benefits from SGLT2 inhibitor therapy in the clinical trial setting (Garber 2020; Perkovic 2019).
Although SGLT2 inhibitors have a strong safety profile, it is important to consider the risks that may arise from treatment. These risks include urinary tract infections and genital mycotic (fungal) infections; diabetic ketoacidosis (particularly when used in combination with insulin or sulfonylureas); hypoglycemia when used in combination with other type 2 diabetes medications; and hypovolemia (Vasilakou 2013; Wilding 2019; Scheen 2019; Sarafidis 2020; Engelhardt 2021; Wang 2020; Horii 2020; Menne 2019). They also carry a risk of dehydration and hypotension due to increased urinary output, especially in the elderly and those being treated with blood pressure-lowering drugs (Garber 2020). Canagliflozin has been shown to increase the risk of amputation and bone fractures (ADA 2019). Careful monitoring for adverse effects is important, particularly in older individuals and those using diuretics or non-steroidal anti-inflammatory drugs (NSAIDs). It is important for those taking SGLT2 inhibitors to be compliant with their treatment regimen and remain mindful of their own signs and symptoms to reduce the risk of adverse effects.
Sulfonylureas. As both insulin and most GLP-1 agonists are injections, high doses of sulfonylureas may be considered for those who are injection averse, as treatment adherence is critical to therapeutic success. Sulfonylureas may also be used in combination with metformin and other medications with complementary mechanisms of action. The risk of hypoglycemia is also high with sulfonylureas and may be further increased through their combined use with other medications such as SGLT2 inhibitors (Jiang 2021).
Sulfonylureas are an older medication class with side effects of weight gain and additional concerns of cardiovascular safety. They have increasingly fallen out of favor as metformin has proven to be optimal first-line therapy, and as the evidence basis grows for the glycemic and cardiorenal benefits of newer drug classes.
Injectable Drugs (Excluding Insulin)
Glucagon-like peptide-1 (GLP-1) receptor agonists. GLP-1 receptor agonists (eg, exenatide, liraglutide) increase insulin secretion while inhibiting glucagon release, but only when blood glucose is high, thus reducing the risk of hypoglycemia. They reduce body weight and lower systolic blood pressure in type 2 diabetics, and increase after-meal satiety. These drugs are administered by subcutaneous injection. Adverse effects associated with GLP-1 agonists include nausea, diarrhea, and vomiting; development of antibodies against the drug; and reactions at the injection site (Garber 2011; Trujillo 2015). Some evidence suggests GLP-1 receptor agonists may be associated with a small increase in risk of pancreatitis or pancreatic cancer, but research thus far has been inconclusive and proven cardiovascular benefits likely outweigh the risks (Drucker 2006; Vilsbøll 2012; Ryder 2013; Nauck 2013; Peng 2016; Cao 2019).
Tirzepatide
Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist (Bailey 2021). The FDA approved tirzepatide as a type 2 diabetes treatment in May 2022 (FDA 2022).
GIP was long ignored as a treatment target in type 2 diabetes because it was believed to have low effectiveness in stimulating insulin secretion. However, newer studies have now revealed that—when hyperglycemia is first sufficiently controlled by another agent such as insulin—GIP effectively stimulates insulin secretion in type 2 diabetics (Bailey 2021). Multiple randomized controlled clinical trials have demonstrated that tirzepatide is superior for glycemic control and weight management in type 2 diabetics, compared to semaglutide 1 mg/week; titrated insulin degludec; or placebo (Ludvik 2021; Frias 2020; Frias 2021; Rosenstock 2021).
SURPASS-4 was a randomized controlled trial that compared the once-weekly tirzepatide (5 mg, 10 mg, or 15 mg) to titrated insulin glargine in nearly 2,000 subjects (Del Prato 2021). This trial followed patients for a minimum of 52 weeks and was designed to establish the safety and efficacy of tirzepatide in patients at high cardiovascular risk, and in whom glycemic management with one or more of three oral diabetes medications (metformin, a sulfonylurea, or an SGLT-2 inhibitor) had failed. All three doses of tirzepatide resulted in greater reductions in weight and HbA1C compared to insulin treatment. The percentage of participants who achieved target HbA1C levels below 5.7% and 7% at 52 weeks was greater with all doses of tirzepatide than with insulin glargine. Additionally, the percentage of participants who developed hypoglycemia was markedly lower in those receiving tirzepatide, although side effects of nausea, diarrhea, loss of appetite, and vomiting were considerably more common.
The SURPASS-5 trial examined tirzepatide (5, 10, and 15 mg) in 475 subjects taking insulin glargine for type 2 diabetes, with or without metformin, over 40 weeks (Dahl 2021). Tirzepatide at all three dosages resulted in clinically significant reductions in weight and HbA1C levels compared with placebo. Those on tirzepatide were nearly three times more likely to achieve a target HbA1C of < 7.0% than those who received placebo. More aggressive HbA1C targets of ≤ 6.5% and < 5.7% were also reached by large percentages of tirzepatide recipients, but relatively infrequently in those who received placebo. There was no significant difference in incidence of events of moderate hypoglycemia with tirzepatide versus placebo, though there were some occurrences of severe hypoglycemia. The most frequent adverse events, which occurred more frequently in the tirzepatide group, were nausea, diarrhea, constipation, and vomiting (Lilly 2022).
Pramlintide. Pramlintide (Symlin) is an injectable drug used along with insulin when after-meal hyperglycemia cannot be controlled with insulin alone (Cohen 2013). Pramlintide is a synthetic version of the hormone amylin, which is normally secreted along with insulin by pancreatic beta cells after a meal. Amylin slows gastric emptying and helps control postprandial glucose spikes (Schmitz 2004; Boyle 2018). Pramlintide is associated with severe hypoglycemia and weight loss. Also, due to the fact that it delays emptying of the stomach, it can delay absorption of drugs taken at the same time (Cohen 2013).
Insulin
Treatment with insulin may be necessary for type 2 diabetics who are already managed on multiple oral medications and are unable to achieve HbA1C goals. Because insulin can more rapidly lower glucose than oral medications, it also may be initiated at the time of diagnosis in individuals with severe hyperglycemia (HbA1C >9.0%). However, along with its potent anti-hyperglycemia effects, insulin also carries a high risk of hypoglycemia, often leads to weight gain, and some types of insulin have been linked to increased rates of several cancers (Karlstad 2013; Hodish 2018). Insulin treatment must be closely monitored with regular glucose measurements (fasting and post-prandial measurements, or via the use of continuous glucose monitoring if available) and adjusted as therapeutic goals are achieved (Garber 2020).
Because of its higher level of safety and ease of use, long-acting basal insulin (a once daily injection), which has the ability to sustain insulin levels up to 24 hours, rather than a short-acting mealtime insulin injection, is preferred for patients who are insulin naïve or at greater risk for hypoglycemia (Garber 2020). Prandial (mealtime) insulin is a short-acting insulin that may be added if post-prandial glucose goals are not being achieved; however, this approach carries a greater risk of hypoglycemia. Thus, treatment should be closely monitored and prandial insulin is not recommended as an initial therapy. Prandial insulin may be considered in patients resistant to other treatments, and if so, will often only be used with the largest meal initially.
Because GLP-1 receptor agonists (typically employed as injectables) increase mealtime insulin secretion and reduce postprandial serum glucose, they may work complementarily with basal insulin treatment to achieve blood sugar goals, especially if basal insulin has not been effective in improving after-meal sugar spikes. While insulin treatment is associated with weight gain, GLP-1 agonists promote weight loss, and may help patients achieve this goal even in combination with insulin (Maiorino 2018). Fixed-ratio combinations of basal insulin along with GLP-1 receptor agonists (as injectables) are available (Garber 2020).
Insulin cannot be taken orally because it would be degraded by stomach acid. Insulin is almost always injected subcutaneously (into the fat under the skin). Specific types of short- and long-acting insulin include (ADA 2015c; Vimalananda 2014):
- long-acting insulin (detemir, glargine) or intermediate-acting insulin (neutral protamine hagedorn [NPH]) to provide a steady basal effect of up to 12‒24 hours;
- rapid-acting insulin (lispro, aspart, insulin glulisine) to be used at mealtime to cover after-meal spikes in blood glucose.
12 Metabolic Surgery for Longstanding Type 2 Diabetes
For people with longstanding diabetes that cannot be adequately controlled with diet and lifestyle changes in conjunction with optimal drug therapy, metabolic surgery (bariatric surgery) may be indicated.
Researchers and physicians have known for many years that weight loss (bariatric) surgery can cause rapid and profound improvement in glucose metabolism among people with diabetes (Ilyas 2020). In addition, bariatric surgery has been associated with long-term improvements in metabolic health leading to reductions in cardiovascular risk factors, cardiovascular events, microvascular complications of diabetes, cancer, and mortality (Cummings 2018). Given that benefits of bariatric surgery can extend well beyond weight loss, it is now frequently referred to as “metabolic surgery” (Cummings 2018; Chen 2019; La Sala 2020).
The benefits of metabolic surgery appear to be sustained for many years. A study that followed 1,156 patients with severe obesity for 12 years found those who underwent gastric bypass surgery at the beginning of the study maintained an average weight loss of 35 kg while those who did not have surgery maintained an average weight loss of 0–2.9 kg. In addition, among participants with diabetes at the beginning of the study, metabolic surgery was associated with a 51% remission rate at the 12-year follow-up (Adams 2017).
Several randomized controlled trials have found metabolic surgery in addition to lifestyle and medical interventions is more effective than intensive medical and lifestyle interventions alone for inducing weight loss, improving metabolic health, and resulting in diabetes remission (Courcoulas 2015; Ikramuddin 2018; Schauer 2017). In one randomized controlled trial that included 60 participants with a BMI of 35 or higher and type 2 diabetes for ≥ 5 years, 75% of those who underwent gastric bypass surgery and 95% of those who underwent a type of metabolic surgery known as biliopancreatic diversion were diabetes-free two years later, but no one treated with non-surgical interventions alone experienced diabetes remission (Mingrone 2012). Ten years later, 25% of those treated with gastric bypass, 50% of those treated with biliopancreatic diversion, and 5.5% of those treated with conventional medical therapy were diabetes-free. Notably, surgically treated patients whose diabetes recurred maintained adequate blood glucose control and had fewer diabetes-related complications at 10 years than medically-treated patients (Mingrone 2021).
In early 2016, 45 worldwide medical and scientific societies endorsed new guidelines that recommend metabolic surgery in type 2 diabetics with a BMI ≥ 40 and in type 2 diabetics with a BMI of 35–39.9 whose hyperglycemia is not adequately controlled by lifestyle and medical therapy. These guidelines, put forth at the 2nd Diabetes Surgery Summit (DSS-II), also suggest metabolic surgery be considered in type 2 diabetic patients with a BMI of 30–34.9 and inadequately controlled hyperglycemia (Rubino 2016). Evidence supporting these guidelines continues to grow, and the safety of metabolic surgery has increased over the past two decades (Arterburn 2020). The 2022 ADA Standards of Medical Care in Diabetes guidelines propose metabolic surgery as an effective way to strongly improve glycemic control which often leads to remission of diabetes, improved quality of life, improved cardiovascular outcomes, and reduced mortality (ADA 2022).
The mechanisms behind the benefits of metabolic surgery are still being investigated. Changes in glucose, lipid, and bile acid metabolism as a result of metabolic surgery are believed to act in concert to improve energy metabolism and glucose tolerance (Xu 2021). Metabolic surgery has also been found to alter production of gut hormones and may cause changes in the gut microbiome, thereby affecting signaling pathways related to appetite, glycemic control, and inflammation (Xu 2021; Valenzano 2020). Type 2 diabetic patients have even been noted to have improved β-cell function after metabolic surgery (Chen 2019; Malin 2016). Another way metabolic surgery may improve health is by modifying adipose tissue-derived microRNA levels. MicroRNAs are small molecules of non-coding RNA, meaning unlike other types of RNA, they are not used for protein assembly. Instead, microRNAs regulate gene expression by preventing translation of targeted protein-coding RNA, in effect, silencing specific parts of the genetic code. Metabolic surgery appears to lead to changes in levels of adipose tissue-derived microRNAs involved in obesity, insulin resistance, systemic inflammation, and the progression from pre-diabetes to type 2 diabetes, and these changes are correlated with clinical benefits (La Sala 2020; Xu 2021). A small clinical trial of 20 patients randomized to metabolic surgery or standard medical therapy found that metabolic surgery can positively affect the gut microbiota. Patients who underwent metabolic surgery showed significantly increased genus richness (ie, diversity) in the gut microbiota compared with those on standard medical therapy, which was correlated with an improvement in metabolic and inflammatory biomarkers (Lau 2021).
Although metabolic surgeries have a good safety profile, problems can occur. The possible adverse outcomes vary somewhat depending on the type of surgery performed; in general, post-surgical problems include short-term complications, such as blood clots, bleeding, leakage, and infection, and long-term complications, such as strictures and obstruction, gallstones, gastroesophageal reflux, and nutritional and vitamin deficiencies. These possible adverse outcomes should be considered alongside the potential metabolic health benefits during the decision-making process for each individual considering metabolic surgery (Arterburn 2020).
13 Novel And Emerging Strategies
As the epidemic of diabetes continues to grow, new therapies are being developed that allow treatment customization, with a focus on weight management and preventing diabetes complications (Mazzola 2012; DeFronzo 2014).
Stem Cell Therapy
Stem cell therapy is aimed at replacing damaged or destroyed insulin-secreting pancreatic beta cells in diabetics with new beta cells developed from human stem cells (Bruin 2015).
Recently, scientists have developed a protocol that can generate pancreatic beta cells. These stem cell-derived beta cells normalized hyperglycemia when transplanted in diabetic mice (Pagliuca 2014). In a separate study, a combination of human stem cell transplantation and antidiabetic drugs was highly effective in improving body weight and glucose metabolism in a mouse model of type 2 diabetes (Bruin 2015).
Glucokinase Activators
Glucokinase is an enzyme that acts as a “glucose sensor,” primarily in the pancreas and liver. It regulates the amount of insulin released in response to glucose in the blood. Small molecules known as glucokinase activators (GKAs) have been developed that enhance the enzymatic activity of glucokinase. Glucokinase activators have been shown to lower glucose levels and stimulate proliferation of pancreatic beta cells in animal models of type 2 diabetes. However, results of recent early-stage clinical trials indicate GKAs lose their efficacy after several months of use. Also, an increased incidence of hypoglycemia and elevated blood fats was observed. Further drug development is needed to address these issues (Nakamura 2015; Rochester 2014).
Smad7 Antisense Oligonucleotides
Emerging technologies are allowing researchers to manipulate specific biochemical pathways more precisely than ever before. One technique that allows selective downregulation of a specific pathway involves antisense oligonucleotides. These small molecules bind to mRNA and prevent it from being translated into a protein in the cell ribosome, thus abrogating the downstream effects of that protein’s signaling (Chan 2006; Visser 2012).
One pathway scientists are interested in targeting in type 1 diabetes is that which results from Smad7 signaling. Smad7 is a protein that interferes with TGF-β signaling inside cells. TGF-β signaling is involved in a broad range of cellular functions and disease processes, including normal pancreatic beta-cell function (Yan 2016; Smart 2006). The interaction of Smad7 and TGF-β signaling is emerging as an important factor in pancreatic beta-cell homeostasis and formation (El-Gohary 2013).
Ongoing research is exploring the potential of antisense oligonucleotides against Smad7 to increase TGF-β signaling and subsequently improve beta-cell function (Monteleone 2013). Interestingly, one Smad7 antisense oligonucleotide, mongersen, was recently shown in a double-blind controlled trial to enhance the 15-day remission rate versus placebo in patients with Crohn’s disease (Monteleone 2015). More research is needed before the role of Smad7 antisense oligonucleotides in the treatment of diabetes is fully understood.
Anti-inflammatory Agents
Chronic, insidious inflammation is a central feature of type 2 diabetes and obesity, and contributes to cardiovascular disease associated with diabetes. Accordingly, anti-inflammatory pharmaceuticals may be therapeutic in these conditions (Esser 2015).
Salsalate has been used for many years to treat pain and inflammation in arthritis, and was recently shown to lower hemoglobin A1C (HbA1C) and markers of inflammation in patients with inadequately controlled type 2 diabetes. Salicylate, the major breakdown product of salsalate, has been shown to activate the metabolic regulator AMPK (adenosine monophosphate-activated protein kinase) in cultured human cells (McPherson 1984; Fleischman 2008; Hardie 2013; Goldfine 2010).
Another anti-inflammatory medication, anakinra (Kineret), blocks the inflammatory cytokine interleukin-1 (IL-1). Anakinra is undergoing clinical trials for safety and effectiveness in diabetes treatment. Neither salsalate nor anakinra are yet FDA approved for diabetes (van Poppel 2014; Esser 2015).
Anti-obesity Medications
Obesity, particularly excess visceral fat, greatly increases risk of developing type 2 diabetes. Two anti-obesity agents have been shown to improve glycemic control in obese individuals with type 2 diabetes (DeFronzo 2014; O’Neil 2012; Garvey 2013; Fonseca 2013).
Lorcaserin (Belviq) is FDA approved for weight loss in obese individuals. It increases satiety and decreases hunger, and is believed to work by influencing serotonin signaling (Gold Standard 2015b). A clinical trial in over 600 patients with type 2 diabetes assessed the effect of lorcaserin on weight loss and metabolic parameters. The study subjects were all undergoing standard treatment with metformin, sulfonylureas, or both; ranged from overweight to morbidly obese; and all received dietary and exercise counseling. Compared with the placebo group, subjects who received lorcaserin were more than twice as likely to lose 5% or more of their body weight, and more than three times as likely to lose 10% or more of their body weight. Fasting glucose and HbA1C both decreased more than twice as much in those receiving lorcaserin than placebo (O’Neil 2012).
Another FDA-approved weight loss medication, a combination of phentermine and topiramate, used in conjunction with lifestyle modification in a late-stage trial, produced significant two-year sustained weight loss in overweight and obese volunteers. Importantly, progression to type 2 diabetes was reduced by up to 76% in nondiabetic subjects treated with phentermine and topiramate (Garvey 2013; Garvey 2012). The combination of phentermine, a psychostimulant, and topiramate, a carbonic anhydrase inhibitor, has been associated with increased heart rate (Alfaris 2015). However, the combination may be safe in people with low-to-moderate cardiovascular risk (Jordan 2014). Phentermine-topiramate should be used cautiously in women of childbearing age because this drug combination may increase risk of oral cleft in children (Alfaris 2015). Other serious side effects of phentermine-topiramate include suicidal behavior and ideation and serious eye problems possibly leading to permanent vision loss (Vivus 2014).
Closed-Loop Artificial Pancreas Systems
A major challenge in the management of type 1 diabetes and late-stage type 2 diabetes is 24-hour glucose and insulin control. Because traditional management strategies necessitate that the patient must be self-reliant for glucose monitoring and insulin delivery, periods when he or she cannot be as vigilant, such as while sleeping, can present problems. Overcoming these barriers and improving the patient experience is the focus of much ongoing research. One solution that appears especially promising is an artificial pancreas and complete closed-loop glucose monitoring and insulin delivery system (Karoff 2016; Heinemann 2016; Russell 2015; Peyser 2014).
With these systems, a glucose monitor and insulin pump are placed under the patient’s skin, and software algorithms closely monitor glucose levels and deliver insulin as necessary to maintain blood sugar near a specified range (Karoff 2016; Peyser 2014; Heinemann 2016; Russell 2015). Some artificial pancreas models also deliver glucagon as needed to help avoid hypoglycemia (Peyser 2014).
Early clinical trials indicate artificial pancreas systems are superior to conventional insulin pump strategies, which require input from the user and therefore are prone to human error (Heinemann 2015; Capel 2014; Haidar 2015; Kovatchev 2014; Oron 2014). Although the FDA has yet to approve a closed-loop artificial pancreas system, research is ongoing and these devices will likely become increasingly available in the coming years as technology improves (FDA 2016).
14 Nutrients
NOTE: Under no circumstances should people suddenly stop taking antidiabetic drugs, especially insulin. Individuals with diabetes should work closely with their healthcare provider before initiating a supplement regimen due to the potential risk of hypoglycemia.
Antiglycation Agents
Advanced glycation end products (AGEs) form when sugars bond with proteins, lipids, and nucleic acids. This process contributes to the toxic effects of high blood sugar (Uribarri 2010; Ceriello 2012). Fortunately, several nutrients can counter these processes.
Benfotiamine. Diabetes and obesity often induce a relative thiamine (vitamin B1) deficiency, which contributes to some of the damaging consequences of hyperglycemia (Beltramo 2008; Page 2011; Via 2012). Benfotiamine is a fat-soluble derivative of thiamine that has much greater bioavailability than other forms of thiamine, and is capable of reaching concentrations in the bloodstream several times that of orally administered thiamine (Greb 1998; Xie 2014). This unique form of vitamin B1 inhibits AGE formation, inflammation, and oxidative stress (Hammes 2003; Du 2008; Balakumar 2010; Shoeb 2012).
A clinical trial in 165 patients with diabetic neuropathy found benfotiamine supplementation for six weeks reduced diabetic neuropathy pain. The benefits were clearer in subjects who consumed 600 mg of benfotiamine daily compared with those who took 300 mg, and in those who took benfotiamine for a longer period of time (Stracke 2008).
In a clinical trial in 13 subjects with type 2 diabetes, participants consumed a high-AGE meal before and after a 3-day course of benfotiamine, 1050 mg per day. The subjects’ vascular and endothelial function were assessed after both high-AGE meals. Signs of vascular dysfunction were completely prevented by benfotiamine administration, and biomarkers of endothelial dysfunction and oxidative stress were significantly reduced (Stirban 2006).
Clinical and animal studies have demonstrated the efficacy of benfotiamine in the treatment of diabetes-related neuropathy, kidney disease, peripheral vascular disease, and retinopathy (Stirban 2006; Chakrabarti 2011; Stracke 1996; Simeonov 1997; Winkler 1999; Haupt 2005; Nikolic 2009).
Carnosine. The peptide carnosine is capable of inhibiting formation of AGEs and even reversing protein glycation (Boldyrev 2013; Seidler 2004). In a study on diabetic mice, carnosine supplementation increased plasma levels of carnosine 20-fold, reduced triglyceride levels by 23%, and increased stability of atherosclerotic lesions (Brown 2014). Carnosine has also been shown to improve the ability of cells to survive in the presence of high glucose concentrations, and improve wound healing in diabetic animals (Ansurudeen 2012). An animal model of diabetes showed carnosine supplementation improved the ability of red blood cells to change their shape as necessitated by mechanical forces encountered during blood flow; this process is impaired in diabetes, contributing to diabetic complications (Yapislar 2012).
Vitamin B6. Vitamin B6—and in particular its active form, pyridoxal 5’-phosphate (PLP)—is involved in several aspects of glucose metabolism and is an effective anti-glycation agent (Mascolo 2020). In addition to preventing protein glycation, PLP is an effective inhibitor of lipid (fat) glycation (Higuchi 2006). Lipid AGEs are elevated in diabetic patients compared with healthy controls, and accumulation of lipid AGEs appears to contribute to vascular diseases related to diabetes (Fishman 2018). High dietary intake of AGEs also contribute to increased cardiovascular disease risk in diabetic patients (Di Pino 2017).
Lower serum levels of PLP have been linked to diabetes incidence and progression, and vitamin B6’s anti-glycation properties may help prevent complications of diabetes (Mascolo 2020). Indeed, higher intake of vitamin B6 has been associated with a lower incidence of diabetic retinopathy among Japanese people with type 2 diabetes (Horikawa 2020).
Interventional studies in humans and animals have been promising as well. For instance, treating 20 type 2 diabetics with 35 mg PLP along with 3 mg activated folate and 2 mg vitamin B12 improved skin sensation in diabetic peripheral neuropathy (Walker 2010). In a randomized controlled trial, 44 obese or overweight women treated with 80 mg of pyridoxine hydrochloride (a form of vitamin B6) for eight weeks experienced improved insulin sensitivity and decreased fat mass compared with placebo (Haidari 2021). Supplementation with PLP significantly decreased high concentrations of glycation-induced toxic compounds in diabetic rats and prevented the progression of diabetic nephropathy (Higuchi 2006; Nakamura 2007). A recent study further identified that diabetic rats treated with PLP experienced a reduction in problems associated with diabetes, including a decline in oxidative stress parameters, reductions in blood glucose levels, and damage recovery in the liver and kidneys (Abdullah 2019).
Phytochemical AMPK Activators
AMPK (adenosine monophosphate-activated protein kinase) is a critical energy sensor in the body. Activation of AMPK helps regulate energy metabolism, increasing fat burning and glucose utilization while blocking fat and cholesterol synthesis (Coughlan 2014; Park, Huh 2014). AMPK activation is a mechanism by which the preeminent antidiabetic drug metformin exerts some of its well-known metabolic benefits (Choi 2013; Yue 2014).
Gynostemma pentaphyllum. Gynostemma pentaphyllum (G. pentaphyllum) is a climbing vine of the family Cucurbitaceae (cucumber or gourd family) that is native to Asian countries including Korea, China, and Japan, where it is used as tea and in traditional medicine. Like metformin, gynostemma extract activates AMPK (Park, Huh 2014).
In animal and human cell cultures, extracts from G. pentaphyllum have been shown to improve insulin sensitivity, reduce levels of glucose and cholesterol, enhance immune function, and inhibit cancer growth (Lu 2008; Yeo 2008; Megalli 2006; Liu, Zhang, 2014). In a randomized controlled trial, an extract from gynostemma modestly reduced body weight and fat mass in obese subjects (Park, Huh 2014). Results from another trial found gynostemma tea improved insulin sensitivity, and lowered fasting glucose nearly ten times more than placebo (Huyen 2013).
In a clinical trial involving 25 diabetics, a gynostemma extract was tested as add-on therapy to the sulfonylurea drug gliclazide. Reductions in plasma glucose and HbA1C were nearly three times greater in the gynostemma extract group compared with placebo. Gynostemma acted by increasing insulin sensitivity rather than stimulating insulin release. It also prevented weight gain and hypoglycemia, which are often associated with sulfonylurea drugs (Huyen 2012).
In a trial in participants with non-alcoholic fatty liver disease, a condition strongly linked to insulin resistance, treatment with gynostemma extract, as an adjunct to diet, resulted in a significant reduction in liver enzymes and insulin levels, a decrease in body mass index, and increased insulin sensitivity (Chou 2006; Utzschneider 2006).
Hesperidin. Hesperidin and related flavonoids are found in a variety of plants, but especially in citrus fruits, particularly their peels (Umeno 2016; Devi 2015). Digestion of hesperidin produces a compound called hesperetin along with other metabolites. These compounds are powerful free radical scavengers and have demonstrated anti-inflammatory, insulin-sensitizing, and lipid-lowering activity (Li 2017; Roohbakhsh 2014). Findings from animal and in vitro research suggest hesperidin’s positive effects on blood glucose and lipid levels may be related in part to activation of the AMP-activated protein kinase (AMPK) pathway (Jia 2015; Rizza 2011; Zhang 2012). Accumulating evidence suggest hesperidin may help prevent and treat a number of chronic diseases associated with aging (Li 2017).
Hesperidin may protect against diabetes and its complications, partly through activation of the AMPK signaling pathway. Coincidentally, metformin, a leading diabetes medication, also activates the AMPK pathway. In a six-week randomized controlled trial on 24 diabetic participants, supplementation with 500 mg of hesperidin per day improved glycemic control, increased total antioxidant capacity, and reduced oxidative stress and DNA injury (Homayouni 2017). Using urinary hesperetin as a marker of dietary hesperidin, another group of researchers found those with the highest level of hesperidin intake had 32% lower risk of developing diabetes over 4.6 years compared to those with the lowest intake level (Sun 2015).
