
Cognitive Decline & Mild Cognitive Impairment
Cognitive Decline & Mild Cognitive Impairment
Last Section Update: 01/2026
Contributor(s): Shayna Sandhaus, PhD; Carrie Decker, ND, MS; Stephen Tapanes, PhD; Maureen Williams, ND
Table of Contents
- Overview
- Introduction
- Background
- Nutrients
- Dietary & Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment (MCI)
- Risk Factors Associated with Cognitive Decline & Mild Cognitive Impairment (MCI)
- Mechanisms Involved in Cognitive Decline & Mild Cognitive Impairment (MCI)
- Nootropic Drugs & Novel Approaches to Cognitive Decline & Mild Cognitive Impairment (MCI)
- Update History
- References
1 Overview
Summary and Quick Facts for Cognitive Decline and Mild Cognitive Impairment
- Cognitive decline generally refers to the typical mild declines in thinking and memory that occur during aging.
- Mild cognitive impairment (MCI) is a condition characterized by changes in cognitive functioning beyond those expected for a person’s age, but which are not bad enough to be described as dementia. People with MCI are about three times more likely to progress to dementia over two to five years than their peers who do not have MCI.
- Dementia is a general term that describes more severe cognitive impairments such as problems with reasoning, judgement, language, and pronounced memory loss that significantly interfere with daily living and independence.
- An estimated 10‒20% of adults aged 65 years and older have MCI.
- This protocol will review many underlying factors that contribute to cognitive decline and MCI and describe several innovative medical strategies, lifestyle and dietary habits, and nutrients that may support brain health and cognition throughout life.
- Eating healthy and exercising, cognitive training, and nutrients such as phosphatidylserine and glyceryl phosphoryl choline have been shown to counteract age-related cognitive decline.
What is Cognitive Decline & Mild Cognitive Impairment?
Aging is associated with a gradual decline in cognitive function and thinking skills. As such, it is common for aging individuals to find that mental tasks take longer to complete and their memory and attention may be diminished. Age-related cognitive decline is a complex process with numerous contributing factors, including cellular senescence, disturbances of the circadian rhythm, and neuroinflammation, among others.
Age-related cognitive decline may progress more than expected for the person’s age but not become severe enough to be called dementia. In this situation, the condition is called mild cognitive impairment (MCI). In contrast, dementia refers to cognitive decline that is severe enough that it becomes debilitating, interfering with the person’s ability to function independently.
Fortunately, proactive lifestyle changes, cognitive training, and nutrients, such as bacopa and huperzine A, have been shown to decrease the rate of intellectual decay and potentially reverse age-related cognitive decline.
Nutrients
- Ginkgo. Numerous clinical trials and meta-analyses have demonstrated Ginkgo biloba’s ability to slow cognitive decline. An expert consensus paper from 2019 concluded that ginkgo is safe and effective and can be recommended alone or in combination with conventional therapies to treat mild cognitive impairment and dementia.
- Bacopa. Bacopa monnieri, a plant used in Ayurvedic medicine for centuries, has been shown in many clinical trials to improve several aspects of cognition.
- Huperzine A. Huperzine A, a compound from the medicinal herb Huperzia serrata, has been shown to inhibit acetylcholinesterase, an enzyme that breaks down the neurotransmitter acetylcholine. Patients with dementia and Alzheimer disease improved their scores on standard cognitive tests after supplementing with huperzine A.
- Acetyl-L-carnitine. Decreasing levels of acetyl-L-carnitine have been associated with a decline in cognitive function. A meta-analysis of data from over 21 studies showed supplementation with acetyl-L-carnitine improved cognitive deficits in patients with mild cognitive impairment and Alzheimer disease.
- Magnesium-L-threonate. Magnesium-L-threonate is a form of magnesium found to effectively raise brain magnesium levels. Preclinical research indicates it can protect brain function and preserve neural connections.
- Phosphatidylserine. Phosphatidylserine is a phospholipid that is an important part of myelin and cell membranes. Clinical trials indicate supplementing with phosphatidylserine can improve cognitive function in aging subjects with cognitive impairment.
- Alpha-glyceryl phosphoryl choline (α-GPC). α-GPC serves as a precursor to the neurotransmitter acetylcholine. Acetylcholine precursors, alone or in combination with acetylcholinesterase inhibitors, are promising for treating dementia.
- Mango leaf extract. Mango leaf extracts rich in mangiferin have anti-inflammatory and neuroprotective properties. An extract standardized to 60% mangiferin was shown in clinical studies to improve various aspects of cognition, including episodic memory and visual information processing.
- Peppermint oil. Peppermint essential oil is rich in monoterpenes, which are known for their benefits to cognition. Peppermint oil inhibits acetylcholinesterase and was shown in a clinical trial to improve performance on a cognitively demanding task and prevent cognitive fatigue.