In a randomized controlled trial, 24 adults with metabolic syndrome were treated with 500 mg of hesperidin per day or placebo for three weeks. After a washout period, the trial was repeated with hesperidin and placebo assignments reversed. Hesperidin treatment improved endothelial function, suggesting this may be one important mechanism behind its benefit to the cardiovascular system. Hesperidin supplementation also led to a 33% reduction in median levels of the inflammatory marker high-sensitivity C-reactive protein (hs-CRP), as well as significant decreases in levels of total cholesterol, apolipoprotein B (apoB), and markers of vascular inflammation, relative to placebo (Rizza 2011). In another randomized controlled trial in overweight adults with evidence of pre-existing vascular dysfunction, 450 mg per day of a hesperidin supplement for six weeks resulted in lower blood pressure and a decrease in markers of vascular inflammation (Salden 2016). Another controlled clinical trial included 75 heart attack patients who were randomly assigned to receive 600 mg hesperidin per day or placebo for four weeks. Those taking hesperidin had significant improvements in levels of high-density lipoprotein (HDL) cholesterol and markers of vascular inflammation and fatty acid and glucose metabolism (Haidari 2015).
Green tea extract. Green tea extract, a major constituent of which is epigallocatechin-3-gallate (EGCG), has been shown to reduce glucose and insulin levels and improve insulin sensitivity. In a rodent model of accelerated aging, EGCG supplementation lowered glucose and insulin levels. EGCG also increased insulin sensitivity, decreased liver fat accumulation, and improved markers of mitochondrial function (Liu, Chan 2015). In animal models of diabetes, green tea has been shown to protect against diabetic retinopathy (Silva, Rosales 2013; Kumar 2012).
In a 16-week randomized controlled trial in 92 subjects with type 2 diabetes and blood lipid abnormalities, participants took 500 mg green tea extract three times daily. The green tea group showed significant increases in insulin sensitivity and HDL cholesterol levels, as well as a significant decrease in serum triglycerides (Liu, Huang, 2014). A two-month trial in 103 healthy postmenopausal women found a significant difference in glucose and insulin levels between a group that took up to 800 mg EGCG per day and a placebo group. Glucose and insulin levels fell in the EGCG group, but rose in the placebo group (Wu 2012). At the end of a four-week trial of catechin-rich green tea in 22 postmenopausal women, those in the green tea group had significantly lower postprandial glucose and significantly better after-meal oxidative stress parameters (Takahashi 2014).
Elevated blood pressure is one of the characteristic cardiovascular risk factors often found in diabetics and prediabetics. A randomized controlled trial administered daily a green tea extract powder containing a total of 544 mg polyphenols to 60 prediabetic subjects. This led to significantly reduced HbA1C and diastolic blood pressure (Fukino 2008). Similarly, a trial in overweight or obese middle-aged men found 800 mg EGCG daily significantly reduced diastolic blood pressure (Brown 2009).
Among additional possible mechanisms underlying green tea’s benefits are suppression of inflammatory genes and mitigation of oxidative damage (Uchiyama 2013; Jang 2013; Yang 2013). Also, green tea, black tea (a rich source of theaflavin polyphenols), and oolong tea have been reported to inhibit the alpha-glucosidase enzyme, causing less carbohydrate to be digested and absorbed (Satoh 2015; Yang 2015; Oh 2015). Other evidence suggests green tea constituents may activate AMPK (Liu, Chan 2015).
Bilberry extract. Closely related to blueberry, bilberry is rich in polyphenols and anthocyanins. In a study in diabetic mice, a bilberry extract reduced blood glucose and enhanced insulin sensitivity by activating AMPK (Ogawa 2014; Takikawa 2010). Bilberry polyphenols also have potent anti-inflammatory and free radical-scavenging actions (Subash 2014; Kolehmainen 2012). Bilberry has also been shown in animal studies to combat diabetic retinopathy (Kim, Kim 2015); and a clinical study in 180 type 2 diabetics showed that bilberry, in combination with several other micronutrients, improved measures of ocular health and visual acuity in subjects with preclinical or early diabetic retinopathy (Moshetova 2015).
In a preliminary controlled trial in eight type 2 diabetic males, a concentrated bilberry extract significantly lowered after-meal glucose and insulin levels compared with placebo. Reduced rates of carbohydrate digestion and absorption likely accounted for these effects. Specifically, bilberry polyphenols may have inhibited the action of alpha-glucosidase, preventing the breakdown of carbohydrates into glucose (Hoggard 2013).
Prevention of Exaggerated Post-Meal Blood Glucose Elevations
Several natural compounds can help prevent post-meal surges in blood glucose. These postprandial glucose spikes increase the risk of cardiometabolic diseases not only for diabetics and prediabetics, but also for people whose fasting glucose level is in the conventional “normal” range. Among the mechanisms that natural substances target to allow tighter control of post-meal glucose levels are alpha-glucosidase inhibition, alpha-amylase inhibition, SGLT1 inhibition, and sucrase inhibition (Van de Laar 2005; Matsuo 1992; Melzig 2007; Kinne 2011; Lee 1982).
Alpha-glucosidase inhibition. The alpha-glucosidase enzymes in the intestine break down carbohydrates into simple sugars so they can be absorbed. Inhibiting alpha-glucosidase reduces the amount of simple sugars available for absorption, mitigating postprandial glucose surges (Tundis 2010).
- White mulberry leaf extract. White mulberry leaf has a long history of use in traditional Chinese medicine for preventing and treating diabetes (Mudra 2007). A component of white mulberry, called 1-deoxynojirimycin, impedes the action of alpha-glucosidase, slowing carbohydrate absorption and preventing post-meal blood sugar spikes (Banu 2015; Naowaboot 2012; Nakanishi 2011). This effect of the white mulberry leaf extract has been demonstrated in healthy subjects, type 2 diabetics, and those with impaired glucose tolerance (Asai 2011; Banu 2015; Mudra 2007).
- Specially roasted coffee and green coffee bean extract. Epidemiologic studies have linked coffee consumption with reduced risk of type 2 diabetes, Alzheimer disease, Parkinson disease, and certain cancers. This association may be explained by the chlorogenic acid content of coffee (Song 2014; Ong 2012; Meng, Cao 2013). Chlorogenic acid, a polyphenol, has demonstrated multiple mechanisms through which it exerts antidiabetic activity, including inhibition of alpha-glucosidase and the glucose-elevating liver enzyme glucose-6-phosphatase, oxidative stress modulation, insulin sensitization, and AMPK activation (Bassoli 2008; Ishikawa 2007; Rodriguez de Sotillo 2006; Simsek 2015; Henry-Vitrac 2010; Andrade-Cetto 2010). Chlorogenic acid also lowers levels of blood lipids (Meng, Cao 2013). Chlorogenic acid’s inhibition of alpha-glucosidase allows it to delay glucose absorption, which can result in a more gradual rise in postprandial glucose levels (Johnston 2003).
- Brown seaweed extract. Metabolic syndrome prevalence is lower in some Asian countries than in other parts of the world, and some researchers suspect dietary brown seaweed may be protecting these populations (Teas 2009). In laboratory experiments, brown seaweed extracts from Ascophyllum nodosum and Fucus vesiculosus inhibited alpha-glucosidase and alpha amylase enzymes (Roy 2011).
A 4-week randomized controlled trial in 36 subjects with impaired fasting glucose found white mulberry leaf extract significantly reduced post-meal glucose and insulin levels (Kim, Ok 2015). A trial in 24 subjects with type 2 diabetes compared a white mulberry leaf product to the sulfonylurea drug glyburide. White mulberry decreased total cholesterol by 12%, LDL cholesterol by 23%, and triglycerides by 16%; raised HDL cholesterol by 18%; and reduced fasting blood glucose and oxidative stress markers. Glyburide only slightly improved glycemic control and triglycerides (Andallu 2001). A clinical study in healthy volunteers found that 1-deoxynojirimycin-enriched white mulberry powder suppressed post-prandial blood glucose surge and lowered insulin levels (Kimura 2007).
In a clinical trial in 42 individuals with type 2 diabetes, 300 mg of a chlorogenic acid-containing plant extract daily for four weeks significantly reduced fasting plasma glucose, C-reactive protein (CRP), and liver enzymes compared with placebo (Abidov 2006). In another trial, a single dose of coffee polyphenols during a glucose-loading test in healthy individuals significantly protected endothelial function (Ochiai 2014). In a mouse study, green coffee bean extract, a rich source of chlorogenic acid, significantly reduced visceral fat accumulation and improved insulin sensitivity, effects that may have been due to suppression of genes associated with fat deposition and inflammation (Song 2014).
Raw green coffee beans are rich in chlorogenic acid (Farah 2008). However, the conventional coffee roasting process appears to significantly reduce the chlorogenic acid content of brewed coffee (Moon 2009; Zapp 2013). Although several studies have shown conventional coffee has meaningful health benefits, consuming a coffee high in chlorogenic acid may extend these benefits further (Johnston 2003; Hemmerle 1997). Fortunately, scientists have developed a method for roasting coffee that yields brewed coffee with higher-than-typical chlorogenic acid content (Zapp 2013). Individuals who wish to attain the most benefit from coffee polyphenols should consume coffee specially prepared to ensure that chlorogenic acid is retained during the roasting process.
In a randomized controlled trial in 23 healthy subjects, a single 500-mg dose of brown seaweed extract caused a 48.3% decrease in post-meal blood sugar spikes. Significant reductions in post-meal insulin concentrations and improved insulin sensitivity were also observed (Paradis 2011).
In a study in diabetic mice, polyphenolic fractions prepared from brown seaweed extract were shown to improve fasting serum glucose levels and blunt the rise in blood glucose following an oral sugar challenge. Compared with untreated mice, the mice given the polyphenol extract exhibited decreased total blood cholesterol. The seaweed-derived polyphenols also restored liver glycogen (stored carbohydrate) content (Zhang 2007).
Alpha-amylase inhibition. Like alpha-glucosidase, alpha-amylase is an enzyme that breaks down larger sugars and starches into smaller molecules that can be rapidly absorbed. Inhibition of alpha-amylase is another way to reduce the rate of sugar absorption (Tundis 2010).
- Sorghum extract. Grain sorghum (Sorghum bicolor) is cultivated for animal and human consumption in several parts of the world, especially Africa, Asia, and Latin America. The grain’s unique protein and starch composition reduce its digestibility and cause it to slow glucose absorption (Poquette 2014). Also, in animal models of diabetes, sorghum inhibited glucose production in the liver (gluconeogenesis) and improved insulin sensitivity. In a laboratory experiment, a flavonoid- and proanthocyanidin-rich sorghum extract inhibited the alpha-amylase enzymes that convert starch into sugars. In a randomized trial in 10 healthy men, muffins made with sorghum were shown to reduce average after-meal glucose and insulin responses (Hargrove 2011; Poquette 2014; Kim 2012; Park 2012).
Additional mechanisms. Some natural products suppress postprandial hyperglycemia via other mechanisms, including inhibition of glucose transporters or sucrase, an enzyme that facilitates digestion and absorption of sucrose (table sugar).
- Phloridzin. Phloridzin is a unique polyphenol found in high concentrations in apples and apple trees. This compound appears to suppress glucose absorption in the intestine by inhibiting sugar transporter systems in the intestine (SGLT1) and kidney (SGLT2). As a result, glucose reabsorption in the kidney is reduced and glucose excretion into the urine is promoted. The oral diabetes medication canagliflozin (Invokana) is also based on this mechanism (Sarnoski-Brocavich 2013; Najafian 2012; Masumoto 2009).
- L-arabinose. L-arabinose is a poorly-absorbed five-carbon sugar found in the cell walls of many plants. L-arabinose inhibits the activity of sucrase, which is an intestinal enzyme that breaks down sucrose (table sugar) into the absorbable sugars glucose and fructose. When l-arabinose is consumed in combination with sucrose, the breakdown of sucrose is delayed, so that glucose is absorbed more slowly, which results in less exaggerated blood glucose and insulin responses. L-arabinose in combination with chromium, a natural insulin sensitizer, significantly lowered circulating glucose and insulin levels in nondiabetic subjects who underwent an oral sucrose challenge (Karley 2005; Kaats 2011; Krog-Mikkelsen 2011).
- Maqui berry extract. Maqui berries (Aristotelia chilensis) are a purple-black fruit native to Chile that have garnered attention for their strong capacity to quench free radicals. They are also the richest known source of highly active polyphenolic compounds called delphinidins. When compared with other berries such as cranberries, blueberries, raspberries, and blackberries, maqui berries were found to be at least three times higher in total polyphenols and to have approximately three times greater free radical-quenching capacity (Watson 2015).
- Clove bud extract. Clove (Syzygium aromaticum), a well-known spice, has shown potential to improve glucose levels and aid in overall metabolism (Tu 2014; Kuroda 2012). An ethanol extract of clove flower buds was found to decrease blood glucose levels in diabetic mice (Kuroda 2012); in diabetic and lipid-disordered mice, supplementation with an alcohol clove extract resulted in lower glucose, triglyceride, free fatty acid, HbA1C levels (Sanae 2014). Its positive effects on metabolism were further demonstrated in an animal model of obesity, in which treatment with an alcohol extract of clove reduced the negative impact of a high-fat diet on body weight as well as lipid, glucose, and insulin levels (Jung 2012).
In a 2014 study to investigate its effects on blood glucose control, a single 200 mg dose of a standardized maqui berry extract 30 minutes prior to ingesting a serving of white rice was found to delay and decrease the rising levels of blood glucose and insulin better than placebo in 10 volunteers with moderate glucose intolerance (Hidalgo 2014). Similarly, single doses of 60 mg, 120 mg, and 180 mg taken one hour before oral glucose tolerance testing effectively improved fasting blood glucose levels as well as glucose and insulin responses in pre-diabetic individuals, with 180 mg having the greatest impact on blood glucose levels (Alvarado, Leschot 2016). To assess its long-term effects, a group of 31 moderately glucose-intolerant subjects were treated with 180 mg standardized maqui berry extract daily for three months. Results showed progressive decreases in HbA1c values at 30, 60, and 90 days. In addition, LDL-cholesterol levels were lower and HDL-cholesterol levels were higher at the end of the trial, indicating improved lipid metabolism (Alvarado, Schoenlau 2016).
Several mechanisms may contribute to the positive effects of maqui berries on glucose regulation. Findings from preclinical research suggest maqui berry extract may decrease glucose production in the liver and increase insulin sensitivity (Rojo 2012). In an animal model of diabetes, maqui berry extract reduced intestinal absorption of glucose (Hidalgo 2014). Other research has indicated that maqui berry extract inhibits the inflammatory signaling between fat cells and immune cells that is associated with the development of insulin resistance (Reyes-Farias 2015).
Findings from a clinical study on human subjects, which are not yet published, add more support for the potential anti-diabetic effects of clove. After 30 days of supplementation with 250 mg per day of a water extract of clove, participants had lower after-meal blood glucose levels. Furthermore, participants with higher blood glucose levels before the trial experienced greater reductions in after-meal glucose levels (AKAY 2017).
In vitro research has shown that a clove extract has insulin-like effects on liver cells, inhibiting the breakdown of stored carbohydrates into glucose and preventing a subsequent rise in levels of circulating glucose (Prasad 2005; Sanae 2014). In muscle cells, clove extract stimulated the conversion of glucose into energy and enhanced mitochondrial function (Tu 2014). Some individual compounds from an ethanol extract of clove have been found to activate cellular pathways that increase glucose and lipid uptake (Kuroda 2012).
Insulin Sensitizers
Chromium. Chromium, a trace mineral, is essential for carbohydrate and fat metabolism, and is believed to act as an insulin-sensitizing agent. Chromium deficiency has been associated with insulin resistance and diabetes (Suksomboon 2014; Anderson 1997). A 2014 study found chromium deficiency was common in people with prediabetes. The authors recommended screening for chromium deficiency in both prediabetics and diabetics, and supplementing if a deficiency was identified (Rafiei 2014).
Evidence suggests chromium supplementation may improve control of blood glucose, raise HDL cholesterol, and lower triglycerides in type 2 diabetes. Chromium has also been shown to significantly lower HbA1C in type 2 diabetics (Suksomboon 2014; Rabinovitz 2004).
Cinnamon. The culinary spice cinnamon has undergone extensive clinical study for its effects on glucose and other metabolic parameters in type 2 diabetes and prediabetes. It has been shown to exert beneficial effects both alone and as an adjunct to standard medical treatment (Allen 2013; Deyno 2019; Kutbi 2022; Lira Neto 2022).
Numerous systematic reviews and meta-analyses have examined evidence from randomized controlled trials, and variously concluded that cinnamon treatment lowers fasting blood sugar, HbA1C, insulin resistance, and triglycerides, while raising HDL cholesterol. In the studies examined in these analyses, cinnamon was used at dosages ranging from 120 mg/day to 14.4 grams per day, in interventions lasting from four to 18 weeks (Allen 2013; Deyno 2019; Moridpour 2024; Zhou 2022).
A randomized placebo-controlled clinical trial tested cinnamon’s efficacy for reducing blood sugar levels in individuals with type 2 diabetes. This trial included 140 participants (68 men and 72 women) with an average age of about 61 and whose HbA1C levels were 6.0% or greater. Study participants received 3 grams daily of cinnamon in capsule form or a matching placebo, in addition to their usual medication, for 90 days. In the cinnamon group, HbA1C was reduced by 0.2% on average, while fasting glucose decreased by 10 mg/dL, compared to the placebo group. These changes were statistically significant (Lira Neto 2022).
A small, randomized, controlled, crossover trial involved 18 participants, average age 51 years, with obesity and prediabetes who underwent a 10-week intervention with cinnamon (4 grams per day) or placebo. The intervention lasted four weeks, followed by a two-week washout period, followed by four weeks in the other group. One important innovation in this trial was that glucose was measured with continuous glucose monitoring rather than single “spot” tests. Twenty-four hour glucose levels were significantly lower during the cinnamon consumption phases, compared to placebo, as were glucose peaks. Additionally, oral glucose tolerance testing revealed decreased triglyceride levels in the cinnamon groups. There was no increase in adverse digestive symptoms in the cinnamon groups, compared with placebo (Zelicha 2024).
Although the specific compounds in cinnamon chiefly responsible for its glucose-lowering and insulin-sensitizing effects remain under investigation, cinnamaldehyde is of particular interest, as are polyphenols and other compounds including proanthocyanidins, flavones, catechins, coumarin, eugenol, beta-caryophyllene, and trans-cinnamic acid. Anti-inflammatory, alleviation of diabetes-induced oxidative stress, and increased hepatic glycogen synthesis mechanisms have also received attention (Zhou 2022; Zelicha 2024).
Omega-3 fatty acids. Omega-3s are essential fatty acids that are incorporated into cell membranes where they can be converted to signaling compounds known as eicosanoids. Omega-3-derived eicosanoids play crucial roles throughout the body. In general, they counter inflammatory signaling and are particularly important to the cardiovascular, nervous, pulmonary, and immune systems (National Institutes of Health Office of Dietary Supplements 2024).
A preclinical study found that omega-3 fatty acids could enhance cells’ ability to remove glucose from the bloodstream, in part by promoting the function of the insulin-regulated glucose transporter GLUT4 (Zhang 2022). Randomized controlled trials have demonstrated that increased omega-3 intake may reduce fasting glucose levels and mitigate insulin resistance (Delpino 2022). Greater omega-3 intake has also been associated with lower risk of cardiovascular complications of type 2 diabetes. These benefits are attributable in part to mechanisms of omega-3 fatty acids, such as improving lipid profiles and counteracting inflammation (Delpino 2022; O’Mahoney 2018; Mason 2020).
A meta-analysis of 30 randomized controlled trials including a total of 1,821 participants found that 20 studies produced at least one significant effect of omega-3 supplementation in relation to type 2 diabetes. The combined analysis showed that, compared with placebo, omega-3 supplementation significantly reduced fasting blood glucose and a measurement of insulin resistance called HOMA-IR; however, there was no significant effect on hemoglobin A1c.The dosages of omega-3 fatty acids used in the included studies generally ranged from 1 to 6 grams daily (Delpino 2022).
In the well-known randomized controlled VITamin D and OmegA-3 (VITAL) trial, a total of 25,835 people supplemented with either one gram of omega-3s (containing 460 mg eicosapentaenoic acid [EPA] and 380 mg docosahexaenoic acid [DHA]), 50 mcg (2,000 IU) vitamin D, both, or a placebo daily for six years. An ancillary analysis of the data from VITAL looked at the effect of omega-3 supplementation on heart failure hospitalization. In type 2 diabetics, the risk of first heart failure hospitalization was 31% lower in the omega-3-supplemented group compared with the placebo group. Among those without diabetes, there was no significant difference in the risk of first heart failure hospitalization between the omega-3 and placebo groups (Djousse 2022).
A meta-analysis evaluated eight randomized controlled trials that assessed cardiovascular outcomes in patients with diabetes who had supplemented with omega-3 fatty acids. These trials enrolled a total of 57,754 people. The combination of EPA and DHA was used in five trials at a dose of 1 gram daily and in one trial at 4 grams daily. Two clinical trials evaluated EPA only at doses of 1.8 and 4 grams (the 4-gram trial used icosapent ethyl, a specific form of EPA used in the FDA-approved drug Vascepa). The median follow-up in these trials ranged from 3.5–6.1 years. The meta-analysis of these studies found that omega-3 supplementation reduced the risk of cardiovascular disease in patients with diabetes by 7% (Huang 2023). An observational study, published after the meta-analysis was completed, found similarly beneficial associations between fish oil supplementation and higher omega-3 levels and better outcomes in people with diabetes. This study examined a dataset of 20,338 participants, followed for an average of 13.2 years, and found that fish oil supplementation was associated with a 10% reduction in risk of macrovascular outcomes and an 11% risk reduction in microvascular outcomes. This study also found that higher plasma omega-3 levels, DHA in particular, were associated with lower risk of macro- and microvascular outcomes. The researchers remarked that “… the favorable associations were partially mediated through improving biomarkers of lipid profile and inflammation” (Tian 2025).
Apolipoprotein-B (apoB) is a protein present in atherogenic lipoproteins and is an indicator of the total number of atherogenic particles in circulation (Lamantia 2024). Elevated levels of apoB-containing lipoproteins are predictive of type 2 diabetes risk. In a 12-week study, 33 non-diabetic adults were stratified around the median plasma apoB into a high-apoB (123 mg/dL) group (n = 17) and a healthy-apoB (85 mg/dL) group (n = 16). Participants in both groups received 2.7 grams of omega-3 fatty acid ethyl esters daily (1,800 mg EPA and 900 mg DHA). At baseline, subcutaneous white-adipose-tissue samples from the high-apoB group secreted markedly more interleukin-1beta (IL-1beta) in response to LDL and other stimuli than tissue from the healthy-apoB group. After supplementation this between-group difference disappeared: post-intervention LDL no longer triggered—and sometimes inhibited—IL-1beta release, even though plasma apoB itself was unchanged. Upon undertaking further investigations to determine the factors mediating these changes, the investigators found that baseline white adipose tissue IL-1beta secretion accounted for much of the association between apoB and several diabetes-related traits, and these links were lost after omega-3 supplementation. Collectively, the data indicate that supplementation with EPA plus DHA blunts the pro-inflammatory NLRP3/IL-1beta response of human adipose tissue, providing a potential mechanism by which omega-3 fatty acid supplementation could mitigate diabetes risk in individuals with elevated apoB (Lamantia 2024; Bissonnette 2023).
Magnesium. Magnesium is involved in more than 600 metabolic reactions and plays a key role in carbohydrate metabolism (de Baaij 2015). Magnesium participates in insulin secretion and function, and low magnesium levels are correlated with insulin resistance (Gums 2004; Bertinato 2015; Paolisso 1990). Low magnesium levels are significantly more common in people with diabetes and impaired glucose tolerance compared with the general population, and higher magnesium levels correlate with lower HbA1C (Hata 2013; Hruby 2014; Hyassat 2014; Galli-Tsinopoulou 2014; Azad 2014). Higher magnesium intake is associated with decreased risk of developing type 2 diabetes (Guerrero-Romero 2014).
Magnesium supplementation has been shown to lower blood levels of glucose and lipids, as well as blood pressure, in type 2 diabetics. Magnesium supplements were also found to lower highly-sensitive C-reactive protein (hs-CRP), a marker of inflammation, in prediabetics with low serum magnesium (Solati 2014; Simental-Mendia 2014).
Magnesium plays a critical role in cardiovascular health. A study in over 13 000 US adults found women with the highest serum magnesium levels had a 56% lower risk of coronary artery disease compared with those with the lowest levels; in men, those with the highest serum magnesium had a 27% less risk compared with those whose levels were lowest. Similarly, women with the lowest dietary magnesium intake had a more than 1.3-fold greater risk of cardiovascular disease (Kolte 2014). One study found that, in diabetic patients with coronary artery or peripheral vascular disease, there was a significant correlation between low magnesium and high fasting plasma glucose and HbA1C (Agrawal 2011).
Dehydroepiandrosterone (DHEA). DHEA is the most abundant adrenal steroid hormone in healthy adults, and a precursor to the sex hormones (androgens and estrogens) (Mayo Clinic 2019; Brahimaj 2017). DHEA levels decline with age. However, youthful DHEA levels, as well as DHEA replacement therapy, have been associated with benefits for cardiovascular health, bone strength, mood, and cognitive function (Samaras 2013; Weiss 2012; Kawano 2003; Jankowski 2006; Weiss 2009; Alhaj 2006).
DHEA has also received attention for its potential to modulate insulin sensitivity. A two-year randomized controlled trial administered 50 mg of DHEA, or placebo, to 125 men and women aged 65 to 75. In those on DHEA, insulin resistance decreased significantly, by 22%, compared with placebo. This effect was accounted for largely by the response of those with abnormal glucose tolerance (Weiss 2011). In another randomized controlled trial of 56 men and women aged 65 to 78 with age-related decreases in DHEA levels, 50 mg DHEA daily for six months resulted in improved insulin sensitivity and decreased body fat (Villareal 2004).
An observational study in which serum DHEA and DHEA-sulfate (DHEA-s) levels were assessed followed over 5,000 Northern European men and women for close to 11 years. Both DHEA and DHEA-s levels were significantly correlated with lower risk of type II diabetes (Brahimaj 2017). Mechanistic studies have found that DHEA modulates insulin signaling pathways, increasing the insulin sensitivity of liver, muscle, and fat cells, which may explain its beneficial metabolic effects (Aoki 2018; Karbowska 2013).
For additional information, please review our DHEA Restoration Therapy protocol.
Oxidative Stress Inhibitors and Anti-Inflammatory Agents
Coenzyme Q10. Coenzyme Q10 (CoQ10) is essential to mitochondrial energy metabolism, and a powerful inhibitor of oxidative stress (Littarru 2007). CoQ10 deficiency has been associated with diabetes (Amin 2014; Kolahdouz 2013; Eriksson 1999). In a randomized controlled trial in 64 type 2 diabetic patients, supplementation with 200 mg CoQ10 per day for 12 weeks decreased serum HbA1C concentration and lowered levels of total and LDL cholesterol (Kolahdouz 2013). A clinical trial in 74 type 2 diabetic subjects found 100 mg CoQ10 twice daily resulted in significantly decreased HbA1C and blood pressure (Hodgson 2002). In a placebo-controlled trial in 23 statin-treated type 2 diabetics, 200 mg CoQ10 per day significantly improved a marker of vascular endothelial dysfunction (Hamilton 2009; Watts 2002).
In an animal model of diabetes, CoQ10 treatment significantly improved insulin resistance, reduced serum levels of insulin and glucose, and increased levels of the energy-regulating hormone adiponectin six-fold (Amin 2014). High levels of adiponectin have been linked to decreased risk of diabetes and cardiovascular complications (Lindberg 2015; Zoico 2004; Yamamoto 2014).
Long-term use of CoQ10 was demonstrated in two animal studies to be protective against progressive diabetic neuropathy. The beneficial effects of CoQ10 may have been attributable to reduction of oxidative damage and inflammation, both key factors implicated in diabetic neuropathy (Zhang, Eber 2013; Shi 2013).
The reduced form of CoQ10—ubiquinol— is absorbed more efficiently than the ubiquinone form (Langsjoen 2008; Hosoe 2007).
Curcumin. Curcumin is a major active component of turmeric, the spice derived from the plant Curcuma longa. Turmeric has been used as a treatment for diabetes in Ayurvedic and traditional Chinese medicine for centuries. Curcumin’s primary mechanisms of action are its ability to neutralize reactive free radicals and reduce inflammation (Nabavi 2015; Zhang, Fu 2013; Meng, Li 2013).
A randomized controlled trial in 240 prediabetic subjects showed curcumin supplementation significantly lowered risk of progressing from prediabetes to type 2 diabetes. During the nine-month trial, none of the prediabetic subjects treated with curcumin progressed to diabetes, whereas over 16% of subjects in the control group were diagnosed with type 2 diabetes. By the end of the study, subjects in the curcumin group had significantly greater insulin sensitivity and beta-cell function, as well as higher adiponectin levels than the placebo group (Chuengsamarn 2012).
Additional experimental studies and human trials indicate curcumin is a promising natural agent for the prevention and treatment of diabetes and its complications. Curcumin appears to increase insulin sensitivity and reduce blood levels of glucose and lipids. It also may protect insulin-producing beta cells in the pancreas (Nabavi 2015; Zhang, Fu 2013).
Resveratrol. Resveratrol, a polyphenol that has received widespread attention for its anti-aging effects, holds promise in type 2 diabetes (Hausenblas 2015; Bruckbauer 2013; Fiori 2013; Tome’-Carneiro 2013; Mozafari 2015). A rigorous review of randomized controlled trials found resveratrol improved systolic blood pressure, HbA1C, and creatinine when used as an adjunct to drug treatment in type 2 diabetes (Hausenblas 2015). In one of these studies, supplementation with resveratrol at 1 g per day for 45 days resulted in a significant decrease in fasting glucose, insulin, and HbA1C and an increase in insulin sensitivity and HDL cholesterol levels. Notably, the improvements in HbA1C and HDL cholesterol were comparable to those achieved by leading antidiabetic drugs (Movahed 2013).
Lipoic acid. Lipoic acid is a free radical scavenger made by the body in small quantities, though levels decline significantly with age (Park, Karuna 2014; Higdon 2012). Lipoic acid may support healthy blood glucose control by activating AMPK, protecting pancreatic beta cells, and augmenting glucose removal from the bloodstream. Lipoic acid has been used for the prevention and treatment of diabetic neuropathy in Germany for several decades (Ziegler 1999; Gomes 2014; Golbidi 2011; Ibrahimpasic 2013).
In a study in subjects with impaired glucose tolerance, arterial flow (a measure of endothelial function) was markedly decreased during fasting and after a glucose challenge. Intravenous administration of 300 mg lipoic acid before the glucose challenge prevented the endothelial dysfunction induced by high blood glucose. Lipoic acid decreases oxygen free radicals, which in excess promote endothelial dysfunction and contribute to diabetes, high blood pressure, and cardiovascular disease (Xiang 2008; Park, Karuna 2014; Gomes 2014).
Lipoic acid comes in two “mirror image” forms labeled “R” and “S.” The R form is the active form produced and used in living systems (Gomes 2014). Inexpensive chemical manufacturing produces equal quantities of R and S lipoic acid, often labeled “R/S lipoic acid” or simply “alpha-lipoic acid” (Flora 2009). Newer precision techniques allow production of a pure, more stable R-lipoic acid supplement, delivering the most bioavailable form. This form is known as sodium R-lipoate, or Na-RALA.
A dose of pure R-lipoic acid provides twice the active ingredient compared with typical alpha-lipoic acid supplements, simply because the whole dose consists of the active “R” molecule. Look for the “R” label to ensure you are getting the most potent form of lipoic acid (Smith 2005; Streeper 1997).
Blueberry extract. Blueberries are a concentrated source of polyphenols and anthocyanins that have multiple antidiabetic effects, including protection of pancreatic beta cells, anti-inflammatory properties, and free-radical-scavenging abilities (Liu, Gao 2015; Martineau 2006; Abidov 2006).
A four-week randomized controlled trial of blueberry supplementation was conducted in 32 obese, insulin-resistant adults without diabetes. Subjects were given 45 g of freeze-dried blueberry powder—the equivalent of two cups of whole blueberries—daily for six weeks. Compared with placebo, blueberry treatment significantly improved insulin sensitivity (Stull 2010).
In a study in rodents fed a high-fructose diet, fasting insulin was elevated, and insulin sensitivity and pancreatic beta-cell function declined. However, when the diet was supplemented with blueberries, these changes were minimized; and when a larger percentage of the diet was derived from blueberries, the effect was greater. Cholesterol and abdominal fat also decreased in the blueberry-fed animals (Khanal 2012).
In a rodent model of diet-induced inflammation similar to that observed in diabetes, the addition of blueberry powder to the animals’ diet prevented inflammatory changes, and protected the mice from developing insulin resistance and high blood sugar (DeFuria 2009). Soybean flour enriched with a concentrate of blueberry polyphenols was shown to reduce hyperglycemia, weight gain, and serum cholesterol in mice. The blueberry-fortified flour also reduced glucose production in the liver by 24‒74% (Roopchand 2013).
In cell culture studies, an anthocyanin-rich blueberry extract exhibited insulin-sensitizing properties, conferred protection against glucose and fatty acid toxicity, and enhanced proliferation of insulin-producing pancreatic beta cells (Martineau 2006; Liu, Gao 2015).
Grape polyphenols. Grapes are a source of polyphenols, proanthocyanidins, and resveratrol, all of which modulate oxidative stress and have been studied in a wide range of health conditions, including high blood pressure, cancer, and Alzheimer disease (Pasinetti 2014; Kaur 2009; Feringa 2011). Grape polyphenols appear to have important antidiabetic effects and protect tissues from the damaging effects of blood sugar elevations.
In a four-week, randomized, placebo-controlled trial in 32 type 2 diabetics, consumption of 600 mg per day of grape seed extract significantly lowered fructosamine compared with placebo. Fructosamine is a test similar to HbA1C that measures blood sugar level over several weeks. Grape seed extract also lowered total cholesterol and hs-CRP, and significantly elevated blood glutathione, one of the body’s primary internal antioxidants (Kar 2009). Another trial found the non-alcoholic portion of red wine, which is rich in grape polyphenols, increased insulin sensitivity and reduced cardiovascular risk (Chiva-Blanch 2013).
A randomized controlled trial administered 2 g of grape polyphenols per day to healthy but overweight first-degree relatives of type 2 diabetics for eight weeks. Subjects were then challenged with substantial doses of fructose, the sugar present in fruit juices and many sweetened beverages. In the placebo group, the fructose challenge resulted in increased oxidative stress and decreased insulin sensitivity and mitochondrial activity. All of these negative effects were prevented in the polyphenol group (Hokayem 2013).
Gamma tocopherol. After-meal blood sugar surges can injure the lining of blood vessels, causing endothelial dysfunction and vascular disease. Gamma tocopherol is a form of vitamin E with both anti-inflammatory and free-radical-scavenging activity. Two trials in healthy men found gamma tocopherol supplementation protected against changes associated with endothelial dysfunction induced by after-meal glucose spikes (Mah, Noh 2013; Masterjohn 2012; Mah, Pei 2013).
Ginkgo biloba. A randomized controlled trial tested the effects of adding Ginkgo biloba to metformin therapy in patients with metabolic syndrome. Forty participants received—in addition to their ongoing metformin treatment—either a Ginkgo biloba extract, administered in a single daily dose as a 120 mg capsule, or a placebo, for 90 days. At completion, participants who received the extract had significant decreases in HbA1c (8%), fasting serum glucose (12%), insulin levels (44%), insulin resistance (19%), body mass index (7%), abdominal body fat (23%), and serum leptin (43%) compared with their levels at the beginning of the study. Several inflammatory markers also showed decreases, including high-sensitivity C-reactive protein (20%), TNF-alpha (48%), and IL-6 (37%). These benefits were not seen in the placebo group. The combination of metformin and Ginkgo biloba extract did not lead to any observable adverse effects during the course of study (Aziz, Hussain, Mahwi, Ahmed 2018).
Another randomized controlled trial showed similar benefits. In the trial, patients with type 2 diabetes, which was poorly controlled with metformin, received Ginkgo biloba extract supplementation. After 90 days, participants who received the extract had decreased blood levels of HbA1c (10.5%), fasting serum glucose (20%), and insulin (28%); and reduced BMI (7%), waist circumference (3%), and abdominal fat (17%) (Aziz, Hussain, Mahwi, Ahmed, Rahman 2018). Previously, a double-blind clinical trial reported that the co-ingestion of 120 mg of a Ginkgo biloba extract together with 500 mg metformin did not significantly change the metabolism of metformin in healthy individuals or in individuals with type 2 diabetes mellitus (Kudolo 2006).
Additional Support
Vitamin D. Vitamin D deficiency has been associated with both diabetes and obesity, and vitamin D status has been shown to be related to glucose metabolism (Lips 2017; Vondra 2021). Higher levels of serum vitamin D were shown to reduce diabetes risk in a diverse population (Chatterjee 2023).
Multiple mechanisms appear to play a role in this relationship. Vitamin D regulates pancreatic beta cell function both directly as well as indirectly by modulating extracellular calcium and the flow of calcium through beta cells. Vitamin D also influences insulin sensitivity in target tissues, including through its effect on the secretion of the hormones leptin and adiponectin. Many of the effects of insulin and glucose are mediated through adipocytes and adipose tissue, and the vitamin D receptor is abundantly expressed in adipocytes and is involved in their regulation (Clemente-Postigo 2015; Mohd 2022). All of these effects could enhance the clearance of glucose from the bloodstream.
Chronic systemic inflammation is a feature of insulin resistance and diabetes, possibly as an effect but with some suggestion that it may actually be causative (Vondra 2021; Mohd 2022; Wu 2020). Immune cells responsible for inflammation express the vitamin D receptor, and vitamin D successfully suppresses chronic inflammation in multiple settings, while vitamin D deficiency is associated with a pro-inflammatory state (Mohd 2022; Ao 2021).
Other potential benefits of vitamin D on glucose metabolism may stem from its positive effects on mitochondrial function, oxidative stress, and epigenetic alterations in beta cells and other insulin-sensitive peripheral tissues (Vondra 2021). Life Extension has long suggested that an optimal target range for 25-hydroxyvitamin D blood levels is between 50 and 80 ng/mL.
A meta-analysis published in 2023 included individual participant-level data from three randomized controlled trials that evaluated the effect of at least two years of vitamin D supplementation on risk of new-onset diabetes among people with prediabetes (Pittas 2023). In total, data from 4,190 participants were included in the analysis.
The form of supplemental vitamin D varied across the three trials. Two trials used cholecalciferol (vitamin D3), and one trial used a synthetic analog of active vitamin D called eldecalcitol, which is a prescription drug for osteoporosis in Japan. One of the vitamin D3 trials used 20,000 IU (500 mcg) weekly, while the other used 4,000 IU (100 mcg) daily. In the whole study population, the mean serum 25-hydroxyvitamin D level was 25 ng/mL at baseline—a level above the threshold for vitamin D deficiency.
After a median follow-up of three years, participants randomized to supplemental vitamin D had a 3.3% absolute reduction in risk of new onset diabetes compared with those who took a placebo. This corresponded with a number needed to treat (NNT) of 30, which means that to prevent one case of new onset diabetes, only 30 people needed to supplement with vitamin D for three years. Moreover, among participants who maintained serum 25-hydroxyvitamin D levels above 50 ng/mL during the follow-up period, the absolute reduction in risk of new onset diabetes was 18.1% compared with those who maintained levels between 20 and 29 ng/mL. Also, participants who took vitamin D were 30% more likely to return to normal glucose regulation during the study period than those who took a placebo.
Importantly, in the two trials that used supplemental vitamin D3, the risk reduction was apparent among participants with a BMI below the median of 31.3 kg/m2, but not among those whose BMI was 31.3 or higher. This suggests that individuals with a higher BMI may need more supplemental vitamin D than leaner individuals and is consistent with previous evidence indicating that obesity may impair formation of active vitamin D.
Folate. In type 2 diabetes, elevated plasma homocysteine is strongly linked to increased risk of cardiovascular disease and death. Homocysteine promotes endothelial dysfunction through a number of different mechanisms. The B vitamin folate, in concert with vitamin B12, lowers homocysteine by converting it back to the amino acid methionine. Both low folate intake and low blood folate levels are strongly associated with high plasma homocysteine (Selhub 2000; Moat 2004; Sudchada 2012; Van Guelpen 2009; Miller 2003; de Bree 2001; Koehler 2001).
A thorough review and analysis of randomized controlled trials assessed the effect of folic acid supplementation on homocysteine levels in type 2 diabetics. In this population, 5 mg per day of folic acid significantly decreased homocysteine levels, to a degree believed to lower the risk of cardiovascular disease, and improved glycemic control (Sudchada 2012).
Genetic predisposition to inefficient conversion of folic acid into the metabolically active 5-methyltetrahydrofolate (5-MTHF) is common (Huo 2015). Supplementation with L-methylfolate (instead of folic acid) avoids this potential problem and is preferable to folic acid.
Irvingia gabonensis. Also known as African mango, Irvingia gabonensis is an African tree that bears mango-like fruit (MMSCC 2015). An extract of irvingia seed has been shown to lower blood glucose and lipid levels, and reduce excess body weight (Ross 2011; Ngondi 2009; Ngondi 2005). In a randomized controlled trial, 150 mg of a proprietary extract from Irvingia gabonensis, taken twice daily for 10 weeks, significantly decreased body weight, body fat, and waist circumference in overweight subjects. There were also improvements in several metabolic parameters related to insulin resistance, including increased adiponectin and decreased leptin and CRP (Ngondi 2009).
In another trial, a combination of extracts from irvingia and Cissus quadrangularis, a West African vine, produced significantly larger reductions in body weight and fat, total cholesterol, LDL cholesterol, and fasting blood glucose compared with the Cissus extract alone (Oben, Ngondi, Momo 2008).
Nicotinamide riboside. Nicotinamide adenine dinucleotide (NAD+) is a critical regulator of cellular energy (Kim, Oh 2015). It is also a cofactor for sirtuin proteins, which are involved in many metabolic activities and associated with longevity. Aging is associated with declining activity of SIRT1, the gene that encodes the sirtuin 1 protein, and preclinical studies have shown increasing SIRT1 expression prolongs lifespan (Poulose 2015). Age-related decline of NAD+ levels has been associated with a reduction in SIRT1 activity (Braidy 2011; Gomes 2013). NAD+ metabolism is also implicated in the causation and complications of diabetes (Yoshino 2011; Canto 2015; Imai 2009).
Supplementation with nicotinamide riboside, an NAD+ precursor, boosts cellular NAD+ levels (Bogan 2008; Khan 2014). Animal research has shown nicotinamide riboside can improve insulin sensitivity, augment the benefits of exercise, combat neurodegeneration, and mitigate the negative effects of a high-fat diet (Chi 2013; Canto 2012). In an animal model of type 2 diabetes, nicotinamide riboside supplementation reduced liver inflammation and improved glucose control (Lee, Hong 2015).
Taurine. Taurine is an amino sulphonic acid found throughout the body; it is especially abundant in the brain, heart, and skeletal muscle. Taurine participates in a variety of important physiological functions, such as promoting cell membrane stability and facilitating healthy nervous system function (Wen 2019). Supplemental taurine is commonly used in sports and energy drinks as a potential energy enhancer to improve exercise performance. Preclinical research has found taurine may counteract some problems that contribute to complications of diabetes (eg, diabetic nephropathy, diabetic cataract, and diabetic cardiomyopathy) (Ito 2012). Observational studies have found that plasma taurine concentrations tend to be lower in those with diabetes compared to non-diabetic controls (Franconi 1994; Sak 2019).
A meta-analysis assessed the effects of taurine supplementation on glycemic markers across five randomized controlled trials that enrolled a combined 209 participants. In these studies, people with diabetes were treated with 500–3,000 mg taurine or placebo daily for 2–16 weeks. Compared with controls, those that took taurine had reduced HbA1C and fasting glucose, and improved in a model of insulin resistance (Tao 2022).
In one study included in the meta-analysis, women with type 2 diabetes had the greatest improvements when taurine was combined with a unique form of strength training called total-body resistance exercise suspension training. This form of training allows for the performance of single and multi-joint exercises using body weight and gravity as resistance. When 500 mg taurine was taken twice daily (in the morning and evening) for eight weeks and combined with this training, participants had greater reductions in body fat percentage and insulin resistance and greater increases in HDL than the groups that only had one or the other or placebo (Samadpour 2021). These findings suggest taurine supplementation in conjunction with strength training may be a promising intervention to support metabolic health in those with diabetes.
A meta-analysis published in 2024 pooled data from 25 randomized controlled trials to assess the effects of taurine on risk factors related to metabolic syndrome (Tzang 2024). A total of 1,024 people were included in the analysis. The taurine dosage used in the studies ranged from 0.5–6 grams daily. The follow-up periods lasted between five days and one year. Compared with the control group, study participants who supplemented with taurine had a significant improvement in the primary outcome of reducing risk of metabolic syndrome, demonstrated by the following weighted mean changes:
- systolic blood pressure
- - 4 mm Hg
- diastolic blood pressure
- -1.5 mm Hg
- triglycerides
- -18.3 mg/dL
- fasting blood glucose
- -5.9 mg/dL
There were also significant improvements in secondary outcomes as shown by the following weighted mean changes:
- LDL cholesterol
- - 6.5 mg/dL
- total cholesterol
- - 8.3 mg/dL
- fasting insulin
- -1.52 µIU/L
- HOMA-IR (calculation of insulin resistance)
- - 0.7
There was no significant effect on HDL, body weight, and BMI.
Vitamin K. Vitamin K, better known for its role in facilitating the incorporation of calcium into bones, may also support healthy blood sugar metabolism and utilization. A few possible mechanisms have been proposed to explain the link between vitamin K and blood sugar metabolism. For instance, vitamin K may reduce proinflammatory cytokines such as TNF-α and IL-6, which are associated with insulin resistance (Manna 2016). Moreover, osteocalcin, a vitamin K-dependent hormone, may improve beta cell function and insulin production (Wei 2014). Clinical evidence suggests that vitamin K2 in the form of menaquinone-7 (MK-7) alters the microbiome in a manner that supports the growth of bacterial species associated with a decreased risk of type 2 diabetes (Mizokami 2017).
An observational study evaluated the risk of type 2 diabetes associated with four genetic variants that may increase circulating phylloquinone (vitamin K1) concentrations. The study examined data from three studies, totaling over 600,000 people—including 69,647 people with type 2 diabetes. Genetically predicted higher concentrations of phylloquinone were associated with a lower risk of type 2 diabetes. The authors found no evidence that these genes had pleiotropy (ie, the genes did not influence other unrelated traits) (Zwakenberg 2019).
In 2023, researchers conducted a systematic review to identify studies on the effect of vitamin K supplementation on blood sugar-related parameters and vitamin K intake on diabetes risk. They then conducted two meta-analyses: one on seven randomized controlled interventional studies and another on five observational studies. The meta-analysis of interventional studies, which included data on 813 participants, showed that vitamin K supplementation significantly reduced fasting blood glucose levels and a measure of insulin resistance (HOMA-IR). However, the magnitude of the effect was small. The dosage of supplemental vitamin K in the included studies ranged from 200 mcg to 1,000 mcg daily and the forms of vitamin K supplemented included K1 and K2 (eg, MK-7). The duration of the trials ranged from four weeks to 36 months and the comparison groups were given either a placebo or a multivitamin without vitamin K. The meta-analysis of observational studies, including nearly 106,000 participants in total, showed that people whose dietary vitamin K intake fell into the highest category had about 21% lower risk of type 2 diabetes than those with the lowest intake (Qu 2023).
In another meta-analysis published in 2023, several nutritional supplements were compared for their effects at managing type 2 diabetes. The review included a total of 170 randomized controlled trials totaling 14,223 people. The duration of the trials was at least two weeks. The included trials compared one of the following nutrients to each other or a placebo (or no treatment): selenium, vanadium, thiamine, niacin, biotin, folic acid, cobalamin, ascorbic acid (vitamin C), vitamin D, vitamin E, vitamin K, zinc, chromium, magnesium, or calcium. The results found that vitamin K supplements were ranked best in reducing hemoglobin A1C and fasting insulin levels with moderate-to-very low certainty evidence (Xia 2023).
Carnitine. Carnitine is an amino acid derivative that participates in fat metabolism by assisting in the transportation and utilization of long-chain fatty acids for energy production within the mitochondria. It is synthesized in the body using the amino acids L-lysine and methionine. Carnitine can also be obtained from dietary sources like red meat (best source), fish, poultry, and milk (Pekala 2011). Evidence suggests carnitine supplementation supports efficient glucose metabolism.
A meta-analysis assessed the pooled effects of carnitine supplementation on glycemic markers across 41 randomized controlled trials that enrolled a total of 2,900 people (Zamani 2022). The results demonstrated modest but statistically significant reductions of fasting blood glucose, hemoglobin A1c, and HOMA-IR (model of insulin resistance) compared with controls. There was no significant overall effect on fasting insulin. A major limitation to the analysis was a high degree of methodological differences between the clinical trials. To help overcome this, the researchers performed a subgroup analysis of specific characteristics shared by individuals within the pooled studies. Those with diabetes had the following mean reductions versus baseline:
- Fasting blood glucose: 6.5 mg/dL
- HbA1c: 0.41%
- HOMA-IR: 0.73
In a more recent meta-analysis, researchers pooled 21 randomized controlled trials that specifically evaluated carnitine supplementation in those with type-2 diabetes. A total of 2,041 people were included. Researchers also evaluated the effects on weight loss and other cardiovascular biomarkers in addition to glycemic markers. For every 1 gram daily of carnitine supplementation, up to 4 grams, the results showed a significant mean reduction in the following compared with controls:
- BMI: 0.37 kg/m3
- Fasting plasma glucose: 3 mg/dL
- HbA1c: 0.16%
- LDL cholesterol: 4 mg/dL
- Total cholesterol: 5 mg/dL
- Triglycerides: 6 mg/dL
The greatest reductions in BMI were observed at 2 grams daily whereas serum triglycerides, total cholesterol, and fasting plasma glucose had the greatest improvements at 4 grams daily (Mirrafiei 2024). The clinical trials used in this meta-analysis also had a high degree of methodological differences; therefore, the results should be interpreted with caution.
2025
- Apr: Updated section on omega-3 fatty acids in Nutrients
- Jan: Added section on metformin for diabetes prevention to Type 2 Diabetes Drugs – Additional Drugs
2024
- Jul: Added section on carnitine to Nutrients
- Jun: Updated section on taurine in Nutrients
- May: Updated section on cinnamon in Nutrients
- Jan: Added section on vitamin K to Nutrients
2023
- Apr: Updated and expanded section on vitamin D in Nutrients
2022
- Nov: Added section on taurine to Nutrients
- Aug: Added section on continuous glucose monitoring to Dietary & Lifestyle Changes for Type 2 Diabetes
- May: Updated section on injectable (non-insulin) type 2 diabetes drugs in Overview of Type 2 Diabetes Drugs
- May: Updated section on tirzepatide in Type 2 Diabetes Drugs – Additional Details
- Feb: Updated section on Metabolic Surgery for Longstanding Type 2 Diabetes
- Feb: Added section on Type 2 Diabetes Drugs – Additional Details
- Feb: Added section on Overview of Type 2 Diabetes Drugs
- Feb: Added section on Starting Drug Therapy for Type 2 Diabetes: What to Expect
- Feb: Updated section on Dietary & Lifestyle Changes for Type 2 Diabetes
- Feb: Updated section on Type 2 Diabetes Treatment: Overview & Goals
- Feb: Added section on tirazepatide to Novel and Emerging Strategies
2021
- Nov: Added section on SGLT2 inhibitors to Treatment of Type 2 Diabetes
- Sept: Updated section on vitamin B6 in Nutrients
- Mar: Added section on Metabolic Surgery for Longstanding Type 2 Diabetes
2016
- Aug: Comprehensive update & review
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
Abd El Aziz M, Cahyadi O, Meier JJ, Schmidt WE, Nauck MA. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: a meta-analysis based on cardiovascular outcomes trials. Diabetes, obesity & metabolism. Apr 2020;22(4):699-704. doi:10.1111/dom.13924
Abdul-Ghani M, Puckett C, Adams J, et al. Durability of Triple Combination Therapy Versus Stepwise Addition Therapy in Patients With New-Onset T2DM: 3-Year Follow-up of EDICT. Diabetes Care. Feb 2021;44(2):433-439. doi:10.2337/dc20-0978
Abdullah KM, Abul Qais F, Hasan H, Naseem I. Anti-diabetic study of vitamin B6 on hyperglycaemia induced protein carbonylation, DNA damage and ROS production in alloxan induced diabetic rats. Toxicol Res (Camb). Jul 1 2019;8(4):568-579. doi:10.1039/c9tx00089e
Abidov M, Ramazanov A, Jimenez Del Rio M, et al. Effect of Blueberin on fasting glucose, C-reactive protein and plasma aminotransferases, in female volunteers with diabetes type 2: double-blind, placebo controlled clinical study. 2006;141:66-72.
ACCORD Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. New England Journal of Medicine. 2008;358(24):2545-2559.
ADA. American Diabetes Association. A1C and eAG. http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/a1c/?loc=keymatch. Last updated 9/29/2014a. Accessed 8/19/2015.
ADA. American Diabetes Association. Glycemic Index and Diabetes. http://www.diabetes.org/food-and-fitness/food/what-can-i-eat/understanding-carbohydrates/glycemic-index-and-diabetes.html?referrer=https://www.google.com/. Last updated 5/14/2014b. Accessed 8/24/2015.
ADA. Standards of medical care in diabetes--2014. Diabetes care. Jan 2014c;37 Suppl 1:S14-80.
ADA. American Diabetes Association. Foundations of Care: Education, Nutrition, Physical Activity, Smoking Cessation, Psychosocial Care, and Immunization. Diabetes Care. 2015a;38(Suppl. 1): S20-S30. http://care.diabetesjournals.org/content/38/Supplement_1/S20.full.pdf
ADA. American Diabetes Association. Checking Your Blood Glucose. http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/checking-your-blood-glucose.html. Last updated 6/17/2015b. Accessed 9/1/2015.
ADA. American Diabetes Association. Insulin Basics. Available at: http://www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-basics.html. Last updated: 3/9/2015c. Accessed 4/10/2015.
ADA. American Diabetes Association. What Are My Options? Available at: http://www.diabetes.org/living-with-diabetes/treatment-and-care/medication/oral-medications/what-are-my-options.html. Last updated: 3/3/2015d. Accessed 4/12/2015.