- Other natural interventions that may benefit brain health and overall cognitive function include polyphenols, melatonin, B vitamins, colostrinin, lithium, and more.
What Dietary & Lifestyle Changes Support Brain Health?
- Switch from a Western-style diet (high in simple sugars and saturated fats) to a healthy dietary pattern such as the Mediterranean, DASH, or MIND diet
- Caloric restriction may improve learning and memory
- Cognitive stimulation and training, including playing chess and speaking more than one language, can enhance cognitive reserve and convey protection against loss of brain function, supporting healthy cognitive function into older age
- Manage stress and get enough quality sleep
- Engage in social activities (strong social networks promote cognitive health)
- Exercise is known to increase levels of brain-derived neurotrophic factor, which can lead to enhanced cognitive function
- Moderate caffeine and coffee consumption (1‒2 cups/day) may convey protection against cognitive decline
What are the Symptoms of Cognitive Decline?
Those who experience cognitive decline may have difficulty with various aspects of cognition, including:
- Planning and organizing
- Following changes in conversation
- Finding words
- Focusing
- Losing items
- Loss of empathy or judgement
- Inappropriate behavior
Additionally, mood changes are common, with depression affecting approximately one-third of those with mild cognitive impairment.
What Increases the Risk of Cognitive Decline?
- Age
- Sedentary lifestyle
- Low level of education
- Smoking
- Obesity
- Insulin resistance/type 2 diabetes
- High blood pressure
- High total and LDL cholesterol
- Depression
- Sleep disorders
- Sleep apnea
- Chronic kidney disease
- Cardiovascular disease
- Cerebrovascular disease including history of stroke
- Traumatic brain injury
- Hearing loss
- Female gender (women seem to be more likely to experience cognitive decline than men)
- Excessive alcohol consumption
- Air pollution
Nootropic Drugs & Novel Approaches to Cognitive Decline
There are not currently any medications specifically approved for age-related cognitive decline. Anti-dementia medications do not appear to prevent progression from mild cognitive impairment to dementia. However, certain medications have been found to have brain-protective or enhancing effects:
- Piracetam and levetiracetam (anti-seizure medications)
- Selegiline (medication used for Parkinson’s, Alzheimer, and major depressive disorder)
- Zileuton (asthma medication)
- Angiotensin receptor blockers (eg, candesartan)
2 Introduction
Cognitive function peaks around age 20 and diminishes steadily over the remaining years of life.1,2 With life expectancies increasing dramatically in the last century, cognitive decline, mild cognitive impairment (MCI), and dementia have become major concerns.3,4
Aging is associated with gradual changes in the brain that impair its function. As a result, older people, even those without overt neurological disease, often find it takes longer to perform mental tasks and experience diminished memory, attention, and abilities to learn, reason, and solve problems.2 Although some cognitive decline occurs during normal aging, its rate of progression is affected by lifestyle, dietary, environmental, and genetic factors.5 Importantly, some of these factors that contribute to the progression of cognitive decline are modifiable.1,6
Some factors that likely contribute to cognitive decline include:
- Stem cell senescence
- Brain oxidative stress and mitochondrial dysfunction
- Neuroinflammation (inflammation in the brain)
- Circadian rhythm and metabolic disturbances
- Vascular dysfunction
- Abnormal protein accumulation in the brain
- Disordered homocysteine metabolism
- Changing hormone levels
- Epigenetic factors—changes in the way genes are expressed
These same mechanisms also appear to contribute to dementia and neurodegenerative diseases like Alzheimer disease and Parkinson disease.1
Much is known about lifestyle factors that work together to promote healthy brain aging, such as eating a nutrient-dense diet (eg, Mediterranean, MIND, or DASH diet), being physically active, reducing stress, getting adequate sleep, and regularly engaging in mentally and socially stimulating activities.1,4,6 In addition, a number of integrative interventions have been identified as having protective effects on brain function.7,8
This protocol will review many underlying factors that contribute to cognitive decline, and describe several novel medical strategies, lifestyle and dietary habits, and integrative interventions that can support healthy cognitive function and brain health throughout life.