ADA. American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care. 2015e;38(Suppl 1): S8-S16. <http://care.diabetesjournals.org/content/38/Supplement_1/S8.full>
ADA. American Diabetes Association. Glycemic Targets. Diabetes Care. 2015f;38(Suppl. 1): S33-S40. http://care.diabetesjournals.org/content/38/Supplement_1/S33.full
ADA. American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care . Jan 2019;42(Suppl 1):S90-S102. doi:10.2337/dc19-S009
ADA. American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care. Jan 2021a;44(Suppl 1):S73-S84. doi:10.2337/dc21-S006
ADA. American Diabetes Association Professional Practice C. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2021b;45(Supplement_1):S60-S82. doi:10.2337/dc22-S005
ADA. American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care . Jan 2021c;44(Suppl 1):S111-S124. doi:10.2337/dc21-S009
ADA. American Diabetes Association. Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clinical Diabetes . 2022;40(1):10-38. doi:10.2337/cd22-as01
Adams RJ, Appleton SL, Hill CL, et al. Independent association of HbA1C and incident cardiovascular disease in people without diabetes. Obesity. 2009;17:559-563.
Adams TD, Davidson LE, Litwin SE, et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. The New England journal of medicine. Sep 21 2017;377(12):1143-1155. doi:10.1056/NEJMoa1700459
Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arteriosclerosis, thrombosis, and vascular biology. Jul 2008;28(7):1225-1236.
Adolfsson P, Parkin CG, Thomas A, Krinelke LG. Selecting the Appropriate Continuous Glucose Monitoring System - a Practical Approach. Eur Endocrinol . Apr 2018;14(1):24-29. doi:10.17925/ee.2018.14.1.24.
Afaghi A, Ziaee A, Afaghi M. Effect of low-glycemic load diet on changes in cardiovascular risk factors in poorly controlled diabetic patients. Indian journal of endocrinology and metabolism. Nov 2012;16(6):991-995.
AFAR. TAME: Targeting Aging with Metformin http://www.afar.org/natgeo/. 4/14/2016.
Agrawal NK, Kant S. Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes. 2014;5(5):697-710.
Agrawal P, Arora S, Bhawna S, et al. Association of macrovascular complications of type 2 diabetes mellitus with serum magnesium levels. Diabetes & Metab Syndr: Clin Res Rev. 2011; 5:41-44.
AHA. American Heart Association. Glycemic Index and Diabetes. http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/HealthyEating/Glycemic-Index-and-Diabetes_UCM_457070_Article.jsp, Copyright 2014. Accessed 8/24/2015.
Ahmadieh H, Azar ST. Liver disease and diabetes: association, pathophysiology, and management. Diabetes research and clinical practice. Apr 2014;104(1):53-62.
Ahmed S, Mahmood Z, Zahid S. Linking insulin with Alzheimer's disease: emergence as type III diabetes. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. Oct 2015;36(10):1763-1769.
AKAY. AKAY Flavours & Aromatics Pvt. Ltd. Supplier Internal Study. Effect of Clovinol on Random Blood Sugar Levels - A Pilot Study. Data on File. 2017.
Albert BB, Derraik JG, Brennan CM, Biggs JB, Smith GC, Garg ML, . . . Cutfield WS. Higher omega-3 index is associated with increased insulin sensitivity and more favourable metabolic profile in middle-aged overweight men. Scientific reports. 2014;4:6697.
Aleem E, Nehrbass D, Klimek F, et al. Upregulation of the insulin receptor and type 1 insulin-like growth factor receptor are early events in hepatocarcinogenesis. Toxicol Pathol. 2011;39(3):524-43.
Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. Jul 2015;23(3):250-256.
Alfaris N, Minnick AM, Hopkins CM, Berkowitz RI, Wadden TA. Combination phentermine and topiramate extended release in the management of obesity. Expert opinion on pharmacotherapy. Jun 2015;16(8):1263-1274.
Alhaj HA, Massey AE, McAllister-Williams RH. Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology (Berl). Nov 2006;188(4):541-551.
Allen RW, Schwartzman E. Cinnamon Use in Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis. The Annals of Family Medicine. 2013;11(5):452. doi:10.1370/afm.1517. http://www.annfammed.org/content/11/5/452.abstract
Alssema M, Schindhelm RK, Dekker JM, et al. Postprandial glucose and not triglyceride concentrations are associated with carotid intima media thickness in women with normal glucose metabolism: The Hoorn prandial study. Atherosclerosis. 2008;196:712-719.
Al-Tabakha MM. Future prospect of insulin inhalation for diabetic patients: The case of Afrezza versus Exubera. Journal of controlled release: official journal of the Controlled Release Society. Jul 26 2015;215:25-38.
Alvarado J, Schoenlau F, Leschot A, Salgad AM, Vigil Portales P. Delphinol(R) standardized maqui berry extract significantly lowers blood glucose and improves blood lipid profile in prediabetic individuals in three-month clinical trial. Panminerva medica.Sep 2016;58(3 Suppl 1):1-6.
Alvarado JL, Leschot A, Olivera-Nappa A, Salgado AM, Rioseco H, Lyon C, Vigil P. Delphinidin-Rich Maqui Berry Extract (Delphinol(R)) Lowers Fasting and Postprandial Glycemia and Insulinemia in Prediabetic Individuals during Oral Glucose Tolerance Tests. BioMed research international. 2016;2016:9070537.
Amin MM, Asaad GF, Abdel Salam RM, et al. Novel CoQ10 antidiabetic mechanisms underlie its positive effect: modulation of insulin and adiponectine receptors, tyrosine kinase, Pl3K, glucose transporters, sRAGE and visfatin in insulin resistant/diabetic rats. PLoS One. 2014;9(2):e89169.
Andallu B, Suryakantham V, Lakshmi Srikanthi B, Kesava Reddy G. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clinica Chimica Acta. 2001;314(1–2):47-53.
Anderson RA, Cheng N, Bryden NA, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46(11):1786-91.
Andrade-Cetto A, Vazquez RC. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. Journal of ethnopharmacology. Jul 6 2010;130(1):93-97.
Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12(22):3483-3489.
Ansurudeen I, Sunkari VG, Grunler J, Peters V, Schmitt CP, Catrina SB, . . . Forsberg EA. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino acids. Jul 2012;43(1):127-134.
Ao T, Kikuta J, Ishii M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules. Nov 3 2021;11(11)doi:10.3390/biom11111624. https://pubmed.ncbi.nlm.nih.gov/34827621/
Aoki K, Terauchi Y. Effect of Dehydroepiandrosterone (DHEA) on Diabetes Mellitus and Obesity. Vitam Horm. 2018;108:355-365.
Arai Y, Takayama M, Abe Y, et al. Adipokines and aging. J Atheroscler Thromb. 2011;18(7):545-50.
Arion JW, Canfield WK, Ramos FC, et al. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose-6-phosphatase. Arch Biochem Biophys. 1997;339(2):315-322.
Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, . . . Crandall JP. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. The Journal of clinical endocrinology and metabolism. Feb 22 2016:jc20153754.
Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and Risks of Bariatric Surgery in Adults: A Review. Jama. Sep 1 2020;324(9):879-887. doi:10.1001/jama.2020.12567
Asai A, Nakagawa K, Higuchi O, Kimura T, Kojima Y, Kariya J, . . . Oikawa S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J Diabetes Investig. Aug 2 2011;2(4):318-323.
Asarani NAM, Reynolds AN, Boucher SE, de Bock M, Wheeler BJ. Cutaneous Complications With Continuous or Flash Glucose Monitoring Use: Systematic Review of Trials and Observational Studies. J Diabetes Sci Technol. Mar 2020;14(2):328-337. doi:10.1177/1932296819870849.
Assefa ST, Yang EY, Chae SY, et al. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants (Basel). Dec 18 2019;9(1)doi:10.3390/plants9010002
Atkinson FS, Foster-Powell K, Brand-Miller JC. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes care. 2008;31(12):2281-2283.
Atzmon G, Pollin TI, Crandall J, et al. Adiponectin levels and genotype: a potential regulator of life span in humans. J Gerontol A Biol Sci Med Sci. 2008;63(5):447-53.
Azad KM, Sutradhar SR, Khan NA, et al. Serum magnesium in hospital admitted diabetic patients. Mymensingh Med J. 2014;23(1):28-34.
Aziz TA, Hussain SA, Mahwi TO, Ahmed ZA. Efficacy and safety of Ginkgo biloba extract as an “add-on” treatment to metformin for patients with metabolic syndrome: a pilot clinical study. Ther Clin Risk Manag. 2018;14:1219-1226. https://pubmed.ncbi.nlm.nih.gov/30034238/
Aziz TA, Hussain SA, Mahwi TO, Ahmed ZA, Rahman HS, Rasedee A. The efficacy and safety of Ginkgo biloba extract as an adjuvant in type 2 diabetes mellitus patients ineffectively managed with metformin: a double-blind, randomized, placebo-controlled trial. Drug Des Devel Ther. 2018;12:735-742. https://pubmed.ncbi.nlm.nih.gov/29670330/
Azoulay L. Incretin-based drugs and adverse pancreatic events: almost a decade later and uncertainty remains. Diabetes care. Jun 2015;38(6):951-953.
Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, . . . Ernst P. Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ (Clinical research ed.). 2016;352:i581.
Azzam H, Malnick S. Non-alcoholic fatty liver disease - the heart of the matter. World journal of hepatology. Jun 8 2015;7(10):1369-1376.
Bahr C, Groner B. The IGF-1 receptor and its contributions to metastatic tumor growth-novel approaches to the inhibition of IGF-1R function. Growth factors (Chur, Switzerland). Mar 2005;23(1):1-14.
Bailey CJ. Tirzepatide: a new low for bodyweight and blood glucose. The lancet Diabetes & endocrinology. Oct 2021;9(10):646-648. doi:10.1016/S2213-8587(21)00217-5
Balakumar P, Rohilla A, Krishan P, et al. The multifaceted therapeutic potential of benfotiamine. Pharmacol Res. 2010;61(6):482-8.
Bales CW, Kraus WE. Caloric restriction: implications for human cardiometabolic health. J Cardiopulm Rehabil Prev. Jul-Aug 2013;33(4):201-208.
Balkau B, Kahn HS, Courbon D, Eschwege E, Ducimetiere P. Hyperinsulinemia predicts fatal liver cancer but is inversely associated with fatal cancer at some other sites: the Paris Prospective Study. Diabetes care. May 2001;24(5):843-849.
Bansal N. Prediabetes diagnosis and treatment: a review. World J Diabetes. 2015;6(2):296-303.
Banu S, Jabir NR, Manjunath NC, et al. Reduction of post-prandial hyperglycemia by mulberry tea in type-2 diabetes patients. Saidi J Biol Sci. 2015;22:32-36.
Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes. 2014;5(6):889-893.
Barbarawi M, Al-Abdouh A, Barbarawi O, Lakshman H, Al Kasasbeh M, Chen K. SGLT2 inhibitors and cardiovascular and renal outcomes: a meta-analysis and trial sequential analysis. Heart failure reviews. Feb 23 2021;doi:10.1007/s10741-021-10083-z
Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. The American journal of clinical nutrition. Mar 2008;87(3):627-637.
Barth E, Albuszies G, Baumgart K, et al. Glucose metabolism and catecholamines. Crit Care Med. 2007;35(9 Suppl):S508-18.
Bassi N, Karagodin I, Wang S, et al. Lifestyle modification for metabolic syndrome: a systematic review. Am J Med. 2014;127:1242.el-1242e10.
Bassoli BK, Cassolla P, Borba-Murad GR, Constantin J, Salgueiro-Pagadigorria CL, Bazotte RB, . . . de Souza HM. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell biochemistry and function. Apr 2008;26(3):320-328.
Basu R, Chandramouli V, Dicke B, et al. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes. 2005;54(7):1942-1948.
Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes care. Jul 2008;31(7):1311-1317.
Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocrine-Related Cancer. 2011;18:R125-R147.
Beltramo E, Berrone E, Tarallo S, Porta M. Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta diabetologica. Sep 2008;45(3):131-141.
Benjamin EM. Self-Monitoring of Blood Glucose: The Basics. Clinical Diabetes. 2002;20(1):45-47.
Berstein LM. Metformin in obesity, cancer and aging: addressing controversies. Aging (Albany NY). 2012;4(5):320-329.
Bertinato J, Wu Xiao C, Ratnayake WM, Fernandez L, Lavergne C, Wood C, Swist E. Lower serum magnesium concentration is associated with diabetes, insulin resistance, and obesity in South Asian and white Canadian women but not men. Food Nutr Res. 2015;59:25974.
Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114-131. https://pubmed.ncbi.nlm.nih.gov/25435019/
Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, . . . Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. The American journal of clinical nutrition. Jul 2014;100(1):218-232.
Bik W, Baranowska-Bik A, Wolinska-Witort E, et al. The relationship between adiponectin levels and metabolic status in centenarian, early elderly, young and obese women. Neuro Endocrinol. 2006;27(4):493-500.
Bik W, Baranowska-Bik A, Wolinska-Witort E, et al. Assessment of adiponectin and its isoforms in Polish centenarians. Experimental Gerontology. 2013;48:401-407.
Bissonnette S, Lamantia V, Ouimet B, et al. Native low-density lipoproteins are priming signals of the NLRP3 inflammasome/interleukin-1β pathway in human adipose tissue and macrophages. Scientific Reports. 2023/11/01 2023;13(1):18848. doi:10.1038/s41598-023-45870-1. https://doi.org/10.1038/s41598-023-45870-1. https://pmc.ncbi.nlm.nih.gov/articles/PMC10620147/pdf/41598_2023_Article_45870.pdf
Bjornholt JV, Erikssen G, Aaser E, et al. Fasting blood glucose: an underestimated risk factor for cardiovascular death. Diabetes Care. 1999;22:45-49.
Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging (Albany NY). 2009;1(3):281-288.
Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, . . . Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. Jun 2007;56(6):1703-1711.
Boden G. Gluconeogenesis and glycogenolysis in health and diabetes. J Investig Med. 2004;52(6):375-378.
Boers I, Muskiet FA, Berkelaar E, Schut E, Penders R, Hoenderdos K, . . . Jong MC. Favourable effects of consuming a Palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids in health and disease. 2014;13:160.
Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115-130. https://pubmed.ncbi.nlm.nih.gov/18429699/
Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiological reviews. Oct 2013;93(4):1803-1845.
Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients. Apr 26 2019;11(5)doi:10.3390/nu11050962
Boniol M, Franchi M, Bota M, et al. Incretin-Based Therapies and the Short-term Risk of Pancreatic Cancer: Results From Two Retrospective Cohort Studies. Diabetes Care. Feb 2018;41(2):286-292. doi:10.2337/dc17-0280
Boscari F, Vettoretti M, Cavallin F, et al. Implantable and transcutaneous continuous glucose monitoring system: a randomized cross over trial comparing accuracy, efficacy and acceptance. J Endocrinol Invest. Jan 2022;45(1):115-124. doi:10.1007/s40618-021-01624-2.
Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254-8.
Boyle JG, Salt IP, McKay GA. Metformin action on AMP-activated protein kinase: a translational research approach to understanding a potential new target. Diabet Med. 2010;27(10):1097-106.
Boyle CN, Lutz TA, Le Foll C. Amylin - Its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Molecular metabolism. Feb 2018;8:203-210. doi:10.1016/j.molmet.2017.11.009
Bozkaya G, Ozgu E, Karaca B. The association between estimated average glucose levels and fasting plasma glucose levels. Clinics (Sao Paulo, Brazil). 2010;65(11):1077-1080.
Brahimaj A, Muka T, Kavousi M, Laven JS, Dehghan A, Franco OH. Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: the Rotterdam Study. Diabetologia. Jan 2017;60(1):98-106.
Braidy N, Guillemin GJ, Mansour H, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in winstar rats. PLoS One. 2011;6(4):e19194. https://pubmed.ncbi.nlm.nih.gov/21541336/
Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, . . . Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. The British journal of nutrition. Mar 2009;101(6):886-894.
Brown BE, Kim CH, Torpy FR, Bursill CA, McRobb LS, Heather AK, . . . van Reyk DM. Supplementation with carnosine decreases plasma triglycerides and modulates atherosclerotic plaque composition in diabetic apo E(-/-) mice. Atherosclerosis. Feb 2014;232(2):403-409.
Bruckbauer A, Zemel MB. Synergistic effects of metformin, reveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes Metab Syndr Obes. 2013;6:93-102.
Bruin JE, Saber N, Braun N, et al. Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem Cell Reports. 2015;4(4):605-620.
Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, . . . Fineman M. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes care. Feb 2016;39(2):198-205.
Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106 Suppl 3:S5-78.
Campbell LK, White JR, Campbell RK. Acarbose: its role in the treatment of diabetes mellitus. The Annals of pharmacotherapy. Nov 1996;30(11):1255-1262.
Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, . . . Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. Jun 6 2012;15(6):838-847.
Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. Jul 7 2015;22(1):31-53.
Cao C, Yang S, Zhou Z. GLP-1 receptor agonists and risk of cancer in type 2 diabetes: an updated meta-analysis of randomized controlled trials. Endocrine . Nov 2019;66(2):157-165. doi:10.1007/s12020-019-02055-z
Capasso I, Esposito E, Pentimalli F, Montella M, Crispo A, Maurea N, . . . Giordano A. Homeostasis model assessment to detect insulin resistance and identify patients at high risk of breast cancer development: National Cancer Institute of Naples experience. Journal of experimental & clinical cancer research: CR. 2013;32:14.
Capel I, Rigla M, Garcia-Saez G, Rodriguez-Herrero A, Pons B, Subias D, . . . Hernando ME. Artificial pancreas using a personalized rule-based controller achieves overnight normoglycemia in patients with type 1 diabetes. Diabetes technology & therapeutics. Mar 2014;16(3):172-179.
Carter P, Gray LJ, Talbot D, Morris DH, Khunti K, Davies MJ. Fruit and vegetable intake and the association with glucose parameters: a cross-sectional analysis of the Let's Prevent Diabetes Study. European journal of clinical nutrition. Jan 2013;67(1):12-17.
Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91(3):813-9.
CDC. Centers for Disease Control and Prevention. Data and Statistics. Available at: http://www.cdc.gov/diabetes/data/index.html. Last updated: 3/4/2015b. Accessed 4/2/2015.
CDC. Centers for Disease Control and Prevention. Diabetes Home. Basics About Diabetes. Available at: http://www.cdc.gov/diabetes/basics/diabetes.html. Last updated: 3/31/2015a. Accessed 4/3/2015.
CDC. Centers for Disease Control and Prevention. Diabetes in Youth. <http://www.cdc.gov/diabetes/risk/age/youth.html>. Last updated 12/9/2014. Accessed 8/12/2015.
Ceriello A. Diabetic complications: from oxidative stress to inflammatory cardiovascular disorders. Medicographia. 2011;33:29-34.
Ceriello A. Point: Postprandial glucose levels are a clinically important treatment target. Diabetes Care. 2010;33(8):1905-1907.
Ceriello A. The emerging challenge in diabetes: the “metabolic memory.” Vascul Pharmacol. 2012;57:133-138.
Ceriello A, Ihnat MA, Thorpe JE. The “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab. 2009;94(2):410-415.
Chakrabarti R, Chen M, Liu W, Chen S. Preventive effects of benfotiamine in chronic diabetic complications. J Diabetes Investig. Apr 7 2011;2(2):123-131.
Chan J, Cheng-Lai A. Inhaled Insulin: A Clinical and Historical Review. Cardiology in review. May/Jun 2017;25(3):140-146. doi:10.1097/CRD.0000000000000143
Chan JH, Lim S, Wong WS. Antisense oligonucleotides: from design to therapeutic application. Clinical and experimental pharmacology & physiology. May-Jun 2006;33(5-6):533-540.
Chatterjee R, Davenport CA, Vickery EM, et al. Effect of intra-trial mean 25(OH)D level on diabetes risk, by race and weight: an ancillary analysis in the D2d randomized study cohort. The American Journal of Clinical Nutrition . 2023/03/29/ 2023;doi:https://doi.org/10.1016/j.ajcnut.2023.03.021. https://pubmed.ncbi.nlm.nih.gov/37001590/
Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A View Beyond HbA1c: Role of Continuous Glucose Monitoring. Diabetes Ther . Jun 2019;10(3):853-863. doi:10.1007/s13300-019-0619-1.
Chen X, Zhang J, Zhou Z. Targeting Islets: Metabolic Surgery Is More than a Bariatric Surgery. Obesity surgery. Sep 2019;29(9):3001-3009. doi:10.1007/s11695-019-03979-1
Chen TH, Li YR, Chen SW, et al. Sodium-glucose cotransporter 2 inhibitor versus metformin as first-line therapy in patients with type 2 diabetes mellitus: a multi-institution database study. Cardiovasc Diabetol. Nov 9 2020;19(1):189. doi:10.1186/s12933-020-01169-3
Chen K, Li F, Cai H, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. Apr 2006;12(4):425-432. https://pubmed.ncbi.nlm.nih.gov/16582918/
Cheng P, Neugaard B, Foulis P, Conlin PR. Hemoglobin A1C as a predictor of incident diabetes. Diabetes care. Mar 2011;34(3):610-615.
Chi Y, Sauve AA. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Current opinion in clinical nutrition and metabolic care. Nov 2013;16(6):657-661.
Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet. 2002;359(9323):2072-7.
Chiu C-T, Chuang D-M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacology & therapeutics. 2010;128(2):281-304.
Chiva-Blanch G, Urpi-Sarda M, Ros E, Valderas-Martinez P, Casas R, Arranz S, . . . Estruch R. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clinical nutrition (Edinburgh, Scotland). Apr 2013;32(2):200-206.
Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J. Dec 2014;38(6):406-415.
Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Molecules and cells. Oct 2013;36(4):279-287.
Chokrungvaranon N, Deer J, Reaven PD. Intensive glycemic control and cardiovascular disease: are there patients who may benefit? Postgraduate medicine. Nov 2011;123(6):114-123.
Chou SC, Chen KW, Hwang JS, et al. The add-on effects of Gynostemma pentaphyllum on nonalcoholic fatty liver disease. Altern Ther Health Med. 2006;12(3):34-9.
Chou HC, Chen WW, Hsiao FY. Acute pancreatitis in patients with type 2 diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors: a population-based nested case-control study. Drug safety : an international journal of medical toxicology and drug experience. Jul 2014;37(7):521-528.
Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, et al. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121-7.
Chung ST, Hsia DS, Chacko SK, Rodriguez LM, Haymond MW. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia. Mar 2015;58(3):596-603.
Clemente-Postigo M, Munoz-Garach A, Serrano M, et al. Serum 25-hydroxyvitamin D and adipose tissue vitamin D receptor gene expression: relationship with obesity and type 2 diabetes. J Clin Endocrinol Metab . Apr 2015;100(4):E591-5. doi:10.1210/jc.2014-3016. https://pubmed.ncbi.nlm.nih.gov/25706239/
Clore JN, Stillman J, Sugerman H. Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes. 2000;49:969-974.
Cohen O, Gillam M. First Consult. Diabetes mellitus type 1 in adults. Available at: www.clinicalkey.com. Copyright by Elsevier BV, Inc. Last updated 10/17/2013. Accessed 3/19/2015.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, . . . Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes care. Dec 2010;33(12):e147-167.
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, . . . Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science (New York, N.Y.). Jul 10 2009;325(5937):201-204.
Coniff RF, Shapiro JA, Robbins D, Kleinfield R, Seaton TB, Beisswenger P, McGill JB. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM. A placebo-controlled dose-comparison study. Diabetes care. Jun 1995;18(6):817-824.
Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. NEJM. 1996;334:292-5.
Costemale-Lacoste JF, Guilloux JP, Gaillard R. The role of GSK-3 in treatment-resistant depression and links with the pharmacological effects of lithium and ketamine: A review of the literature. L'Encephale. Apr 2016;42(2):156-164.
Coughlan KA, Valentine RJ, Ruderman NB, et al. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241-253.
Courcoulas AP, Belle SH, Neiberg RH, et al. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA surgery. Oct 2015;150(10):931-40. doi:10.1001/jamasurg.2015.1534
Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes care. Feb 1999;22(2):233-240.
Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006;169(5): 1505-1522. https://pubmed.ncbi.nlm.nih.gov/17071576/
Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia. Feb 2018;61(2):257-264. doi:10.1007/s00125-017-4513-y
Currie CJ, Poole CD, Gale EAM. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766-1777.
Dahl D, Onishi Y, Norwood P, Huh R, Patel H, RodrÍGuez Á. 80-LB: Tirzepatide, a Dual GIP/GLP-1 Receptor Agonist, Is Effective and Safe When Added to Basal Insulin for Treatment of Type 2 Diabetes (SURPASS-5). Diabetes. 2021;70(Supplement_1)doi:10.2337/db21-80-LB
Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends in immunology. Jan 2004;25(1):4-7.
Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods . Mar 9 2019;8(3)doi:10.3390/foods8030092
Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. The American journal of medicine. Jan 1997;102(1):99-110.
Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14(9):884-9.
de Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1-46. doi: 10.1152/physrev.00012.2014. https://pubmed.ncbi.nlm.nih.gov/25540137/
de Bree A, Verschuren WM, Blom HJ, Kromhout D. Association between B vitamin intake and plasma homocysteine concentration in the general Dutch population aged 20-65 y. The American journal of clinical nutrition. Jun 2001;73(6):1027-1033.
De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimer’s Dement. 2014;10(Suppl):S26-32.
DeFronzo RA, Triplitt CL, Abdul-Ghani M, et al. Novel agents for the treatment of type 2 diabetes. Diabetes Spectrum. 2014;27(2):100-112.
DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes care. Nov 2009;32 Suppl 2:S157-163.
de Heer J, Goke B. Are incretin mimetics and enhancers linked to pancreatitis and malignant transformations in pancreas? Expert opinion on drug safety. Nov 2014;13(11):1469-1481.
de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B12 deficiency: randmised placebo controlled trial. BMJ. 2010;340:c2181.
DeFuria J, Bennett G, Strissel KJ, Perfield JW, 2nd, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. The Journal of nutrition. Aug 2009;139(8):1510-1516.
Delamater AM. Clinical Use of Hemoglobin A1C to Improve Diabetes Management. Clinical Diabetes. January 1, 2006 2006;24(1):6-8.
de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101-1113.
Del Bene A, Ciolli L, Borgheresi L, et al. Is type 2 diabetes related to leukoaraiosis? an updated review. Acta Neurol Scand. 2015;Mar 16. Doi:10.1111/ane.12398.
Delgado H, Lehmann T, Bobbioni-Harsch E, Ybarra J, Golay A. Acarbose improves indirectly both insulin resistance and secretion in obese type 2 diabetic patients. Diabetes & metabolism. Jun 2002;28(3):195-200.
Delpino FM, Figueiredo LM, da Silva BGC, et al. Omega-3 supplementation and diabetes: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62(16):4435-4448. doi:10.1080/10408398.2021.1875977. https://pubmed.ncbi.nlm.nih.gov/33480268/
Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. Nov 13 2021;398(10313):1811-1824. doi:10.1016/S0140-6736(21)02188-7
Derdemezis CS, Lovegrove JA. Glycemic index, glycemic control and beyond. Current pharmaceutical design. 2014;20(22):3620-3630.
Derosa G, Maffioli P. Efficacy and safety profile evaluation of acarbose alone and in association with other antidiabetic drugs: a systematic review. Clin Ther. 2012;34(6):1221-1236.
Despres JP, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334.15:952-958.
De Tata V. Age-related impairment of pancreatic beta-cell function: pathophysiological and cellular mechanisms. Front Endocrinol (Lausanne). 2014;5:138.
Devi KP, Rajavel T, Nabavi SF, Setzer WN, Ahmadi A, Mansouri K, Nabavi SM. Hesperidin: A promising anticancer agent from nature. Industrial Crops and Products. 2015;76:582-589.
Deyno S, Eneyew K, Seyfe S, et al. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A meta-analysis and meta-regression. Diabetes Research and Clinical Practice. 2019/10/01/ 2019;156:107815. doi:https://doi.org/10.1016/j.diabres.2019.107815. https://www.sciencedirect.com/science/article/pii/S0168822719307065
Diabetes.co.uk. Treatment: Medication. Metformin Side Effects http://www.diabetes.co.uk/diabetes-medication/metformin-side-effects.html. Copyright 2016. Accessed 8/8/2016.
Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes care. Dec 2002;25(12):2165-2171.
Dicembrini I, Montereggi C, Nreu B, Mannucci E, Monami M. Pancreatitis and pancreatic cancer in patientes treated with Dipeptidyl Peptidase-4 inhibitors: An extensive and updated meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. Jan 2020;159:107981. doi:10.1016/j.diabres.2019.107981
Didyuk O, Econom N, Guardia A, Livingston K, Klueh U. Continuous Glucose Monitoring Devices: Past, Present, and Future Focus on the History and Evolution of Technological Innovation. J Diabetes Sci Technol. May 2021;15(3):676-683. doi:10.1177/1932296819899394.
Dimsdale JE, Moss J. Short-term catecholamine response to psychological stress. Psychosomatic medicine. Sep 1980;42(5):493-497.
Dineley KT, Jahrling JB, Denner L. Insulin resistance in Alzheimer’s disease. Neurobiol Dis. 2014;Dec;72Pt A:92-103.
Ding D, Qiu J, Li X, et al. Hyperglycemia and mortality among patients with coronary artery disease. Diabetes Care. 2014;37(2):546-54.
Di Pino A, Currenti W, Urbano F, et al. High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutr Metab Cardiovasc Dis. Nov 2017;27(11):978-984. doi:10.1016/j.numecd.2017.06.014
Djiogue S, Nwabo Kamdje AH, Vecchio L, et al. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer. 2013;20:R1-R17.
Djousse L, Biggs ML, Lemaitre N, et al. Plasma omega-3 fatty acids and incident diabetes in older adults. Am J Clin Nutr. 2011;94(2):527-533.
Djousse L, Cook NR, Kim E, et al. Diabetes Mellitus, Race, and Effects of Omega-3 Fatty Acids on Incidence of Heart Failure Hospitalization. JACC Heart failure. Apr 2022;10(4):227-234. doi:10.1016/j.jchf.2021.12.006. https://pubmed.ncbi.nlm.nih.gov/35361440/
Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients. Sep 2013;5(9):3551-3562.
Donnan K, Segar L. SGLT2 inhibitors and metformin: Dual antihyperglycemic therapy and the risk of metabolic acidosis in type 2 diabetes. European journal of pharmacology . Mar 5 2019;846:23-29. doi:10.1016/j.ejphar.2019.01.002
Dowling RJO, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Medicine. 2011;9:33.
Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. Journal of the American College of Cardiology. Aug 13 2013;62(7):569-576.
Draznin B. Mechanism of the mitogenic influence of hyperinsulinemia. Diabetology & Metabolic Syndrome. 2011;3:10.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. The Lancet. 2006;368(9548):1696-1705.
Du X, Edelstein D, Brownlee M. Oral benfotiamine plus alpha-lipoic acid normalises complication-causing pathways in type 1 diabetes. Diabetologia. Oct 2008;51(10):1930-1932.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, . . . Huang GD. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. New England Journal of Medicine. 2009;360(2):129-139.
Dworatzek PD, Arcudi K, Gougeon R, Husein N, Sievenpiper JL, Williams SL. Nutrition therapy. Canadian journal of diabetes. Apr 2013;37 Suppl 1:S45-55.
Edgerton DS, Cardin S, Pan C, et al. Effects of insulin deficiency or excess on hepatic gluconeogenesis flux during glycogenolytic inhibition in the conscious dog. Diabetes. 2002;51(11):3151-3162.
Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C. Pancreatic Safety of Incretin-Based Drugs — FDA and EMA Assessment. New England Journal of Medicine. 2014;370(9):794-797.
El-Gohary Y, Tulachan S, Wiersch J, Guo P, Welsh C, Prasadan K, . . . Gittes G. A Smad Signaling Network Regulates Islet Cell Proliferation. Diabetes. 2013;63(1):224-236.
Eldar-Finkelman H, Martinez A. GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS. Front Mol Neurosci. 2011;4:32.
Engelhardt K, Ferguson M, Rosselli JL. Prevention and Management of Genital Mycotic Infections in the Setting of Sodium-Glucose Cotransporter 2 Inhibitors. Ann Pharmacother. Apr 2021;55(4):543-548. doi:10.1177/1060028020951928
Esposito K, Giugliano D. Mediterranean diet and type 2 diabetes. Diabetes/metabolism research and reviews. Mar 2014;30 Suppl 1:34-40.
Esser N, Paquot N, Scheen AL, Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24(3):283-307.
Eriksson JG, Forsen TJ, Mortensen SA, et al. The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. BioFactors 9. 1999;315-318.
Farah A, Monteiro M, Donangelo CM, Lafay S. Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans. The Journal of nutrition. 2008;138(12):2309-2315.
Farsi PF, Djazayery A, Eshraghian MR, Koohdani F, Saboor-Yaraghi AA, Derakhshanian H, . . . Djalali M. Effects of supplementation with omega-3 on insulin sensitivity and non-esterified free fatty acid (NEFA) in type 2 diabetic patients. Arquivos brasileiros de endocrinologia e metabologia. Jun 2014;58(4):335-340.
FDA. U.S. Food and Drug Administration. Safety. Exubera (insulin human [rDNA origin] Inhalation Powder September 2008. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/Safety-RelatedDrugLabelingChanges/ucm122978.htm. Last updated: 6/19/2009. Accessed 6/21/2015.
FDA. U.S. Food and Drug Administration. News and Events. FDA News Release. FDA approves Afrezza to treat diabetes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm403122.htm. Updated: 6/30/2014. Accessed 4/19/2015.
FDA. What is the pancreas? What is an artificial pancreas device system? http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ArtificialPancreas/ucm259548.htm. 2016. 3/30/2016.
FDA. U.S. Food and Drug Administration. FDA News Release. FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes. 5/13/2022. Accessed 5/18/2022. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes
Feingold KR. Oral and Injectable (Non-Insulin) Pharmacological Agents for the Treatment of Type 2 Diabetes. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. MDText.com, Inc. Copyright ©2000-2021; 2000.
Ferensztajn-Rochowiak E, Rybakowski JK. The effect of lithium on hematopoietic, mesenchymal and neural stem cells. Pharmacological reports: PR. Apr 2016;68(2):224-230.
Feringa HH, Laskey DA, Dickson JE, Coleman CI. The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. Journal of the American Dietetic Association. Aug 2011;111(8):1173-1181.
Feskens EJM, Kromhout D. Glucose tolerance and the risk of cardiovascular disease: The Zutphen Study. J Clin Epidemiol. 1992;45(11):1327-1334.
Feuers RJ, Desai VG, Chen FX, Hunter JD, Duffy PH, Oriaku ET. Effects of dietary restriction on insulin resistance in obese mice. J Am Aging Assoc. Apr 2000;23(2):95-101.
Fiori JL, Shin YK, Kim W, et al. Resveratrol prevents beta-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62(10):3500-13.
Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Molecular medicine (Cambridge, Mass). Nov 23 2018;24(1):59. doi:10.1186/s10020-018-0060-3
Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes care. Feb 2008;31(2):289-294.
Fleming LW, Fleming JW, Davis CS. Afrezza: an inhaled approach to insulin delivery. J Am Assoc Nurse Pract. 2015;Mar 12. doi: 10.1002/2327-6924.12247.
Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative medicine and cellular longevity. Sep-Oct 2009;2(4):191-206.
Fonseca V, Levinson P, Toth DW, Gillam M. First Consult. Diabetes mellitus type 2 in adults. Available at: www.clinicalkey.com. Copyright by Elsevier BV, Inc. Last updated 8/26/2013. Accessed 3/19/2015.
Forouzandeh F, Salazar G, Patrushev N, et al. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. J Am Heart Assoc. 2014;3(6):e001202.
Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, . . . Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. Dec 10 2015;528(7581):262-266.
Fossati P, Romon-Rousseaux M. Insulin and HDL-cholesterol metabolism. Diabete Metab. 1987;13(3 Pt 2):390-394. https://pubmed.ncbi.nlm.nih.gov/3308570/
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clinical Diabetes. 2008;26(2):77-82.
Franciosi M, Lucisano G, Lapice E, et al. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8(8):e71583.
Franconi F, Bennardini F, Mattana A, et al. Taurine levels in plasma and platelets in insulin-dependent and non-insulin-dependent diabetes mellitus: correlation with platelet aggregation. Adv Exp Med Biol. 1994;359:419-24. doi:10.1007/978-1-4899-1471-2_45
Franekova V, Angin Y, Hoebers NT, Coumans WA, Simons PJ, Glatz JF, . . . Larsen TS. Marine omega-3 fatty acids prevent myocardial insulin resistance and metabolic remodeling as induced experimentally by high insulin exposure. Am J Physiol Cell Physiol. Feb 15 2015;308(4):C297-307.
Frias JP, Nauck MA, Van J, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes, obesity & metabolism. Jun 2020;22(6):938-946. doi:10.1111/dom.13979
Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. The New England journal of medicine. Aug 5 2021;385(6):503-515. doi:10.1056/NEJMoa2107519
Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. European journal of clinical nutrition. Aug 2008;62(8):953-960.
Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-Carbohydrate Diets and All-Cause and Cause-Specific MortalityTwo Cohort Studies. Annals of Internal Medicine. 2010;153(5):289-298.
GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519-524.
Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and cellular endocrinology. Mar 25 2010;316(2):129-139.
Galli-Tsinopoulou A, Maggana I, Kyrgios I, et al. Association between magnesium concentration and HbA1C in children and adolescents with type 1 diabetes mellitus. J Diabetes. 2014;6(4):369-77.
Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl2):S279-S284.
Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2020 Executive Summary. Endocr Pract. Jan 2020;26(1):107-139. doi:10.4158/CS-2019-0472
Garvey WT. Phentermine and topiramate extended-release: a new treatment for obesity and its role in a complications-centric approach to obesity medical management. Expert Opin Drug Saf. 2013;12(5):741-56.
Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, . . . Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. The American journal of clinical nutrition. Feb 2012;95(2):297-308.
Gerstein HC, Pais P, Pogue J, et al. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999;33:612-9.
Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr., . . . Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. The New England journal of medicine. Mar 3 2011;364(9):818-828.
Gerstein HC, Coleman RL, Scott CAB, et al. Impact of Acarbose on Incident Diabetes and Regression to Normoglycemia in People With Coronary Heart Disease and Impaired Glucose Tolerance: Insights From the ACE Trial. Diabetes Care . Sep 2020;43(9):2242-2247. doi:10.2337/dc19-2046
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058-1070.
Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. Journal of neurology. Mar 2004;251(3):261-268.
Giri B, Dey S, Das T, et al. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother. 2018;107:306-328. https://pubmed.ncbi.nlm.nih.gov/30098549/
Glossmann HH, Lutz OMD. Metformin and Aging: A Review. Gerontology. 2019;65(6):581-590. doi:10.1159/000502257
Godsland IF. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin Sci (Lond). 2009;118(Pt 5):315-332.
Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol. 2011;2:69.
Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, . . . Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation. Nov 15 1996;94(10):2402-2409.
Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Annals of internal medicine. Mar 16 2010;152(6):346-357.
Gold Standard. Drug Monograph. Acarbose. www.clinicalkey.com. Copyright 2015a Elsevier, Inc. Accessed 8/12/2015.
Gold Standard. Drug Monograph. Lorcaserin. Copyright 2015b Elsevier, Inc. www.clinicalkey.com. Accessed 7/17/2015.
Gold Standard. Drug Monograph. Metformin. Copyright 2015c Elsevier, Inc. www.clinicalkey.com. Accessed 7/16/2015.
Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014;6:80.
Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, . . . Sinclair DA. Declining NAD(+) Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell. 2013;155(7):1624-1638.
Gonzalez-Reyes RE, Aliev G, Avila-Rodrigues M, Barreto GE. Alterations in Glucose Metabolism on Cognition: A Possible Link Between Diabetes and Dementia. Current pharmaceutical design. 2016;22(7):812-818.
Gossens GH. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 2017;10(3):207-215. https://pubmed.ncbi.nlm.nih.gov/28564650/
Gotsis E, Anagnostis P, Mariolis A, Vlachou A, Katsiki N, Karagiannis A. Health benefits of the Mediterranean Diet: an update of research over the last 5 years. Angiology. Apr 2015;66(4):304-318.
Gower BA, Goss AM. A lower-carbohydrate, higher-fat diet reduces abdominal and intermuscular fat and increases insulin sensitivity in adults at risk of type 2 diabetes. The Journal of nutrition. Jan 2015;145(1):177s-183s.
Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94:1187-1195.
Greb A, Bitsch R. Comparative bioavailability of various thiamine derivatives after oral administration. International journal of clinical pharmacology and therapeutics. Apr 1998;36(4):216-221.
Greer EL, Dowlatshahi D, Banko MR, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17(19):1646-56.
Gregg EW. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35(6):1252-1257.
Gu S, Shi J, Tang Z, et al. Comparison of glucose lowering effect of metformin and acarbose in type 2 diabetes mellitus: a meta-analysis. PLoS One. 2015;10(5):e0126704.
Guerrero-Romero F, Rodriguez-Moran M. [Oral magnesium supplementation: an adjuvant alternative to facing the worldwide challenge of type 2 diabetes?] Cir Cir. 2014;82(3):282-9.
Gums JG. Magnesium in cardiovascular and other disorders. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. Aug 1 2004;61(15):1569-1576.
Guo J, Zhao X. [Free-radical theory and the antisenescence effect of fruits and vegetables]. Wei sheng yan jiu = Journal of hygiene research. May 30 1999;28(3):190-192.
Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60(6):910-20.
Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. The lancet. Diabetes & endocrinology. Jan 2015;3(1):17-26.
Haidari F, Heybar H, Jalali MT, Ahmadi Engali K, Helli B, Shirbeigi E. Hesperidin supplementation modulates inflammatory responses following myocardial infarction. Journal of the American College of Nutrition.2015;34(3):205-211.
Haidari F, Mohammadshahi M, Zarei M, Haghighizadeh MH, Mirzaee F. The Effect of Pyridoxine Hydrochloride Supplementation on Leptin, Adiponectin, Glycemic Indices, and Anthropometric Indices in Obese and Overweight Women. Clin Nutr Res. Jul 2021;10(3):230-242. doi:10.7762/cnr.2021.10.3.230
Halabi A, Sen J, Huynh Q, Marwick TH. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol. Aug 5 2020;19(1):124. doi:10.1186/s12933-020-01100-w
Halmos T, Suba I. [Alzheimer's disease and diabetes - the common pathogenesis]. Neuropsychopharmacologia Hungarica: a Magyar Pszichofarmakologiai Egyesulet lapja = official journal of the Hungarian Association of Psychopharmacology. Mar 2016;18(1):5-19.
Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Current diabetes reviews. Nov 2006;2(4):367-373.
Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes care. May 2009;32(5):810-812.
Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevens experiemental diabetic retinopathy. Nat Med. 2003;9(3):294-9.
Handa K, Inukai K, Onuma H, Kudo A, Nakagawa F, Tsugawa K, . . . Ishida H. Long-term low carbohydrate diet leads to deleterious metabolic manifestations in diabetic mice. PloS one. 2014;9(8):e104948.
Hanefeld M. The role of acarbose in the treatment of non-insulin-dependent diabetes mellitus. Journal of diabetes and its complications. Jul-Aug 1998;12(4):228-237.
Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: a meta-analysis of seven long-term studies. Eur Heart J. 2004;25(1):10-6.
Hansawasdi C, Kawabata J. Alpha-glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia. Dec 2006;77(7-8):568-573.
Hanssen NM, Beulens JW, van Dieren S, Scheijen JL, van der AD, Spijkerman AM, . . . Schalkwijk CG. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: a case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes. Jan 2015;64(1):257-265.
Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62(7):2164-2172.
Hargrove JL, Greenspan P, Hartle DK, et al. Inhibition of aromatase and alpha-amylase by flavonoids and proanthocyanidins from Sorghum bicolor bran extracts. J Med Food. 2011;14(7-8):799-807.
Hata A, Doi Y, Ninomiya T, Mukai N, Hirakawa Y, Hata J, . . . Kiyohara Y. Magnesium intake decreases Type 2 diabetes risk through the improvement of insulin resistance and inflammation: the Hisayama Study. Diabetic medicine: a journal of the British Diabetic Association. Dec 2013;30(12):1487-1494.
Haupt E, Ledermann H, Kopcke W. Benfotiamine in the treatment of diabetic polyneuropathy--a three-week randomized, controlled pilot study (BEDIP study). International journal of clinical pharmacology and therapeutics. Feb 2005;43(2):71-77.
Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol Nutr Food Res. 2015;59:147-159.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. The New England journal of medicine . Oct 8 2020;383(15):1436-1446. doi:10.1056/NEJMoa2024816
Heinemann L. The failure of exubera: are we beating a dead horse? Journal of diabetes science and technology. May 2008;2(3):518-529.
Heinemann L, Benesch C, DeVries JH. AP@home: The Artificial Pancreas Is Now at Home. J Diabetes Sci Technol. Feb 16 2016.
Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin Pump Risks and Benefits: A Clinical Appraisal of Pump Safety Standards, Adverse Event Reporting, and Research Needs A Joint Statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes care. 2015;38(4):716-722.
Hemmerle H, Burger HJ, Below P, Schubert G, Rippel R, Schindler PW, . . . Herling AW. Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. Journal of medicinal chemistry. Jan 17 1997;40(2):137-145.
Henry RR, Mudaliar SR, Howland WC 3rd, et al. Inhaled insulin using the AERx Insulin Diabetes Management System in healthy and asthmatic subjects. Diabetes Care. 2003;26(3):754-9.
Henry-Vitrac C, Ibarra A, Roller M, Merillon JM, Vitrac X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. Journal of agricultural and food chemistry. Apr 14 2010;58(7):4141-4144.
Hidalgo J, Flores C, Hidalgo MA, Perez M, Yanez A, Quinones L, . . . Burgos RA. Delphinol(R) standardized maqui berry extract reduces postprandial blood glucose increase in individuals with impaired glucose regulation by novel mechanism of sodium glucose cotransporter inhibition. Panminerva medica.Jun 2014;56(2 Suppl 3):1-7.
Higdon J, Drake V, Liu S. Linus Pauling Institute. Micronutrient Information Center. Glycemic Index and Glycemic Load. Data on file.
Higdon J, Drake V, Angelo G. Linus Pauling Insitute. Micronutrient Information Center. Essential Fatty Acids. Data on file.
Higdon J, Drake V, Hagen TM. Linus Pauling Institute. Micronutrient Information Center. Lipoic Acid. Data on file.
Higuchi O, Nakagawa K, Tsuzuki T, et al. Aminophospholipid glycation and its inhibitor screening system: a new role of pyridoxal 5’-phosphate as the inhibitor. J Lipid Res. 2006;47(5):964-74.
Hipkiss AR. Glycation, ageing and carnosine: are carnivorous diets beneficial? Mech Ageing Dev. 2005;126:1034-1039.
Hiraga T, Myoui A, Hashimoto N, Sasaki A, Hata K, Morita Y, . . . Yoneda T. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer research. Aug 15 2012;72(16):4238-4249.
Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocrine reviews. Aug 2013;34(4):463-500.
Hodgson JM, Watts GF, Playford DA, et al. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56(11):1137-42.
Hodish I. Insulin therapy, weight gain and prognosis. Diabetes, Obesity and Metabolism . 2018;20(9):2085-2092.
Hoehn AN, Stockert AL. The Effects of Cinnamomum Cassia on Blood Glucose Values are Greater than those of Dietary Changes Alone. Nutr Metab Insights. 2012;5:77-83.
Hoggard N, Cruickshank M, Moar, K, et al. A single supplement of a standardized bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by lifestyle. J Nutr Sci. 2013;2:e22.
Hokayem M, Blond E, Vidal H, Lambert K, Meugnier E, Feillet-Coudray C, . . . Avignon A. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes care. Jun 2013;36(6):1454-1461.
Homayouni F, Haidari F, Hedayati M, Zakerkish M, Ahmadi K. Hesperidin Supplementation Alleviates Oxidative DNA Damage and Lipid Peroxidation in Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytotherapy research: PTR.Aug 14 2017.
Horii T, Oikawa Y, Kunisada N, Shimada A, Atsuda K. Real-world risk of hypoglycemia-related hospitalization in Japanese patients with type 2 diabetes using SGLT2 inhibitors: a nationwide cohort study. BMJ Open Diabetes Res Care . Nov 2020;8(2):e001856. doi:10.1136/bmjdrc-2020-001856
Horikawa C, Aida R, Kamada C, et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: analysis of data from the Japan Diabetes Complications Study (JDCS). European journal of nutrition. Jun 2020;59(4):1585-1594. doi:10.1007/s00394-019-02014-4
Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regulatory toxicology and pharmacology: RTP. Feb 2007;47(1):19-28.
Hou G, Zhang S, Zhang X, et al. Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Treat. 2013;137:807-816.
Hruby A, Meigs JB, O’Donnel CJ, et al. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care. 2014;37(2):419-27.
Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes, obesity & metabolism. Apr 2017;19(4):524-536. doi:10.1111/dom.12849
Huang L, Zhang F, Xu P, et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Outcomes in Patients with Diabetes: A Meta-analysis of Randomized Controlled Trials. Adv Nutr. Jul 2023;14(4):629-636. doi:10.1016/j.advnut.2023.04.009. https://pubmed.ncbi.nlm.nih.gov/35361440/
Huang, Y, Cai X, Qiu M, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57(11):2261-9.
Huang CC, Weng SF, Tsai KT, Chen PJ, Lin HJ, Wang JJ, . . . Hsu CC. Long-term Mortality Risk After Hyperglycemic Crisis Episodes in Geriatric Patients With Diabetes: A National Population-Based Cohort Study. Diabetes care. May 2015;38(5):746-751.
Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K, . . . Bierhaus A. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Molecular medicine (Cambridge, Mass.). May-Jun 2008;14(5-6):301-308.
Hung CT. Effects of high-fructose (90%) corn syrup on plasma glucose, insulin, and C-peptide in non-insulin-dependent diabetes mellitus and normal subjects. Taiwan yi xue hui za zhi. Journal of the Formosan Medical Association. Sep 1989;88(9):883-885.
Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313(13):1325-35.
Hur KY, Lee MS. Gut Microbiota and Metabolic Disorders. Diabetes Metab J. Jun 2015;39(3):198-203.
Huyen VTT, Phan DV, Thang P, et al. Antidiabetic effects of add-on Gynostemma pentaphyllum extract therapy with sulfonylureas in type 2 diabetic patients. Evid Based Complement Alternat Med. 2012;2012:452313.
Huyen VTT, Phan DV, Thang P, et al. Gynostemma pentaphyllum tea improves insulin sensitivity in type 2 diabetic patients. J Nutr Metab. 2013;2013:765383.
Hyassat D, Al Sitri E, Batieha A, et al. Prevalence of hypomagnesaemia among obese type 2 diabetic patients attending the National Center for Diabetes, Endocrinology and Genetics (NCDEG). Int J Endocrinol Metab. 2014;12(3):e17796.
Ibrahimpasic K. Alpha lipoic acid and glycaemic control in diabetic neuropathies at type 2 diabetes treatment. Med Arch. 2013;67(1):7-9.
IDF. International Diabetes Federation. Diabetes Misconceptions. Available at: http://www.idf.org/worlddiabetesday/toolkit/gp/diabetes-misconceptions. Last updated: 2014. Accessed 4/15/2015.
Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle Intervention and Medical Management With vs Without Roux-en-Y Gastric Bypass and Control of Hemoglobin A1c, LDL Cholesterol, and Systolic Blood Pressure at 5 Years in the Diabetes Surgery Study. Jama. Jan 16 2018;319(3):266-278. doi:10.1001/jama.2017.20813
Ilyas S, Al-Refai R, Maharjan R, Diaz Bustamante L, Ghattas KN, Khan S. Bariatric Surgery and Type 2 Diabetes Mellitus: Assessing Factors Leading to Remission. A Systematic Review. Cureus. Aug 23 2020;12(8):e9973. doi:10.7759/cureus.9973
Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Frontiers in bioscience (Landmark edition). 2009;14:2983-2995.
Indulekha K, Anjana RM, Surendar J, Mohan V. Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113). Clin Biochem. Mar 2011;44(4):281-287.
Innes KE, Vincent HK, Taylor AG. Chronic stress and insulin resistance-related indices of cardiovascular death risk, part I: neurophysiological responses and pathological sequelae. Altern Ther Health Med. 2007 Jul-Aug;13(4):46-52. https://pubmed.ncbi.nlm.nih.gov/17658122/
Inzucchi SE, Sherman RS, Goldman L (ed.), SchaferAI (ed.). Goldman's Cecil Medicine, Twenty-Fourth Edition. Chapter 236: Type 1 Diabetes Mellitus; 1475-1488. Copyright 2012a Saunders, an imprint of Elsevier, Inc. Available at: www.clinicalkey.com Accessed: 3/19/2015.
Inzucchi SE, Sherman RS, Goldman L (ed.), SchaferAI (ed.). Goldman's Cecil Medicine, Twenty-Fourth Edition. Chapter 237: Type 2 Diabetes Mellitus; 1489-1499. Copyright 2012b Saunders, an imprint of Elsevier, Inc. Available at: www.clinicalkey.com Accessed: 3/19/2015.
Inzucchi SE. Diagnosis of Diabetes. New England Journal of Medicine. 2012c;367(6):542-550.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, . . . Matthews DR. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). 2012d;35(6):1364-1379.
Inzucchi SE, Docherty KF, Kober L, et al. Dapagliflozin and the Incidence of Type 2 Diabetes in Patients With Heart Failure and Reduced Ejection Fraction: An Exploratory Analysis From DAPA-HF. Diabetes Care. Feb 2021;44(2):586-594. doi:10.2337/dc20-1675
Ishikawa A, Yamashita H, Hiemori M, Inagaki E, Kimoto M, Okamoto M, . . . Natori Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J Nutr Sci Vitaminol (Tokyo). Apr 2007;53(2):166-173.
Ito T, Schaffer SW, Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids. May 2012;42(5):1529-39. doi:10.1007/s00726-011-0883-5
Jain R, Kabadi U, Kabadi M. Is beta-cell failure in type 2 diabetes mellitus reversible? International journal of diabetes in developing countries. Jan 2008;28(1):1-5.
Jakus V, Sandorova E, Kalninova J, et al. Monitoring of glycation, oxidative stress and inflammation in relation to the occurrence of vascular complications in patients with type 2 diabetes mellitus. Physiol Res. 2014;63:297-309.
Jang HJ, Ridgeway SD, Kim JA. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am J Physiol Endocrinol Metab. 2013;305(12):E1444-51.
Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, . . . Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab. Aug 2006;91(8):2986-2993.
Janssen J. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int J Mol Sci. 2021;22(15):7797. https://pubmed.ncbi.nlm.nih.gov/34360563/
Jayaram S, Hariharan RS, Madhavan R, Periyandavar I, Samra SS. A prospective, parallel group, open-labeled, comparative, multi-centric, active controlled study to evaluate the safety, tolerability and benefits of fixed dose combination of acarbose and metformin versus metformin alone in type 2 diabetes. The Journal of the Association of Physicians of India. Nov 2010;58:679-682, 687.
JDC. Joslin Diabetes Center. Healthy Eating: Why Does Fat Increase Blood Glucose? http://blog.joslin.org/2011/09/why-does-fat-increase-blood-glucose/. 9/27/2011. Accessed 6/7/2016
Jellinger PS. Focus on incretin-based therapies: targeting the core defects of type 2 diabetes. Postgrad Med. 2011;123(1):53-65.
Jia S, Hu Y, Zhang W, Zhao X, Chen Y, Sun C, . . . Chen K. Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-A(y) mice. Food Funct.Mar 2015;6(3):878-886.
Jiang M, Liu Q, Jiang T, Nizigiyimana P, Lei M. Adding Sodium-Glucose Co-Transporter 2 Inhibitors to Sulfonylureas and Risk of Hypoglycemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Frontiers in endocrinology . 2021;12:713192. doi:10.3389/fendo.2021.713192
Johnson AB, Webster JM, Sum CF, Heseltine L, Argyraki M, Cooper BG, Taylor R. The impact of metformin therapy on hepatic glucose production and skeletal muscle glycogen synthase activity in overweight type II diabetic patients. Metabolism: clinical and experimental. Sep 1993;42(9):1217-1222.
Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. The American journal of clinical nutrition. Oct 2003;78(4):728-733.
Jonsson T, Granfeldt Y, Ahren B, Branell UC, Palsson G, Hansson A, . . . Lindeberg S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovascular diabetology. 2009;8:35.
Jordan J, Astrup A, Engeli S, Narkiewicz K, Day WW, Finer N. Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity. Journal of hypertension. Jun 2014;32(6):1178-1188.
Jorgensen L, Jenssen T, Joakimsen O, et al. Glycated hemoglobin level is strongly related to the prevalence of carotid artery plaques with high echogenicity in nondiabetic individuals: the Tromso study. Circulation. 2004;110:466-470.
Joseph JI. Review of the Long-Term Implantable Senseonics Continuous Glucose Monitoring System and Other Continuous Glucose Monitoring Systems. J Diabetes Sci Technol. Jan 2021;15(1):167-173. doi:10.1177/1932296820911919.
Joshi SR, Ramachandran A, Chadha M, et al. Acarbose plus metformin fixed-dose combination in the management of type 2 diabetes. Expert Opin Pharmacother. 2014;15(11):1611-1620.
Josse RG, McGuire AJ, Saal GB. A review of the economic evidence for acarbose in the prevention of diabetes and cardiovascular events in individuals with impaired glucose tolerance. Int J Clin Pract 2006;60(7):847-855.
Juanola-Falgarona M, Salas-Salvado J, Ibarrola-Jurado N, Rabassa-Soler A, Diaz-Lopez A, Guasch-Ferre M, . . . Bullo M. Effect of the glycemic index of the diet on weight loss, modulation of satiety, inflammation, and other metabolic risk factors: a randomized controlled trial. The American journal of clinical nutrition. Jul 2014;100(1):27-35.
Jung CH, Ahn J, Jeon TI, Kim TW, Ha TY. Syzygium aromaticum ethanol extract reduces high-fat diet-induced obesity in mice through downregulation of adipogenic and lipogenic gene expression. Experimental and therapeutic medicine. Sep 2012;4(3):409-414.
Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. Novartis Foundation symposium. 2004;262:247-260; discussion 260-268.
Kaats GR, Keith SC, Keith PL, et al. A combination of l-arabinose and chromium lowers circulating glucose and insulin levels after an acute oral sucrose challenge. Nutr J. 2011;10:42.
Kabagambe EK, Judd SE, Howard VJ, Zakai NA, Jenny NS, Hsieh M, . . . Cushman M. Inflammation biomarkers and risk of all-cause mortality in the Reasons for Geographic And Racial Differences in Stroke cohort. American journal of epidemiology. Aug 1 2011;174(3):284-292.
Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. Oct 2012;55(10):2622-2630.
Kajbaf F, De Broe ME, Lalau JD. Therapeutic Concentrations of Metformin: A Systematic Review. Clinical pharmacokinetics. Apr 2016;55(4):439-459.
Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am. 2013;42(2):333-347.
Kanie T, Mizuno A, Takaoka Y, et al. Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis. The Cochrane database of systematic reviews . Oct 25 2021;10:CD013650. doi:10.1002/14651858.CD013650.pub2
Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabetic medicine: a journal of the British Diabetic Association. May 2009;26(5):526-531.
Karbowska J, Kochan Z. Effects of DHEA on metabolic and endocrine functions of adipose tissue. Hormone molecular biology and clinical investigation. Aug 2013;14(2):65-74.
Karley AJ, Ashford DA, Minto LM, Pritchard J, Douglas AE. The significance of gut sucrase activity for osmoregulation in the pea aphid, Acyrthosiphon pisum. Journal of insect physiology. Dec 2005;51(12):1313-1319.
Karlstad O, Starup Linde J, Vestergaard P, et al. Use of insulin and insulin analogs and risk of cancer—systematic review and meta-analysis of observational studies. Current drug safety. 2013;8(5):333-348.
Karoff P. Harvard Paulson School Communications. In: Harvard gazette [online]. Artificial pancreas system aimed at type 1 diabetes mellitus. http://news.harvard.edu/gazette/story/2016/01/artificial-pancreas-system-aimed-at-type-1-diabetes-mellitus/. 1/4/2016. Accessed 3/30/2016.
Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Annals of translational medicine. Jun 2014;2(6):57. doi:10.3978/j.issn.2305-5839.2014.06.01
Kato M, Noda M, Suga H, et al. Fasting plasma glucose and incidence of diabetes – implication for the threshold for impaired fasting glucose: results from the population-based Omiya MA Cohort study. J Atheroscler Thromb. 2009;16:857-861.
Kaur J. A comprehensive review on metabolic syndrome. Cardiology research and practice. 2014;2014:943162.
Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. The Journal of nutrition. Sep 2009;139(9):1806S-1812S.
Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375-C391. https://pubmed.ncbi.nlm.nih.gov/33356944/
Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, . . . Ogawa H. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. Jul 2003;88(7):3190-3195.
Kelly CT, Mansoor J, Dohm GL, Chapman WH, 3rd, Pender JRt, Pories WJ. Hyperinsulinemic syndrome: the metabolic syndrome is broader than you think. Surgery. Aug 2014;156(2):405-411.
Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, . . . Suomalainen A. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO molecular medicine. Jun 2014;6(6):721-731.
Khanal RC, Howard LR, Wilkes SE, Rogers TJ, Prior RL. Effect of dietary blueberry pomace on selected metabolic factors associated with high fructose feeding in growing Sprague-Dawley rats. Journal of medicinal food. Sep 2012;15(9):802-810.
Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470-512.
Kim B, Backus C, Oh S, et al. Hyperglycemia-induced tau cleavage in vitro and in vivo: a possible link between diabetes and Alzheimer’s disease. J Alzheimers Dis. 2013;34(3):727-39.
Kim J, Park Y. Antidiabetic effect of sorghum extract on hepatic gluconeogenesis of streptozotocin-induced diabetic rats. Nutr Metab. 2012;9:106.
Kim J, Kim CS, Lee YM, Sohn E, Jo K, Kim JS. Vaccinium myrtillus extract prevents or delays the onset of diabetes--induced blood-retinal barrier breakdown. International journal of food sciences and nutrition. Mar 2015;66(2):236-242.
Kim JY, Ok HM, Kim J, Park SW, Kwon SW, Kwon O. Mulberry leaf extract improves postprandial glucose response in prediabetic subjects: a randomized, double-blind placebo-controlled trial. Journal of medicinal food. Mar 2015;18(3):306-313.
Kim HJ, Oh GS, Shen A, Lee SB, Khadka D, Pandit A, . . . So HS. Nicotinamide adenine dinucleotide: An essential factor in preserving hearing in cisplatin-induced ototoxicity. Hear Res. Aug 2015;326:30-39.
Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, . . . Miyazawa T. Food-Grade Mulberry Powder Enriched with 1-Deoxynojirimycin Suppresses the Elevation of Postprandial Blood Glucose in Humans. Journal of agricultural and food chemistry. 2007;55(14):5869-5874.
King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E. Glycogen synthase kinase-3 inhibitors: Rescuers of cognitive impairments. Pharmacology & therapeutics. Jan 2014;141(1):1-12.
Kinne RK, Castaneda F. SGLT inhibitors as new therapeutic tools in the treatment of diabetes. Handbook of experimental pharmacology. 2011(203):105-126.
Kiraly O, Gong G, Olipitz W, et al. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015;11(2):e1004901.
Kishore P. Merck Manual. Professional Version. Diabetes Mellitus (DM). Available at: http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Last update: 6/2014. Accessed 4/2/2015.
Kjaer M, Secher NH, Galbo H. Physical stress and catecholamine release. Bailliere's clinical endocrinology and metabolism. May 1987;1(2):279-298.
Klonoff DC. The Beneficial Effects of a Paleolithic Diet on Type 2 Diabetes and Other Risk Factors for Cardiovascular Disease. Journal of Diabetes Science and Technology. 2009;3(6):1229-1232.
Knapen LM, van Dalem J, Keulemans YC, van Erp NP, Bazelier MT, De Bruin ML, . . . Driessen JH. Use of incretin agents and risk of pancreatic cancer: a population-based cohort study. Diabetes, obesity & metabolism. Mar 2016;18(3):258-265.
Kocsis T, Molnár B, Németh D, et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Scientific reports.2020;10(1):11787.
Koehler KM, Baumgartner RN, Garry PJ, Allen RH, Stabler SP, Rimm EB. Association of folate intake and serum homocysteine in elderly persons according to vitamin supplementation and alcohol use. The American journal of clinical nutrition. 2001;73(3):628-637.
Koh ES, Han K, Nam YS, et al. Renal outcomes and all-cause death associated with sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL 3 Korea). Diabetes, obesity & metabolism . Feb 2021;23(2):455-466. doi:10.1111/dom.14239
Kolahdouz Mohammadi R, Hosseinzadeh-Attar MJ, Eshraghian MR, et al. The effect of coenzyme Q10 supplementation on metabolic status of type 2 diabetic patients. Minerva Gastroenterol Dietol. 2013;59(2):231-6.
Kolehmainen M, Mykkanen O, Kirjavainen PV, et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol Nutr Food Res. 2012;56(10):1501-10.
Koloverou E, Esposito K, Giugliano D, et al. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism Clinical and Experimental. 2014;63:903-911.
Kolte D, Vijayaraghavan K, Khera S, et al. Role of magnesium in cardiovascular diseases. Cardiol Rev. 2014;22(4):182-92.
Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, . . . Doyle FJ, 3rd. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes care. Jul 2014;37(7):1789-1796.
Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Current Opinion in Lipidology. 2008;19:235-241.
Krog-Mikkelsen I, Hels O, Tetens I, et al. The effects of L-arabinose on intestinal sucrose activity: dose-response studies in vitro and in humans. Am J Clin Nutr. 2011;94(2):472-478.
Kromhout D, Geleijnse JM, de Goede J, Oude Griep LM, Mulder BJ, de Boer MJ, . . . Giltay EJ. n-3 fatty acids, ventricular arrhythmia-related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes care. Dec 2011;34(12):2515-2520.
Kudolo GB, Wang W, Elrod R, et al. Short-term ingestion of Ginkgo biloba extract does not alter whole body insulin sensitivity in non-diabetic, pre-diabetic or type 2 diabetic subjects—a randomized double-blind-controlled crossover study. Clin Nutr. 2006;25(1):123-134. https://pubmed.ncbi.nlm.nih.gov/16293352/
Kumar B, Gupta SK, Nag TC, et al. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 2012;47(2):103-8.
Kuroda M, Mimaki Y, Ohtomo T, Yamada J, Nishiyama T, Mae T, . . . Kawada T. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. Journal of natural medicines. Apr 2012;66(2):394-399.
Kutbi EH, Sohouli MH, Fatahi S, et al. The beneficial effects of cinnamon among patients with metabolic diseases: A systematic review and dose-response meta-analysis of randomized-controlled trials. Critical Reviews in Food Science and Nutrition. 2022;62(22):6113-6131. doi:10.1080/10408398.2021.1896473. https://doi.org/10.1080/10408398.2021.1896473
Lagger G, Chambouleyron M, Correia JC, Sittarame F, Miganne G, Lasserre Moutet A, Golay A. [The cure of type 2 diabetes and patient education]. Revue medicale suisse. Mar 25 2015;11(467):715-716, 718-719.
Lai YJ, Hu HY, Chen HH, Chou P. Dipeptidyl Peptidase-4 Inhibitors and the Risk of Acute Pancreatitis in Patients With Type 2 Diabetes in Taiwan: A Population-Based Cohort Study. Medicine. Oct 2015;94(43):e1906.
Lamantia V, Bissonnette S, Beaudry M, et al. EPA and DHA inhibit LDL-induced upregulation of human adipose tissue NLRP3 inflammasome/IL-1beta pathway and its association with diabetes risk factors. Sci Rep. Nov 7 2024;14(1):27146. doi:10.1038/s41598-024-73672-6. https://pubmed.ncbi.nlm.nih.gov/39511203/
LaMoia TE, Shulman GI. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev. Jan 28 2021;42(1):77-96. doi:10.1210/endrev/bnaa023
Langsjoen PH, Langsjoen AM. Supplemental ubiquinol in patients with advanced congestive heart failure. BioFactors (Oxford, England). 2008;32(1-4):119-128.
La Sala L, Pontiroli AE. Prevention of Diabetes and Cardiovascular Disease in Obesity. International journal of molecular sciences. Oct 31 2020;21(21)doi:10.3390/ijms21218178
Lau E, Belda E, Picq P, et al. Gut microbiota changes after metabolic surgery in adult diabetic patients with mild obesity: a randomised controlled trial. Diabetol Metab Syndr. May 21 2021;13(1):56. doi:10.1186/s13098-021-00672-1
Lee Y, Berglund ED, XinXin Y, et al. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc Natl Acad Sci USA. 2014;111(36):13217-13222.
Lee SM. The effect of chronic alpha-glycosidase inhibition on diabetic nephropathy in the db/db mouse. Diabetes. Mar 1982;31(3):249-254.
Lee TC, Ivester P, Hester AG, Sergeant S, Case LD, Morgan T, . . . Chilton FH. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids in health and disease. 2014;13:196.
Lee HJ, Hong YS, Jun W, Yang SJ. Nicotinamide Riboside Ameliorates Hepatic Metaflammation by Modulating NLRP3 Inflammasome in a Rodent Model of Type 2 Diabetes. Journal of medicinal food. May 14 2015.
Lee SH, Min KJ. Caloric restriction and its mimetics. BMB reports. Apr 2013;46(4):181-187.
Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, . . . Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochemical and biophysical research communications. Feb 3 2006;340(1):291-295.
Lee M, Sun J, Han M, et al. Nationwide Trends in Pancreatitis and Pancreatic Cancer Risk Among Patients With Newly Diagnosed Type 2 Diabetes Receiving Dipeptidyl Peptidase 4 Inhibitors. Diabetes Care. Nov 2019;42(11):2057-2064. doi:10.2337/dc18-2195
Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, . . . Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. Mar 2009;203(1):206-213.
Lehrke M, Broedl UC, Biller-Friedmann IM, et al. Serum concentrations of cortisol, interleukin 6, leptin and adiponectin predict stress induced insulin resistance in acute inflammatory reactions. Critical Care 2008;12:R157.
Levitan EB, Song Y, Ford ES, et al. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147-55.
Lexicomp. Drug information monographs. UpToDate: https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information. Accessed Jan. 6, 2022.
Li C, Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Critical reviews in food science and nutrition. Feb 11 2017;57(3):613-631.
Li S, Flint A, Pai JK, Forman JP, Hu FB, Willett WC, . . . Rimm EB. Low carbohydrate diet from plant or animal sources and mortality among myocardial infarction survivors. J Am Heart Assoc. Oct 2014;3(5):e001169.
Li L, Shen J, Bala MM, Busse JW, Ebrahim S, Vandvik PO, . . . Sun X. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ (Clinical research ed.). 2014;348:g2366.
Li X, Song D, Leng SX. Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin Interv Aging. 2015;10:549-560.
Lilly. Medicines in Development. Updated 1/31/2022. Accessed 2/7/2022, https://www.lilly.com/discovery/clinical-development-pipeline
Lilly. News Release: Lily Reports Robust Third-Quarter 2021 Financial Results as Pipeline Success Strengthens Future Growth Potential. Updated 10/26/2021a. Accessed 2/7/2022. https://investor.lilly.com/news-releases/news-release-details/lilly-reports-robust-third-quarter-2021-financial-results
Lilly. News Release: Tirzepatide significantly reduced A1C and body weight in people with type 2 diabetes in two phase 3 trials from Lilly's SURPASS program. Updated 2/17/2021. Accessed 2/10/2022, https://investor.lilly.com/news-releases/news-release-details/tirzepatide-significantly-reduced-a1c-and-body-weight-people
Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. Oct 2011;54(10):2506-2514.
Lin H-J, Lee B-C, Ho Y-L, et al. Postprandial glucose improves the risk prediction of cardiovascular death beyond the metabolic syndrome in the nondiabetic population. Diabetes Care. 2009;32:1721-1726.
Lindberg S, Skov Jensen J, Bjerre M, et al. Adiponectin, type 2 diabetes and cardiovascular risk. Eur J Prev Cardiol. 2015;22(3):276-283.
Lips P, Eekhoff M, van Schoor N, et al. Vitamin D and type 2 diabetes. J Steroid Biochem Mol Biol. Oct 2017;173:280-285. https://www.ncbi.nlm.nih.gov/pubmed/27932304
Lira Neto JCG, Damasceno MMC, Ciol MA, et al. Efficacy of Cinnamon as an Adjuvant in Reducing the Glycemic Biomarkers of Type 2 Diabetes Mellitus: A Three-Month, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Journal of the American Nutrition Association. 2022;41(3):266-274. doi:10.1080/07315724.2021.1878967. https://doi.org/10.1080/07315724.2021.1878967
Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37(1):31-7.
Liu XY, Zhang N, Chen R, Zhao JG, Yu P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. Journal of diabetes and its complications . Nov-Dec 2015;29(8):1295-303.
Liu J, Zhang L, Ren Y, Gao Y, Kang L, Qiao Q. Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor-bearing mice. International journal of biological macromolecules. Aug 2014;69:1-4.
Liu HW, Chan YC, Wang MF, Wei CC, Chang SJ. Dietary (-)-epigallocatechin-3-gallate supplementation counteracts aging-associated skeletal muscle insulin resistance and fatty liver in senescence-accelerated mouse. Journal of agricultural and food chemistry. Jul 8 2015.
Liu CY, Huang CJ, Huang LH, Chen IJ, Chiu JP, Hsu CH. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial. PloS one. 2014;9(3):e91163.
Liu J, Gao F, Ji B, et al. Anthocyanins-rich extract of wild Chinese blueberry protects glucolipotoxicity-induced INS832/13 beta-cell against dysfunction and death. J Food Sci Technol. 2015;52(5):3022-9.
Lobo V, Patil A, Phatik A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118-126.
Lontchi-Yimagou E, Sobngwi E, Matsha TE, et al. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435-444.
Lopez-Alarcon M, Perichart-Perera O, Flores-Huerta S, et al. Excessive refines carbohydrates and scarce micronutrients intakes increase inflammatory mediators nd insulin resistance in prepubertal and pubertal obese children independently of obesity. Mediators Inflamm. 2014;2014:849031.
Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP. The alpha-amylase and alpha-glucosidase inhibitory effects of Irish seaweed extracts. Food chemistry. Dec 1 2013;141(3):2170-2176.
Lu HF, Chen YS, Yang JS, et al. Gypenosides induced G0/G1 arrest via inhibition of cyclin E and induction of apoptosis via activation of caspases-3 and -9 in human lung cancer A-549 cells. In Vivo. 2008;22(2):215-21.
Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. Aug 14 2021;398(10300):583-598. doi:10.1016/s0140-6736(21)01443-4
Luevano-Contreras C, Garay-Sevilla ME, Wrobel K, et al. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2013;52(1):22-26.
Madonna R, De Caterina R. Atherogenesis and diabetes: focus on insulin resistance and hyperinsulinemia. Rev Esp Cardiol. 2012;65(4):309-313.
Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nature reviews Endocrinology . Oct 2020;16(10):545-555. doi:10.1038/s41574-020-0381-5
Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. The Journal of clinical investigation. Oct 1992;90(4):1323-1327.
Mah E, Noh SK, Ballard KD, et al. Supplementation of a gamma-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J Nutr Biochem. 2013;24:196-203.
Mah E, Pei R, Guo Y, et al. Gamma-tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic Biol Med. 2013;65:1291-9.
Mahajan R, Gupta K. Revisiting Metformin: Annual Vitamin B12 Supplementation may become Mandatory with Long-Term Metformin Use. Journal of young pharmacists: JYP. Oct 2010;2(4):428-429.
Maiorino MI, Chiodini P, Bellastella G, et al. Free and fixed-ratio combinations of basal insulin and GLP-1 receptor agonists versus basal insulin intensification in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes, obesity & metabolism . Sep 2018;20(9):2309-2313. doi:10.1111/dom.13343
Malekshahi Moghadam A, Saedisomeolia A, Djalali M, Djazayery A, Pooya S, Sojoudi F. Efficacy of omega-3 fatty acid supplementation on serum levels of tumour necrosis factor-alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singapore medical journal. Sep 2012;53(9):615-619.
Malik FS, Taplin CE. Insulin therapy in children and adolescents with type 1 diabetes. Paediatric drugs. Apr 2014;16(2):141-150.
Malin SK, Kashyap SR. Effects of various gastrointestinal procedures on β-cell function in obesity and type 2 diabetes. Surg Obes Relat Dis . Jul 2016;12(6):1213-9. doi:10.1016/j.soard.2016.02.035
Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review. Nutrition (Burbank, Los Angeles County, Calif). Jul-Aug 2016;32(7-8):732-9. doi:10.1016/j.nut.2016.01.011. https://www.ncbi.nlm.nih.gov/pubmed/27133809
Maqbool M, Mobashir M, Hoda N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer's disease. European journal of medicinal chemistry. Jan 1 2016;107:63-81.
Martineau LC, Couture A, Spoor D, et al. Antidiabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phyomedicine. 2006;13(9-10):612-23.
Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192.
Mascolo E, Verni F. Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms. International journal of molecular sciences. May 23 2020;21(10):3669. doi:10.3390/ijms21103669
Masharani U, Sherchan P, Schloetter M, Stratford S, Xiao A, Sebastian A, . . . Frassetto L. Metabolic and physiologic effects from consuming a hunter-gatherer (Paleolithic)-type diet in type 2 diabetes. European journal of clinical nutrition. Aug 2015;69(8):944-948.
Mason RP, Libby P, Bhatt DL. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arteriosclerosis, thrombosis, and vascular biology. May 2020;40(5):1135-1147. doi:10.1161/atvbaha.119.313286. https://pubmed.ncbi.nlm.nih.gov/32212849/
Masterjohn C, Mah E, Guo Y, et al. Gamma-tocopherol abolishes postprandial increases in plasma methylglyoxal following an oral dose of glucose in healthy, college-aged men. J Nutr Biochem. 2012;23:292-298.
Masters CJ, Crane DI. On the role of the peroxisome in ontogeny, ageing and degenerative disease. Mechanisms of ageing and development. May 12 1995;80(2):69-83.
Masumoto S, Akimoto Y, Oike H, et al. Dietary phloridzin reduces blood glucose levels and reverses Sglt1 expression in the small intestine in Streptozotocin-induced diabetic mice. J Agric Food Chem. 2009;57:4651-4656.
Matsui T, Ishikawa T, Ito H, Okamoto M, Inoue K, Lee MC, . . . Soya H. Brain glycogen supercompensation following exhaustive exercise. The Journal of physiology. Feb 1 2012;590(Pt 3):607-616.
Matsuo T, Odaka H, Ikeda H. Effect of an intestinal disaccharidase inhibitor (AO-128) on obesity and diabetes. The American journal of clinical nutrition. Jan 1992;55(1 Suppl):314s-317s.
Mayo Clinic. Diseases and Conditions page. Type 1 diabetes. <http://www.mayoclinic.org/diseases-conditions/type-1-diabetes/basics/definition/CON-20019573?p=1>. 8/2/2014. Accessed 8/12/2015.
Mayo Clinic. DHEA. https://www.mayoclinic.org/drugs-supplements-dhea/art-20364199. 09/20/2019.
Mayo JJ, Kravitz L. Glycemic Index: Weight Loss Sham or Sensation? https://www.unm.edu/~lkravitz/Article%20folder/glycemicUNM.html. Accessed 3/31/2016
Mazza A, Fruci B, Garinis GA, et al. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404.
Mazzola N. Review of current and emerging therapies in type 2 diabetes mellitus. Am J Manag Care. 2012;18(1Suppl):S17-26.
McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. Mar 2016;59(3):426-435.
McEwen B, Morel-Kopp M, Tofler G, et al. Effect of omega-3 fish oil on cardiovascular risk in diabetes. Diabetes Educ. 2010;36(4):565-584.
McGill JB, Peters A, Buse JB, et al. Comprehensive Pulmonary Safety Review of Inhaled Technosphere((R)) Insulin in Patients with Diabetes Mellitus. Clinical drug investigation. Oct 2020;40(10):973-983. doi:10.1007/s40261-020-00958-8
McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, Dahl M. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open medicine: a peer-reviewed, independent, open-access journal. 2011;5(1):e35-48.
McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. The Journal of clinical endocrinology and metabolism. Nov 2011;96(11):E1756-1760.
McPherson TC. Salsalate for arthritis: a clinical evaluation. Clinical therapeutics. 1984;6(4):388-403.
McRae MP. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. Journal of chiropractic medicine.2018;17(1):44-53.
Megalli S, Davies NM, Roufogalis BD. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J Pharm Pharm Sci. 2006;9(3):281-91.
Meigs JB, Nathan DM, Wilson PW, Cupples LA, Singer DE. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance. The Framingham Offspring Study. Ann Intern Med. Apr 1 1998;128(7):524-533.
Meister B. Control of food intake via leptin receptors in the hypothalamus. Vitamins and hormones. 2000;59:265-304.
Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes, obesity & metabolism. 2013;15(1):15-27.
Melzig MF, Funke I. [Inhibitors of alpha-amylase from plants--a possibility to treat diabetes mellitus type II by phytotherapy?]. Wiener medizinische Wochenschrift (1946). 2007;157(13-14):320-324.
Mendez CE, Walker RJ, Eiler CR, Mishriky BM, Egede LE. Insulin therapy in patients with type 2 diabetes and high insulin resistance is associated with increased risk of complications and mortality. Postgrad Med. Aug 2019;131(6):376-382. doi:10.1080/00325481.2019.1643635
Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, . . . Elahi D. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes care. 2000;23(8):1162-1167.
Meng B, Li J, Cao H. Antioxidant and anti-inflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des. 2013;19(11):2101-13.
Meng S, Cao J, Feng Q, et al. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complement Alternat Med. 2013;2013:801457.
Menne J, Dumann E, Haller H, Schmidt BMW. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: A systematic review and meta-analysis. PLoS Med. Dec 2019;16(12):e1002983. doi:10.1371/journal.pmed.1002983
Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-82.
Miklossy J, Qing H, Radenovic A, Kis A, Vileno B, Laszlo F, . . . McGeer PL. Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiology of aging. Sep 2010;31(9):1503-1515.
Milder TY, Stocker SL, Abdel Shaheed C, et al. Combination Therapy with an SGLT2 Inhibitor as Initial Treatment for Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Clin Med. Jan 4 2019;8(1)doi:10.3390/jcm8010045
Milenkovic T, Bozhinovska N, Macut D, et al. Mediterranean Diet and Type 2 Diabetes Mellitus: A Perpetual Inspiration for the Scientific World. A Review. Nutrients. Apr 15 2021;13(4)doi:10.3390/nu13041307
Miles JM, Rule AD, Borlaug BA. Use of metformin in diseases of aging. Curr Diab Rep. 2014;14:490.
Miller AL. The methionine-homocysteine cycle and its effects on cognitive diseases. Alternative medicine review: a journal of clinical therapeutic. Feb 2003;8(1):7-19.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. The New England journal of medicine . Apr 26 2012;366(17):1577-85. doi:10.1056/NEJMoa1200111
Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. The Lancet. 2021;397(10271):293-304. doi:10.1016/S0140-6736(20)32649-0
Mirrafiei A, Jayedi A, Shab-Bidar S. The Effects of L-Carnitine Supplementation on Weight Loss, Glycemic Control, and Cardiovascular Risk Factors in Patients With Type 2 Diabetes: A Systematic Review and Dose-response Meta-Analysis of Randomized Controlled Trials. Clin Ther. May 2024;46(5):404-410. doi:10.1016/j.clinthera.2024.03.002. https://pubmed.ncbi.nlm.nih.gov/38594107/
Misbin RI. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes care. Jul 2004;27(7):1791-1793.
Mittal K, Katare DP. Shared links between type 2 diabetes mellitus and Alzheimer's disease: A review. Diabetes Metab Syndr. Feb 11 2016.
Miyazawa T, Nakagawa K, Shimasaki S, et al. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42(4):1163-70.
Mizokami A, Kawakubo-Yasukochi T, Hirata M. Osteocalcin and its endocrine functions. Biochemical pharmacology. May 15 2017;132:1-8. doi:10.1016/j.bcp.2017.02.001. https://www.ncbi.nlm.nih.gov/pubmed/28189726
MMSCC. Memorial Sloan Kettering Cancer Center. Integrative Medicine/About Herbs, Botanicals & Other Products. https://www.mskcc.org/cancer-care/treatments/symptom-management/integrative-medicine/herbs/disclaimer?destination=cancer-care%2Fintegrative-medicine%2Fherbs%2Firvingia-gabonensis. Copyright 2015. Accessed 9/9/15.
Moat SJ, Lang D, McDowell IF, Clarke ZL, Madhavan AK, Lewis MJ, Goodfellow J. Folate, homocysteine, endothelial function and cardiovascular disease. The Journal of nutritional biochemistry. Feb 2004;15(2):64-79.
Moghaddam E, Vogt JA, Wolever TM. The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. The Journal of nutrition. Oct 2006;136(10):2506-2511.
Mohanty RR, Das S. Inhaled Insulin - Current Direction of Insulin Research. Journal of clinical and diagnostic research: JCDR. Apr 2017;11(4):OE01-OE02. doi:10.7860/JCDR/2017/23626.9732
Mohd Ghozali N, Giribabu N, Salleh N. Mechanisms Linking Vitamin D Deficiency to Impaired Metabolism: An Overview. International journal of endocrinology . 2022;2022:6453882. doi:10.1155/2022/6453882. https://pubmed.ncbi.nlm.nih.gov/35859985/
Monami M, Candido R, Pintaudi B, Targher G, Mannucci E. Effect of metformin on all-cause mortality and major adverse cardiovascular events: An updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis . Mar 10 2021;31(3):699-704. doi:10.1016/j.numecd.2020.11.031
Monteleone G, Buchwald P, Inverardi L, Pileggi A, Ricordi C, Tomei A, Inventors; Nogra Pharma Limited, University of Miami, assignee. Methods of treating diabetes and/or promoting survival of pancreatic islets after transplantation. US patent 14/394,9992013. International Filing Date 4/18/2013.
Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, . . . Pallone F. Mongersen, an Oral SMAD7 Antisense Oligonucleotide, and Crohn’s Disease. New England Journal of Medicine. 2015;372(12):1104-1113.
Moon JK, Yoo HS, Shibamoto T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: correlation with coffee acidity. Journal of agricultural and food chemistry. Jun 24 2009;57(12):5365-5369.
Mooradian AD, Thurman JE. Drug therapy of postprandial hyperglycaemia. Drugs. Jan 1999;57(1):19-29.
Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17-29.
Moridpour AH, Kavyani Z, Khosravi S, et al. The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes mellitus: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Phytother Res. Jan 2024;38(1):117-130. doi:10.1002/ptr.8026. https://www.ncbi.nlm.nih.gov/pubmed/37818728
Morris G, Berk M. The Putative Use of Lithium in Alzheimer's Disease. Current Alzheimer research. Feb 18 2016.
Moshetova LK, Vorob'eva IV, Alekseev IB, Mikhaleva LG. [Results of the use of antioxidant and angioprotective agents in type 2 diabetes patients with diabetic retinopathy and age-related macular degeneration]. Vestnik oftalmologii. May-Jun 2015;131(3):34-40, 42-34.
Movahed A, Nabipour I, Lieben Louis X, et al. Antihyperlgycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid Based Complement Alternat Med. 2013;2013.
Mozafari M, Nekooeian AA, Panjeshahin MR, et al. The effects of resveratrol in rats with simultaneous type 2 diabetes and renal hypertension: a study of antihypertensive mechanisms. Iran J Med. 2015;40(2):152-60.
Mudaliar S, Henry RR. Inhaled insulin in patients with asthma and chronic obstructive pulmonary disease. Diabetes technology & therapeutics. Jun 2007;9 Suppl 1:S83-92.
Mudra M, Ercan-Fang N, Zhong L, Furne J, Levitt M. Influence of Mulberry Leaf Extract on the Blood Glucose and Breath Hydrogen Response to Ingestion of 75 g Sucrose by Type 2 Diabetic and Control Subjects. Diabetes care. 2007;30(5):1272-1274.
Muntoni S, Muntoni S, Draznin B. Effects of chronic hyperinsulinemia in insulin-resistant patients. Curr Diabetes Reports. 2008;8:233-238.
Muti P, Quattrin T, Grant BJ, et al. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1361-8.
Nabavi SF, Thiagarajan R, Rastrelli L, et al. Curcumin: a natural product for diabetes and its complications. Curr Top Med Chem. 2015;Jun 19.
Nagai R, Shirakawa J, Ohno R, et al. Inhibition of AGEs formation by natural products, Amino Acids. 2014;46(2):261-6.
Nagel AK, Ahmed-Sarwar N, Werner PM, Cipriano GC, Van Manen RP, Brown JE. Dipeptidyl Peptidase-4 Inhibitor-Associated Pancreatic Carcinoma: A Review of the FAERS Database. The Annals of pharmacotherapy. Jan 2016;50(1):27-31.
Najafian M, Jahromi MZ, Nowroznejhad J, et al. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol Biol Rep. 2012;39:5299-5306.
Nakamura A, Terauchi Y. Present status of clinical deployment of glycokinase activators. J Diabetes Invest. 2015;6:124-132.
Nakamura S, Li H, Adijiang A, et al. Pyridoxal phosphate prevents progression of diabetic neuropathy. Nephrol Dial Transplant. 2007;22(8):2165-74.
Nakanishi H, Onose S, Kithara E, et al. Effect of environmental conditions on the alpha-glucosidase inhibitory activity of mulberry leaves. Biosci Biotechnol Biochem. 2011;75(12):2293-6.
Naowaboot J, Pannangpetch P, Kukongviriyapan V, et al. Mulberry leaf extract stimulates glucose uptake and GLUT4 translocation in rat adipocytes. Am J Chin Med. 2012;40(1):163-75.
Napolitano A, Miller S, Nicholls AW, Baker D, Van Horn S, Thomas E, . . . Nunez DJ. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PloS one. 2014;9(7):e100778.
Nasri H, Rafieian-Kopaei M. Metformin: current knowledge. J Res Med Sci. 2014;19(7):658-664.
Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753-759.
National Institiutes of Health Office of Dietary Supplements. Omega-3 Fatty Acids: Fact Sheet for Professionals. Updated 12/17/2024. Accessed 4/17/2025. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/
Nauck MA, Friedrich N. Do GLP-1–Based Therapies Increase Cancer Risk? Diabetes care. 2013;36(Supplement 2):S245-S252.
Nawale RB, Mourya VK, Bhise SB. Non-enzymatic glycation of proteins: a cause for complications in diabetes. Indian J Biochem Biophys. 2006;43:337-344.
NDIC. National Diabetes Information Clearinghouse. Causes of Diabetes. Available at: http://diabetes.niddk.nih.gov/dm/pubs/causes/. Last updated: 8/27/2014. Accessed 4/20/2015.
Neuen BL, Arnott C, Perkovic V, et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: A meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes, obesity & metabolism. Feb 2021;23(2):382-390. doi:10.1111/dom.14226
Neumiller JJ, Campbell RK. Technosphere insulin: an inhaled prandial insulin product. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. Jun 2010;24(3):165-172.
Ngondi JL, Etoundi BC, Nyangono CB, et al. IGOB131, a novel seed extract of the West African plant Irvingia gabonensis, significantly reduces body weight and improves metabolic parameters in overweight humans in a randomized double-blind placebo controlled investigation. Lipids Health Dis. 2009;8:7.
Ngondi JL, Oben JE, Minka SR. The effect of Irvingia gabonensis seeds on body weight and blood lipids of obese subjects in Cameroon. Lipids Health Dis. 2005;4:12.
Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (Lausanne). 2012;3:170.
Niafar M, Hai F, Porhomayon J, et al. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10(1):93-102.
Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519-524.
NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Diagnosis of Diabetes and Prediabetes. <http://www.niddk.nih.gov/health-information/health-topics/Diabetes/diagnosis-diabetes-prediabetes/Pages/index.aspx>. 6/2014c. Accessed 8/12/2015.
NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Insulin Resistance and Prediabetes. <http://www.niddk.nih.gov/health-information/health-topics/Diabetes/insulin-resistance-prediabetes/Pages/index.aspx>. 6/2014a. Accessed 8/12/2015.
NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Health Information page. What I need to know about Gestational Diabetes. http://www.niddk.nih.gov/health-information/health-topics/Diabetes/gestational-diabetes/Pages/index.aspx. 9/2014b. Accessed 4/25/2016
NIDDK. National Institute of Diabetes and Digestive Kidney Diseases. Am I at Risk for Type 2 Diabetes? Taking Steps to Lower Your Risk of Getting Diabetes. http://www.niddk.nih.gov/health-information/health-topics/Diabetes/type-2-diabetes-taking-steps-lower-your-risk-diabetes/Pages/index.aspx. 6/2012. Accessed 9/1/2015.
Nikolic A, Kacar A, Lavrnic D, Basta I, Apostolski S. [The effect of benfothiamine in the therapy of diabetic polyneuropathy]. Srp Arh Celok Lek. Nov-Dec 2009;137(11-12):594-600.
Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. British journal of cancer. Feb 2 2001;84(3):417-422.
NLM. U.S. National Library of Medicine. Meglitinide analogues for type 2 diabetes mellitus. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0012926/. Copyright 2009 by Cochrance Collaboration. Accessed 8/17/2015.
Norman J. Symptoms, Diagnosis, and Treatments of Type 1 Diabetes http://www.endocrineweb.com/conditions/type-1-diabetes/type-1-diabetes 6/7/2016.
Noto H, Goto A, Tsujimoto T, Noda M. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PloS one. 2013;8(1):e55030.
Nowlin SY, Hammer MJ, D’Eramo Melkus G. Diet, inflammation, and glycemic control in type 2 diabetes: an integrative review of the literature. J Nutr Metab. 2012;2012:542698.
NPG. Nature Publishing Group. Role of insulin-like growth factor 1 in bone metastasis. BoneKEy reports. 2012;1:170.
Nussey S, Whitehead S. Chapter 4: The adrenal gland. In: Endocrinology: An Integrated Approach. Oxford: BIOS Scientific Publishers; 2001. http://www.ncbi.nlm.nih.gov/books/NBK26/.
Nwaneri C, Copper H, Bowen-Jones D. Mortality in type 2 diabetes mellitus. Br J Diabetes and Vascular Disease. 2013;13(4):192-207.
Oben JE, Ngondi JL, Momo CN, et al. The use of Cissu quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double-blind placebo-controlled study. Lipids Health Dis. 2008;7:12.
Oben JE, Ngondi JL, Blum K. Inhibition of Irvingia gabonensis seed extract (OB131) on adipogenesis as mediated via down regulation of the PPARgamma and leptin genes and up-regulation of the adiponectin gene. Lipids in health and disease. 2008;7:44.
Ochiai R, Sugiura Y, Shioya Y, Otsuka K, Katsuragi Y, Hashiguchi T. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutrition research (New York, N.Y.). Feb 2014;34(2):155-159.
O'Connor PJ, Sperl-Hillen JM. ePocrates online. Type 2 diabetes mellitus in adults. Available at: https://online.epocrates.com/u/291124/Type+2+diabetes+mellitus+in+adults. Last updated 1/12/2015. Accessed 3/19/2015.
Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Western-style fast food intake and cardiometabolic risk in an Eastern country. Circulation. Jul 10 2012;126(2):182-188.
Ogawa K, Kuse Y, Tsuruma K, Kobayashi S, Shimazawa M, Hara H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC complementary and alternative medicine. 2014;14:120.
Oh J, Jo SH, Kim JS, Ha KS, Lee JY, Choi HY, . . . Kim YC. Selected tea and tea pomace extracts inhibit intestinal alpha-glucosidase activity in vitro and postprandial hyperglycemia in vivo. International journal of molecular sciences. 2015;16(4):8811-8825.
O'Mahoney LL, Matu J, Price OJ, et al. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovasc Diabetol. Jul 7 2018;17(1):98. doi:10.1186/s12933-018-0740-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6035402/pdf/12933_2018_Article_740.pdf
O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM-DM Study. Obesity. 2012;20:1426-1436.
Ong KW, Hsu A, Tan BKH. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7(3):e32718.
Orgel E, Mittelman SD. The links between insulin resistance, diabetes, and cancer. Curr Diab Rep. 2013;13(2):213-22.
Oron T, Farfel A, Muller I, Miller S, Atlas E, Nimri R, Phillip M. A remote monitoring system for artificial pancreas support is safe, reliable, and user friendly. Diabetes technology & therapeutics. Nov 2014;16(11):699-705.
Ortuno-Sahagun D, Pallas M, Rojas-Mayorquin AE. Oxidative stress in aging: advances in proteomic approaches. Oxidative medicine and cellular longevity. 2014;2014:573208.
Ota T. Obesity-induced inflammation and insulin resistance. Frontiers in endocrinology. 2014;5:204.
Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Human pathology. Aug 2003;34(8):803-808.
Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. International journal of clinical practice. Jun 2011;65(6):684-690.
Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428-439.
Pai JK, Cahill LE, Hu FB, et al. Hemoglobin A1C is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2:e000077.
Palau-Rodriguez M, Tulipani S, Isabel Queipo-Ortuno M, Urpi-Sarda M, Tinahones FJ, Andres-Lacueva C. Metabolomic insights into the intricate gut microbial-host interaction in the development of obesity and type 2 diabetes. Front Microbiol. 2015;6:1151.
Paolisso G, Scheen A, D'Onofrio F, Lefebvre P. Magnesium and glucose homeostasis. Diabetologia. Sep 1990;33(9):511-514.
Paradis ME, Couture P, Lamarche B. A randomized crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl Physiol Nutr Metab. 2011;36(6):913-9.
Park JH, Lee SH, Chung IM, et al. Sorghum extract exerts an antidiabetic effect by improving insulin sensitivity via PPAR-gamma in mice fed a high-fat diet. Nutr Res Pract. 2012;6(4):322-327.
Park S, Karuna U, Jeoung NH, et al. Physiological and therapeutic application of alpha lipoic acid. Curr Med Chem. 2014;21(32):3636-45.
Park SH, Huh TL, Kim SY, et al. Antiobesity effect of Gynostemma pentaphyllum extract (actiponin): a randomized, double-blind, placebo-controlled trial. Obesity. 2014;22:63-71.
Park SA. A common pathogenic mechanism linking type-2 diabetes and Alzheimer's disease: evidence from animal models. Journal of clinical neurology (Seoul, Korea). Mar 2011;7(1):10-18.
Pasanisi P, Oliverio A, Baldassari I, et al. Metformin Treatment With or Without Mediterranean Diet for the Prevention of Age-Related Diseases in People With Metabolic Syndrome: The MeMeMe Randomized Trial. Diabetes Care. Feb 1 2025;48(2):265-272. doi:10.2337/dc24-1597. https://pubmed.ncbi.nlm.nih.gov/39641916/
Pasinetti GM, Wang J, Ho L, Zhao W, Dubner L. Roles of Resveratrol and Other Grape-Derived Polyphenols in Alzheimer's Disease Prevention and Treatment. Biochimica et biophysica acta. Oct 11 2014.
Pekala J, Patkowska-Sokola B, Bodkowski R, et al. L-carnitine--metabolic functions and meaning in humans life. Curr Drug Metab. Sep 2011;12(7):667-78. doi:10.2174/138920011796504536. https://pubmed.ncbi.nlm.nih.gov/21561431/
Peng H, Want LL, Aroda VR. Safety and Tolerability of Glucagon-Like Peptide-1 Receptor Agonists Utilizing Data from the Exenatide Clinical Trial Development Program. Current diabetes reports. May 2016;16(5):44.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. The New England journal of medicine . Jun 13 2019;380(24):2295-2306. doi:10.1056/NEJMoa1811744
Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nature reviews. Endocrinology. Mar 2014;10(3):143-156.
Perraudeau F, McMurdie P, Bullard J, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care.2020;8(1).
Peyser T, Dassau E, Breton M, Skyler JS. The artificial pancreas: current status and future prospects in the management of diabetes. Annals of the New York Academy of Sciences. Apr 2014;1311:102-123.
Pinto LC, Rados DV, Barkan SS, Leitao CB, Gross JL. Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: A meta-analysis with trial sequential analysis. Sci Rep. Jan 15 2018;8(1):782. doi:10.1038/s41598-017-19055-6
Pittas AG, Kawahara T, Jorde R, et al. Vitamin D and Risk for Type 2 Diabetes in People With Prediabetes: A Systematic Review and Meta-analysis of Individual Participant Data From 3 Randomized Clinical Trials. Ann Intern Med . Mar 2023;176(3):355-363. doi:10.7326/M22-3018. https://www.ncbi.nlm.nih.gov/pubmed/36745886
Poquette NM, Gu X, Lee SO. Grain sorghum muffin reduces glucose and insulin responses in men. Food Funct. 2014;5(5):894-9.
Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care. 2012;35(12):2438-2442.
Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochimica et biophysica acta. Nov 2015;1852(11):2442-2455.
Prasad RC, Herzog B, Boone B, Sims L, Waltner-Law M. An extract of Syzygium aromaticum represses genes encoding hepatic gluconeogenic enzymes. Journal of ethnopharmacology. Jan 04 2005;96(1-2):295-301.
Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. The Journal of clinical investigation. 2006;116(7):1802-1812.
Pryor R, Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. The Biochemical journal. Nov 1 2015;471(3):307-322.
Qu B, Yan S, Ao Y, Chen X, Zheng X, Cui W. The relationship between vitamin K and T2DM: a systematic review and meta-analysis. Food Funct. Oct 2 2023;14(19):8951-8963. doi:10.1039/d3fo02943c. https://www.ncbi.nlm.nih.gov/pubmed/37724446
Rabinovitz H, Friedensohn A, Leibovitz A, et al. Effect of chromium supplementation on blood glucose and lipid levels in type 2 diabetes mellitus elderly patients. Int J Vitam Nutr Res. 2004;74(3):178-82.
Rafiei R, Habyby Z, Fouladi L, et al. Chromium level in prediction of diabetes in pre-diabetic patients. Adv Biomed Res. 2014;3:235.
Rahman K. Studies on free radicals, antioxidants, and co-factors. Clinical interventions in aging. 2007;2(2):219-236.
Rana S, Blowers EC, Natarajan A. Small molecule adenosine 5’-monophosphate activated protein kinase (AMPK) modulators and human diseases. J Med Chem. 2015;58:2-29.
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. Dec 1988;37(12):1595-1607.
Rehman MB, Tudrej BV, Soustre J, et al. Efficacy and safety of DPP-4 inhibitors in patients with type 2 diabetes: Meta-analysis of placebo-controlled randomized clinical trials. Diabetes Metab. Feb 2017;43(1):48-58. doi:10.1016/j.diabet.2016.09.005
Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56(9):1898-1906.
Reyes-Farias M, Vasquez K, Ovalle-Marin A, Fuentes F, Parra C, Quitral V, . . . Garcia-Diaz DF. Chilean native fruit extracts inhibit inflammation linked to the pathogenic interaction between adipocytes and macrophages. Journal of medicinal food.May 2015;18(5):601-608.
Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. Jan 2014;13:38-45.
Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, . . . Quon MJ. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. The Journal of clinical endocrinology and metabolism.May 2011;96(5):E782-792.
Rochester CD, Akiyode O. Novel and emerging diabetes mellitus drug therapies for the type 2 diabetes patient. World J Diabetes. 2014;5(3):305-315.
Rodriguez de Sotillo DV, Hadley M, Sotillo JE. Insulin receptor exon 11+/- is expressed in Zucker (fa/fa) rats, and chlorogenic acid modifies their plasma insulin and liver protein and DNA. The Journal of nutritional biochemistry. Jan 2006;17(1):63-71.
Roden M. Hepatic glucose production and insulin resistance. Wien Med Wochenschr. 2008;158/19-20:558-561.
Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, . . . Raskin I. In Vitro and in Vivo Anti-Diabetic Effects of Anthocyanins from Maqui Berry (Aristotelia chilensis). Food chemistry. Mar 15 2012;131(2):387-396.
Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin--a mini-review. Life sciences. Sep 15 2014;113(1-2):1-6.
Roopchand DE, Kuhn P, Rojo LE, et al. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol Res. 2013;68(1):59-67.
Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. Jul 10 2021;398(10295):143-155. doi:10.1016/S0140-6736(21)01324-6
Roshanov PS, Dennis BB. Incretin-based therapies are associated with acute pancreatitis: Meta-analysis of large randomized controlled trials. Diabetes research and clinical practice. Dec 2015;110(3):e13-17.
Ross SM. African mango (IGOB131): a proprietary seed extract of Irvingia gabonensis is found to be effective in reducing body weight and improving parameters in overweight humans. Holist Nurs Pract. 2011;25(4):215-217.
Roy MC, Anguenot R, Fillion C, et al. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res Int. 2011;44:3026-3029.
Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, . . . Cummings DE. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes care. Jun 2016;39(6):861-877.
Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. The Journal of clinical investigation. Jul 2013;123(7):2764-2772.
Russell SJ. Review: progress of artificial pancreas devices towards clinical use: the first outpatient studies. Curr Opin Endocrinol Obes. 2015;22:106-111.
Rybakowski JK. Effect of Lithium on Neurocognitive Functioning. Current Alzheimer research. Apr 15 2016.
Ryder RE. The potential risks of pancreatitis and pancreatic cancer with GLP-1-based therapies are far outweighed by the proven and potential (cardiovascular) benefits. Diabetic medicine: a journal of the British Diabetic Association. Oct 2013;30(10):1148-1155.
Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. 2014;38(5):330-6.
Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism: clinical and experimental. Jan 2015;64(1):35-46.
Sak D, Erdenen F, Müderrisoglu C, et al. The Relationship between Plasma Taurine Levels and Diabetic Complications in Patients with Type 2 Diabetes Mellitus. Biomolecules. Mar 11 2019;9(3)doi:10.3390/biom9030096
Salden BN, Troost FJ, de Groot E, Stevens YR, Garces-Rimon M, Possemiers S, . . . Masclee AA. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. The American journal of clinical nutrition. Dec 2016;104(6):1523-1533.
Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl). 2011;89(7):667-76.
Samadpour Masouleh S, Bagheri R, Ashtary-Larky D, et al. The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes. Nutrients. Nov 5 2021;13(11)doi:10.3390/nu13113958
Samaras N, Samaras D, Frangos E, Forster A, Philippe J. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuvenation research. 2013;16(4):285-294.
Sanae F, Kamiyama O, Ikeda-Obatake K, Higashi Y, Asano N, Adachi I, Kato A. Effects of eugenol-reduced clove extract on glycogen phosphorylase b and the development of diabetes in db/db mice. Food & function.Feb 2014;5(2):214-219.
Sandhir R, Gupta S. Molecular and biochemical trajectories from diabetes to Alzheimer's disease: A critical appraisal. World journal of diabetes. Sep 25 2015;6(12):1223-1242.
Sano R, Shinozaki Y, Ohta T. Sodium-glucose cotransporters: Functional properties and pharmaceutical potential. J Diabetes Investig. Jul 2020;11(4):770-782. doi:10.1111/jdi.13255
Sarafidis PA, Ortiz A. The risk for urinary tract infections with sodium-glucose cotransporter 2 inhibitors: no longer a cause of concern? Clin Kidney J. Feb 2020;13(1):24-26. doi:10.1093/ckj/sfz170
Sarbolouki S, Javanbakht MH, Derakhshanian H, et al. Eicosapentaenoic acid improves insulin sensitivity in overweight type 2 diabetes mellitus patients: a double-blind randomized clinical trial. Singapore Med J. 2013;54(7):387-90.
Sarnoski-Brocavich S, Hilas O. Canagliflozin (Invokana), a Novel Oral Agent For Type-2 Diabetes. P & T : a peer-reviewed journal for formulary management. Nov 2013;38(11):656-666.
Sasaki S, Inoguchi T. The role of oxidative stress in the pathogenesis of diabetic vascular complications. Diabets Metab. 2012;36(4):255-261.
Satoh T, Igarashi M, Yamada S, et al. Inhibitory effect of black tea and its combination with acarbose on small intestinal alpha-glucosidase activity. J Ethnopharmacol. 2015;161:147-55.
Schaafsma D, Gosens R, Ris JM, et al. Insulin induces airway smooth muscle contraction. Br J Phrmacol. 2007;150(2):136-42.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. The New England journal of medicine . Feb 16 2017;376(7):641-651. doi:10.1056/NEJMoa1600869
Scheen AJ, Paquot N. Metformin revisited: a critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab. 2013;39:179-190.
Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert opinion on drug safety . Apr 2019;18(4):295-311. doi:10.1080/14740338.2019.1602116
Schmitz O, Brock B, Rungby J. Amylin Agonists: A Novel Approach in the Treatment of Diabetes. Diabetes. December 1, 2004 2004;53(suppl 3):S233-S238.
Schurman L, McCarthy AD, Sedlinsky C, et al. Metformin reverts deleterious effects of advanced glycation end-products on osteoblastic cells. Exp Clin Endocrinol Diabetes. 2008;116(6):333-40.
Seidler NW, Yeargans GS, Morgan TG. Carnosine disaggregates glycated alpha-crystallin: an in vitro study. Archives of biochemistry and biophysics. Jul 1 2004;427(1):110-115.
Selam JL. Inhaled insulin: promises and concerns. J Diabetes Sci Technol. 2008;2(2):311-5.
Selhub J, Jacques PF, Bostom AG, Wilson PW, Rosenberg IH. Relationship between plasma homocysteine and vitamin status in the Framingham study population. Impact of folic acid fortification. Public health reviews. 2000;28(1-4):117-145.
Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? The journals of gerontology. Series A, Biological sciences and medical sciences. Sep 2010;65(9):963-975.
Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829-41.
Shahbazian H. World diabetes day; 2013. Journal of renal injury prevention. 2013;2(4):123-124.
Sheard NF, Clark NG, Brand-Miller JC, Franz MJ, Pi-Sunyer FX, Mayer-Davis E, . . . Geil P. Dietary Carbohydrate (Amount and Type) in the Prevention and Management of Diabetes: A statement by the American Diabetes Association. Diabetes care. September 1, 2004 2004;27(9):2266-2271.
Shi TJ, Zhang MD, Zeberg H, et al. Coenzyme Q10 prevents peripheral neuropathy and attenuates neuron loss in the db-/db- mouse, a type 2 diabetes model. Proc Natl Acad Sci USA. 2013;11092):690-5.
Shimabukuro M, Higa N, Chinen I, et al. Effects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients” a randomized crossover study. J Clin Endocrinol Metab. 2006;91(3):837-42.
Shimoda M, Kaku K. Controversy about the relationship between sulfonylurea use and cardiovascular events and mortality. J Diabetes Investig. Feb 9 2016.
Shoeb M, Ramana KV. Anti-inflammatory effects of benfotiamine are mediated through the regulation of the arachidonic acid pathway in macrophages. Free radical biology & medicine. Jan 1 2012;52(1):182-190.
Siekmeier R, Scheuch G. Inhaled insulin--does it become reality? Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. Dec 2008;59 Suppl 6:81-113.
Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutrition reviews.2013;71(12):790-801.
Silva KC, Rosales MA, Hamassaki DE, et al. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(2):1325-36.
Silverii GA, Monami M, Mannucci E. Sodium-glucose co-transporter-2 inhibitors and all-cause mortality: A meta-analysis of randomized controlled trials. Diabetes, obesity & metabolism. Apr 2021;23(4):1052-1056. doi:10.1111/dom.14286
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation decreases C-reactive protein levels in subjects with prediabetes and hypomagnesemia: a clinical randomized double-blind placebo-controlled trial. Arch Med Res. 2014;45(4):325-30.
Simeonov S, Pavlova M, Mitkov M, Mincheva L, Troev D. Therapeutic efficacy of "Milgamma" in patients with painful diabetic neuropathy. Folia medica. 1997;39(4):5-10.
Simm A. Protein glycation during aging and in cardiovascular disease. J Proteomics. 2013;92:248-259.
Simons LA, Friedlander Y, McCallum J, Simons J. Fasting plasma glucose in non-diabetic elderly women predicts increased all-causes mortality and coronary heart disease risk. Australian and New Zealand journal of medicine. Feb 2000;30(1):41-47.
Simsek M, Quezada-Calvillo R, Ferruzzi MG, Nichols BL, Hamaker BR. Dietary phenolic compounds selectively inhibit the individual subunits of maltase-glucoamylase and sucrase-isomaltase with the potential of modulating glucose release. Journal of agricultural and food chemistry. Apr 22 2015;63(15):3873-3879.
Smart NG, Apelqvist AA, Gu X, Harmon EB, Topper JN, MacDonald RJ, Kim SK. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS biology. Feb 2006;4(2):e39.
Smith JR, Thiagaraj HV, Seaver B, Parker KK. Differential activity of lipoic acid enantiomers in cell culture. Journal of herbal pharmacotherapy. 2005;5(3):43-54.
Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience. 2006;8(4):383-395.
Singh SK. Post-prandial hyperglycemia. Indian J Endocrinol Metab. 2012;16(Suppl 2):S245-S247.
Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417-1435.
Soare A, Weiss EP, Pozzilli P. Benefits of caloric restriction for cardiometabolic health, including type 2 diabetes mellitus risk. Diabetes/metabolism research and reviews. Mar 2014;30 Suppl 1:41-47.
Soffer DE, Marston NA, Maki KC, et al. Role of apolipoprotein B in the clinical management of cardiovascular risk in adults: An Expert Clinical Consensus from the National Lipid Association. J Clin Lipidol. Sep-Oct 2024;18(5):e647-e663. doi:10.1016/j.jacl.2024.08.013. https://pubmed.ncbi.nlm.nih.gov/39256087/
Solati M, Ouspid E, Hosseini S, et al. Oral magnesium supplementation in type II diabetic patients. Med J Islam Repub Iran. 2014;July 15;28:67.
Song SJ, Choi S, Park T. Decaffeinated green coffee bean extract attenuates diet-induced obesity and insulin resistance in mice. Evid Based Complement Alternat Med. 2014;2014:Article ID 718379.
Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17(6):813-22.
Sprague JE, Arbelaez AM. Glucose counterregulatory responses to hypoglycemia. Pediatric endocrinology reviews: PER. Sep 2011;9(1):463-473; quiz 474-465.
Standl E, Theodorakis MJ, Erbach M, et al. On the potential of acarbose to reduce cardiovascular disease. Cardiovascular Diabetology. 2014;13:81.
Stattin P, Bjor O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30(3):561-567.
Steven S, Lim EL, Taylor R. Population response to information on reversibility of Type 2 diabetes. Diabetic medicine: a journal of the British Diabetic Association. Apr 2013;30(4):e135-138.
Steyers CM, 3rd, Miller FJ, Jr. Endothelial dysfunction in chronic inflammatory diseases. International journal of molecular sciences. 2014;15(7):11324-11349.
Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes care. 2006;29(9):2064-2071.
Stirban A, Nandrean S, Gotting C, Tamler R, Pop A, Negrean M, . . . Tschoepe D. Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. The American journal of clinical nutrition. Mar 2010;91(3):808-813.
Strack T. Metformin: a review. Drugs of today (Barcelona, Spain: 1998). Apr 2008;44(4):303-314.
Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG. Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. Nov 2008;116(10):600-605.
Stracke H, Lindemann A, Federlin K. A benfotiamine-vitamin B combination in treatment of diabetic polyneuropathy. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 1996;104(4):311-316.
Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. The American journal of physiology. Jul 1997;273(1 Pt 1):E185-191.
Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. The Journal of nutrition. Oct 2010;140(10):1764-1768.
Suarez EA, Koro CE, Christian JB, Spector AD, Araujo AB, Abraham S. Incretin-mimetic therapies and pancreatic disease: a review of observational data. Current medical research and opinion. Dec 2014;30(12):2471-2481.
Subash S, Essa MM, Al-Adawi S, Memon MA, Manivasagam T, Akbar M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural regeneration research. Aug 15 2014;9(16):1557-1566.
Sudchada P, Saokaew S, Sridetch S, et al. Effects of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;98(1):151-8.
Suksomboon N, Poolsup N, Yuwanakorn A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J Clin Parm Ther. 2014;39:292-306.
Sun Q, Wedick NM, Tworoger SS, Pan A, Townsend MK, Cassidy A, . . . van Dam RM. Urinary Excretion of Select Dietary Polyphenol Metabolites Is Associated with a Lower Risk of Type 2 Diabetes in Proximate but Not Remote Follow-Up in a Prospective Investigation in 2 Cohorts of US Women. The Journal of nutrition. Jun 2015;145(6):1280-1288.
Szablewski L. Diabetes mellitus: influences on cancer risk. Diabetes Metab Res Rev. 2014;30(7):543-53.
Taheri S, Zaghloul H, Chagoury O, et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol.2020;8(6):477-489.
Takahashi K, Kamada C, Yoshimura H, Okumura R, Iimuro S, Ohashi Y, . . . Ito H. Effects of total and green vegetable intakes on glycated hemoglobin A1C and triglycerides in elderly patients with type 2 diabetes mellitus: the Japanese Elderly Intervention Trial. Geriatrics & gerontology international. Apr 2012;12 Suppl 1:50-58.
Takahashi M, Miyashita M, Suzuki K, Bae SR, Kim HK, Wakisaka T, . . . Yasunaga K. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. The British journal of nutrition. Nov 14 2014;112(9):1542-1550.
Takikawa M, Inoue S, Horio F, et al. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140(3):527-533.
Tao X, Zhang Z, Yang Z, Rao B. The effects of taurine supplementation on diabetes mellitus in humans: A systematic review and meta-analysis. Food Chem (Oxf) . Jul 30 2022;4:100106. doi:10.1016/j.fochms.2022.100106
Tasyurek HM, Altunbas HA, Balci MK, et al. Incretins: their physiology and application in the treatment of diabetes mellitus. Diabetes Metab Res Rev. 2014;30:354-371.
Tat V, Forest CP. The role of SGLT2 inhibitors in managing type 2 diabetes. JAAPA : official journal of the American Academy of Physician Assistants . Jun 2018;31(6):35-40. doi:10.1097/01.JAA.0000533660.86287.04
Teas J, Baldeon ME, Chiriboga DE, Davis JR, Sarries AJ, Braverman LE. Could dietary seaweed reverse the metabolic syndrome? Asia Pacific journal of clinical nutrition. 2009;18(2):145-154.
Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism: clinical and experimental. Jul 2013;62(7):922-934.
Tian B, Deng Y, Cai Y, Han M, Xu G. Efficacy and safety of Combination Therapy with Sodium-glucose Transporter 2 Inhibitors and Renin-Angiotensin System Blockers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association . Feb 19 2021;doi:10.1093/ndt/gfab048
Tian S, Guo T, Qian F, et al. Fish Oil, Plasma n-3 PUFAs, and Risk of Macro- and Microvascular Complications Among Individuals With Type 2 Diabetes. J Clin Endocrinol Metab. Apr 22 2025;110(5):e1687-e1696. doi:10.1210/clinem/dgae482. https://academic.oup.com/jcem/article-abstract/110/5/e1687/7712628?redirectedFrom=fulltext
Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, . . . Rudich A. Normal fasting plasma glucose levels and type 2 diabetes in young men. The New England journal of medicine. Oct 6 2005;353(14):1454-1462.
Toft I, Jenssen T. Type 2 diabetic patients have increased gluconeogenic efficiency to substrate availability, but intact autoregulation of endogenous glucose production. Scandinavian journal of clinical and laboratory investigation. 2005;65(4):307-320.
Tognon G, Lissner L, Saebye D, Walker KZ, Heitmann BL. The Mediterranean diet in relation to mortality and CVD: a Danish cohort study. The British journal of nutrition. Jan 14 2014;111(1):151-159.
Tome’-Carneiro J, Larrosa M, Yanez-Gascon MJ, et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res. 2013;72:69-82.
Trefts E, Shaw RJ. AMPK: restoring metabolic homeostasis over space and time. Mol Cell. Sep 16 2021;81(18):3677-3690. doi:10.1016/j.molcel.2021.08.015
Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19-28.
Tu Z, Moss-Pierce T, Ford P, Jiang TA. Syzygium aromaticum L. (Clove) extract regulates energy metabolism in myocytes. Journal of medicinal food.Sep 2014;17(9):1003-1010.
Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini reviews in medicinal chemistry. Apr 2010;10(4):315-331.
Tzang CC, Chi LY, Lin LH, et al. Taurine reduces the risk for metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrition & diabetes. May 16 2024;14(1):29. doi:10.1038/s41387-024-00289-z. https://pubmed.ncbi.nlm.nih.gov/38755142/
Tzimas P, Petrou A, Laou E, Milionis H, Mikhailidis DP, Papadopoulos G. Impact of metabolic syndrome in surgical patients: should we bother? British journal of anaesthesia. Jun 23 2015.
Uchiyama Y, Suzuki T, Mochizuki K, et al. Dietary supplementation with a low dose of (-)-epigallocatechin-3-gallate reduces pro-inflammatory responses in peripheral leukocytes of non-obese type 2 diabetic GK rats. J Nutr Sci Vitaminol (Tokyo). 2013;59(6):541-7.
Udupa A, Nahar P, Shah S, et al. A comparative study of effects of omega-3 fatty acids, alpha lipoic acid and vitamin E in type 2 diabetes mellitus. Ann Med Health. 2013;3:442-6.
UIE. University of Illinois Extension. Your Guide to Diet & Diabetes. What Impacts Blood Glucose Levels? Available at: http://extension.illinois.edu/diabetes2/subsection.cfm?SubSectionID=26. Last updated: June 2014. Accessed 5/31/2015.
Umeno A, Horie M, Murotomi K, Nakajima Y, Yoshida Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules. May 30 2016;21(6).
UMMC. University of Maryland Medical Center. Diabetes. <http://umm.edu/health-information/medical-reference-guide/medical-encyclopedia/articles/diabetes>. 8/5/2014. Accessed 8/12/2015.
Unger J, Parkin C. Hypoglycemia in insulin-treated diabetes: a case for increased vigilance. Postgraduate medicine. Jul 2011;123(4):81-91.
UOS. University of Sydney. Not All Carbohydrate Foods Are Equal. http://www.glycemicindex.com/. Copyright 2015. Accessed 3/31/2016
Uribarri J, Woodruff S, Goodman S. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911-16.
Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753-4761.
Valenzano A, Tartaglia N, Ambrosi A, et al. The Metabolic Rearrangements of Bariatric Surgery: Focus on Orexin-A and the Adiponectin System. J Clin Med . Oct 16 2020;9(10)doi:10.3390/jcm9103327
Vallianou NG, Stratigou T, Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens, Greece) . Jun 2019;18(2):141-144. doi:10.1007/s42000-019-00093-w
Van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;18(2):CD003639.
Van Guelpen B, Hultdin J, Johansson I, et al. Plasma folate and totlal homocysteine levels are associated with the risk of myocardial infarction, independently of each other and of renal function. J Intern Med. 2009;266(2):182-95.
van Heijst JW, Niessen HW, Hoekman K, Schalkwijk CG. Advanced glycation end products in human cancer tissues: detection of Nepsilon-(carboxymethyl)lysine and argpyrimidine. Annals of the New York Academy of Sciences. Jun 2005;1043:725-733.
Van Poppel PC, van Asseldonk EJ, Holst JJ, et al. The interleukin-1 receptor antagonist anakinra improves first-phase insulin secretion and insulinogenic index in subjects with impaired glucose tolerance. Diabetes Obes Metab. 2014;16(12):1269-73.
Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. Aug 20 2013;159(4):262-74. doi:10.7326/0003-4819-159-4-201308200-00007
Venkataraman K, Khurana S, Tai TC. Oxidative stress in aging--matters of the heart and mind. International journal of molecular sciences. 2013;14(9):17897-17925.
Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. European journal of clinical nutrition. Dec 2007;61 Suppl 1:S122-131.
Via M. The Malnutrition of Obesity: Micronutrient Deficiencies That Promote Diabetes. ISRN Endocrinology. 2012;2012:103472.
Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. Nov 10 2004;292(18):2243-2248.
Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical research ed.). 2012-01-11 13:01:56 2012;344.
Vimalananda V, Garg R. ePocrates online. Type 1 diabetes mellitus. Available at: https://online.epocrates.com/u/291125/Type+1+diabetes+mellitus. Last updated 8/12/2014. Accessed 3/19/2015.
Violett B, Horman S, Leclerc J, et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45(4):276-295.
Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012;122(6):253-270.
Visser ME, Witztum JL, Stroes ESG, Kastelein JJP. Antisense oligonucleotides for the treatment of dyslipidaemia. European heart journal. 2012;33(12):1451-1458.
Vivus. Qsymia. Highlights of prescribing information. https://qsymia.com/patient/include/media/pdf/prescribing-information.pdf. Last updated 10/2014. Accessed 4/15/16.
Vlassara H, Uribarri J. Advanced gkycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453.
Von Kriegstein E. Inhaled insulin for diabetes mellitus. N Engl J Med. 2007;356:2106-2108.
Vondra K, Hampl R. Vitamin D and new insights into pathophysiology of type 2 diabetes. Hormone molecular biology and clinical investigation. Mar 1 2021;42(2):203-208. doi:10.1515/hmbci-2020-0055. https://www.ncbi.nlm.nih.gov/pubmed/33655734
Walker MJ Jr, Morris LM, Cheng D. Improvement of cutaneous sensitivity in diabetic peripheral neuropathy with combination L-methylfolate, methylcobalamin, and pyridoxal 5’-phosphate. Rev Neurol Dis. 2010;7(4):132-9.
Wamil M, McMurray JJV, Scott CAB, et al. Predicting heart failure events in patients with coronary heart disease and impaired glucose tolerance: Insights from the Acarbose Cardiovascular Evaluation (ACE) trial. Diabetes Res Clin Pract . Dec 2020;170:108488. doi:10.1016/j.diabres.2020.108488
Wang JS, Huang CN, Hung YJ, Kwok CF, Sun JH, Pei D, . . . Sheu WH. Acarbose plus metformin fixed-dose combination outperforms acarbose monotherapy for type 2 diabetes. Diabetes research and clinical practice. Oct 2013;102(1):16-24.
Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell biochemistry and biophysics. Jun 2014;69(2):213-218.
Wang KM, Isom RT. SGLT2 Inhibitor-Induced Euglycemic Diabetic Ketoacidosis: A Case Report. Kidney Med. Mar-Apr 2020;2(2):218-221. doi:10.1016/j.xkme.2019.12.006
Watson RR, Schonlau F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol(R): a review. Minerva cardioangiologica.Apr 2015;63(2 Suppl 1):1-12.
Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia. Mar 2002;45(3):420-426.
Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes. Mar 2014;63(3):1021-31. doi:10.2337/db13-0887. https://www.ncbi.nlm.nih.gov/pubmed/24009262
Weickert MO, Pfeiffer AFH. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J Nutr.2018;148(1):7-12.
Weinstock RS. Patient education: Blood glucose monitoring in diabetes (Beyond the Basics). Updated May 20th 2021. Accessed July 1st, 2022. https://www.uptodate.com/contents/blood-glucose-monitoring-in-diabetes-beyond-the-basics?search=glucose%20monitoring&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2#H16
Weiss EP, Shah K, Fontana L, Lambert CP, Holloszy JO, Villareal DT. Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. Am J Clin Nutr. May 2009;89(5):1459-1467.
Weiss EP, Villareal DT, Ehsani AA, Fontana L, Holloszy JO. Dehydroepiandrosterone replacement therapy in older adults improves indices of arterial stiffness. Aging Cell. Oct 2012;11(5):876-884.
Weiss EP, Villareal DT, Fontana L, Han DH, Holloszy JO. Dehydroepiandrosterone (DHEA) replacement decreases insulin resistance and lowers inflammatory cytokines in aging humans. Aging (Albany NY). May 2011;3(5):533-542.
Wen C, Li F, Zhang L, et al. Taurine is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Mol Nutr Food Res. Jan 2019;63(2):e1800536. doi:10.1002/mnfr.201800536
Wexler DJ, Nathan DM, Mulder JE. Initial management of hyperglycemia in adults with type 2 diabetes mellitus. UpToDate. Updated May 20, 2021a. Accessed Jan. 10, 2022, https://www.uptodate.com/contents/initial-management-of-hyperglycemia-in-adults-with-type-2-diabetes-mellitus?s#H1
Wexler DJ, Nathan DM, Mulder JE. Metformin in the treatment of adults with type 2 diabetes mellitus. UpToDate. Updated Mar. 15, 2021b. Accessed Jan. 10, 2022, https://www.uptodate.com/contents/metformin-in-the-treatment-of-adults-with-type-2-diabetes-mellitus#H1
Wheeler ML, Pi-Sunyer FX. Carbohydrate issues: type and amount. J Am Diet Assoc. 2008;108(4 Suppl 1):S34-9.
White JR, Davis SN, Cooppan R, Davidson MB, Mulcahy K, Manko GA, Nelinson D. Clarifying the Role of Insulin in Type 2 Diabetes Management. Clinical Diabetes. 2003;21(1):14-21.
Whitlatch HB, Gaddam S, Ferri FF. Ferri's Clinical Advisor. Diabetes Mellitus. Available at: www.clinicalkey.com. Copyright 2015. Accessed 3/19/2015.
Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19-39.
Wilding J. SGLT2 inhibitors and urinary tract infections. Nature reviews Endocrinology . Dec 2019;15(12):687-688. doi:10.1038/s41574-019-0275-6
Williams JA. GLP-1 mimetic drugs and the risk of exocrine pancreatic disease: Cell and animal studies. Pancreatology: official journal of the International Association of Pancreatology (IAP) ... [et al.]. Jan-Feb 2016;16(1):2-7.
Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes care. Jan 1994;17(1):30-36.
Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Archives of internal medicine. Oct 1987;147(10):1749-1753.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, . . . Group tLAR. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals With Type 2 Diabetes. Diabetes care. 2011;34(7):1481-1486.
Winkler G, Pal B, Nagybeganyi E, Ory I, Porochnavec M, Kempler P. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittel-Forschung. Mar 1999;49(3):220-224.
Witters LA. The blooming of the French lilac. Journal of Clinical Investigation. 2001;108(8):1105-1107.
Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, . . . Fuchs CS. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. Journal of the National Cancer Institute. Jul 17 2013;105(14):1027-1035.
Wong YV, Cook P, Somani BK. The association of metabolic syndrome and urolithiasis. International journal of endocrinology. 2015;2015:570674.
Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer prevention research (Philadelphia, Pa.). Mar 2012;5(3):393-402.
Wu H, Ballantyne CM. Metabolic Inflammation and Insulin Resistance in Obesity. Circulation Research. 2020;126(11):1549-1564. doi:10.1161/CIRCRESAHA.119.315896. https://pubmed.ncbi.nlm.nih.gov/32437299/
Xi B, Li S, Liu Z, Tian H, Yin X, Huai P, . . . Steffen LM. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis. PloS one. 2014;9(3):e93471.
Xia J, Yu J, Xu H, et al. Comparative effects of vitamin and mineral supplements in the management of type 2 diabetes in primary care: A systematic review and network meta-analysis of randomized controlled trials. Pharmacological Research. Feb 2023;188:106647. doi:https://doi.org/10.1016/j.phrs.2023.106647. https://pubmed.ncbi.nlm.nih.gov/36638933/
Xiang GD, Sun HL, Zhao LS, et al. The antioxidant alpha-lipoic acid improves endothelial dysfunction induced by acute hyperglycemia during OGTT in impaired glucose tolerance. Clin Endocrinol. 2008;68:716-723.
Xie F, Cheng Z, Li S, Liu X, Guo X, Yu P, Gu Z. Pharmacokinetic study of benfotiamine and the bioavailability assessment compared to thiamine hydrochloride. Journal of clinical pharmacology. Jun 2014;54(6):688-695.
Xu H, Hu MB, Bai PD, et al. Proinflammatory cytokines in prostate cancer development and progression promoted by high-fat diet. Biomed Res Int. 2015;2015:249741.
Xu G, Song M. Recent advances in the mechanisms underlying the beneficial effects of bariatric and metabolic surgery. Surg Obes Relat Dis. Jan 2021;17(1):231-238. doi:10.1016/j.soard.2020.08.028
Yadav A, Kataria MA, Saini V, et al. Role of leptin and adiponectin in insulin resistance. Clinica Chimica Acta. 2013;417:80-84.
Yamamoto S, Matsushita Y, Nakagawa T, et al. Circulating adiponectin levels and risk of type 2 diabetes in the Japanese. Nutrition & Diabetes. 2014;4:e130.
Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769.
Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288-95.
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH, Chen YG. Smad7 Protein Interacts with Receptor-regulated Smads (R-Smads) to Inhibit Transforming Growth Factor-beta (TGF-beta)/Smad Signaling. The Journal of biological chemistry. Jan 1 2016;291(1):382-392.
Yang J, Han Y, Chen C, et al. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-alphaB signaling in human umbilical vein endothelial cells. Life Sci. 2013;92(10):589-97.
Yang X, Kong F. Evaluation of the in vitro alpha-glucosidase inhibitory activity of green tea polyphenols and different tea types. J Sci Food Agric. 2015;Feb 24. Doi:10.1002/jsfa.7147.
Yapislar H, Aydogan S. Effect of carnosine on erythrocyte deformability in diabetic rats. Arch Physiol Biochem. Dec 2012;118(5):265-272.
Yatsunami K, Ichida M, Onodera S. The relationship between 1-deoxynojirimycin content and alpha-glucosidase inhibitory activity in leaves of 276 mulberry cultivars (Morus spp.) in Kyoto, Japan. Journal of natural medicines. Jan 2008;62(1):63-66.
Yeo J, Kang YJ, Jeon SM, et al. Potential hypoglycemic effect of an ethanol extract of Gynostemma penatphyllum in C57BL/KsJ-db/db mice. J Med Food. 2008;11(4):709-16.
Yoo JY, Kim SS. Probiotics and prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients. 2016;18(8):173.
Yoshino J, Mills KF, Yoon MJ, Imai S-i. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell metabolism. 2011;14(4):528-536.
Yu H, Zhong X, Gao P, et al. The Potential Effect of Metformin on Cancer: An Umbrella Review. Systematic Review. Frontiers in endocrinology. 2019-September-18 2019;10(617)doi:10.3389/fendo.2019.00617
Yue W, Yang CS, DiPaola RS, Tan XL. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer prevention research (Philadelphia, Pa.). Apr 2014;7(4):388-397.
Zamani M, Pahlavani N, Nikbaf-Shandiz M, et al. The effects of L-carnitine supplementation on glycemic markers in adults: A systematic review and dose-response meta-analysis. Front Nutr. 2022;9:1082097. doi:10.3389/fnut.2022.1082097. https://pubmed.ncbi.nlm.nih.gov/36704801/
Zapp L, Slaga TJ, Zhao J, Lange M, Inventors. Method for Enhancing Post-Processing Content of Beneficial Compounds in Beverages Naturally Containing Same. US patent 8,357,419 B2. Jan. 22. 2013.
Zeki Al Hazzouri A, Stone KL, Haan MN, et al. Liptin. Mild Cognitive Impairment, and Dementia Among Elderly Women. J Gerontol a Biol Sci Med Sci. 2013;68(2):175-180.
Zelicha H, Yang J, Henning SM, et al. Effect of cinnamon spice on continuously montored glycemic response in adults with prediabetes: a 4-week randomized controlled crossover trial. The American Journal of Clinical Nutrition. 2024;119(3):649-657. doi:https://doi.org/10.1016/j.ajcnut.2024.01.008. https://www.sciencedirect.com/science/article/pii/S000291652400008X
Zeymer U. Cardiovascular benefits of acarbose in impaired glucose tolerance and type 2 diabetes. Int J Cardiol. 2006;107(1):11-20.
Zhang J, Tiller C, Shen J, Wang C, Girouard GS, Dennis D, . . . Ewart HS. Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Canadian journal of physiology and pharmacology. Nov 2007;85(11):1116-1123.
Zhang J, Sun C, Yan Y, Chen Q, Luo F, Zhu X, . . . Chen K. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food chemistry. Dec 01 2012;135(3):1471-1478.
Zhang DW, Fu M, Gao SH, et al. Curcumin and diabetes: a sytematic review. Evid Based Complement Alternat Med. 2013;2013:636053.
Zhang Y, He L, Chen X, Shentu P, Xu Y, Jiao J. Omega-3 polyunsaturated fatty acids promote SNAREs mediated GLUT4 vesicle docking and fusion. J Nutr Biochem. Mar 2022;101:108912. doi:10.1016/j.jnutbio.2021.108912. https://pubmed.ncbi.nlm.nih.gov/34801692/
Zhang YP, Eber A, Yuan Y, et al. Prophylactic and antinociceptive effects of coenzyme Q10 on diabetic neuropathic pain in a mouse model of type 1 diabetes. Anesthesiology. 2013;118(4):945-54.
Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science (New York, NY).2018;359(6380):1151.
Zhou Q, Lei X, Fu S, et al. Efficacy of cinnamon supplementation on glycolipid metabolism in T2DM diabetes: A meta-analysis and systematic review. Front Physiol. 2022;13:960580. doi:10.3389/fphys.2022.960580. https://pubmed.ncbi.nlm.nih.gov/36505061/
Zhou Y, Rui L. Leptin signaling and leptin resistance. Frontiers of medicine. Jun 2013;7(2):207-222.
Ziegler D, Reljanovic M, Mehnert H, Gries FA. Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 1999;107(7):421-430.
Zoico E, Di Francesco V, Mazzali G, et al. Adipocytokines, fat distribution, and insulin resistance in elderly men and women. J Gerontol: Medical Sciences. 2004;59A(9):935-939.
Zuo H, Shi Z, Yuan B, et al. Association between serum leptin concentrations and insulin resistance: a population-based study in China. PLoS ONE. 2013;8(1):e54615.
Zwakenberg SR, Remmelzwaal S, Beulens JWJ, et al. Circulating Phylloquinone Concentrations and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes. Jan 2019;68(1):220-225. doi:10.2337/db18-0543. https://pubmed.ncbi.nlm.nih.gov/30352877/