3 Background
The brain contains approximately 100 billion interconnected neurons, which collectively assimilate information received from nerves throughout the body and external stimuli. In addition to neurons, the brain is home to specialized cells known as glial cells, mainly astrocytes and microglia, which play numerous essential support roles.9,10 Glial cells also participate in vital signaling processes within the brain.11
The Aging Brain
With age, the number of brain neurons decreases and the cells and tissues that support them deteriorate slowly after age 20 and more rapidly after age 60. By age 90, brain mass has been found to be decreased by 11% compared with individuals in their 50s.5 The majority of neuronal loss is in the cerebral cortex, where most information processing occurs, and the hippocampus, a brain structure involved in memory and learning.2,12,13
Aging is associated with functional brain changes as well. For instance, cerebral blood flow decreases and production of neurotransmitters is reduced. Also, the integrity of the blood‒brain barrier, which controls movement of cells and molecules into and out of blood vessels in the brain, weakens,2,14 and the phospholipid-rich myelin sheaths that protect neurons and facilitate signal transmission deteriorate.13,15
These age-related brain changes manifest in the diminished mental abilities and thinking skills typically associated with old age, namely reduced short-term and episodic memory, difficulty recalling words, slower reaction times, and possibly depressed mood.2
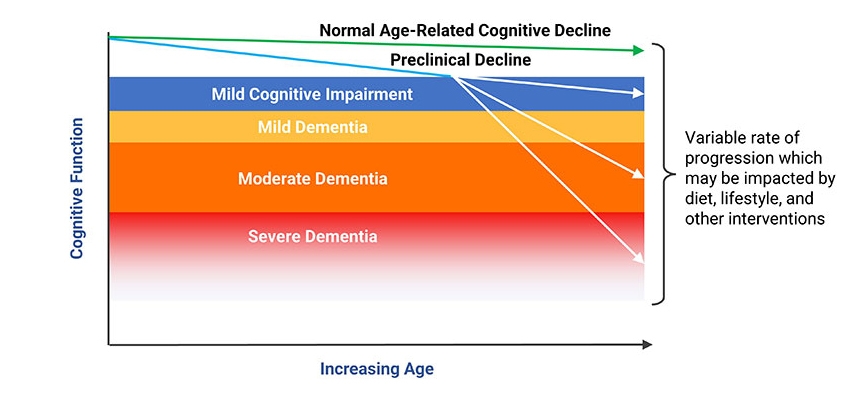
From Age-Related Cognitive Decline to Mild Cognitive Impairment and Dementia
Age-related cognitive decline describes the natural decline in ability to learn, remember, and process information. Mild cognitive impairment (MCI) is the condition characterized by cognitive changes that are more than expected for age, but not debilitating. It is estimated that 10–20% of adults aged 65 years and older have MCI.16,17 There are two broad classifications of MCI: amnestic MCI and non-amnestic MCI.547
- Amnestic MCI describes cognitive changes that include memory problems that are not significant enough to be classified as dementia. Amnestic MCI is the most common type of MCI and is often considered a precursor to Alzheimer disease, although not all people with amnestic MCI will progress to Alzheimer disease.
- Non-amnestic MCI describes cognitive changes in domains other than memory, such as language, executive functioning, and visual-spatial skills. This form of MCI is about half as common as amnestic MCI.
When cognitive decline becomes severe enough to interfere with social and occupational function and the ability to live independently, the condition is called dementia.17,18 Dementia affects approximately 5–10% of US adults age 65 and older.17,19 Alzheimer disease is the most common form of dementia in the elderly, followed by cerebrovascular dysfunction.14 Importantly, most people with age-related losses in cognitive function never develop these more advanced conditions.18,20
Distinguishing between normal age-related cognitive issues and mild cognitive impairment is challenging. A comprehensive assessment that includes a history of cognitive changes, physical exam, neurological exam, and cognitive function testing is essential to an accurate diagnosis.18 A standardized assessment of cognitive function known as the Mini-Mental State Examination (MMSE) is one such example of a test that may be performed. The MMSE is scored on a 30-point scale and assesses verbal, memory, and constructional functions. An MMSE score of less than 24 is commonly considered abnormal and indicative of dementia; scores of 24–27 may indicate MCI and increased risk of progression to dementia in the future (see Figure 1).548-550 Laboratory testing, including screening for hypothyroidism and vitamin B12 deficiency, will help determine if common treatable conditions are contributing factors to cognitive decline.
| Table 1: Domains of cognitive decline and functional signs of impairment17 | |
|---|---|
| Cognitive Domain | Signs of Impairment |
| Executive function |
Difficulties with:
|
| Attention |
|
| Visuospatial skills |
|
| Language |
|
| Memory and learning |
|
| Social cognition |
|
4 Nutrients
Ginkgo
Ginkgo (Ginkgo biloba) is perhaps the most widely studied and commonly used integrative therapy for supporting cognitive function. Ginkgo extracts have been shown to reduce oxidative stress, decrease neuroinflammation, improve microcirculation, modulate neurotransmitter activity, and promote neuroplasticity.228,229 Animal research suggests ginkgo may stimulate neural stem cell proliferation and activity.230
Numerous randomized controlled trials, systematic reviews, and meta-analyses have concluded that ginkgo, usually at a dose of 120‒240 mg per day, can slow cognitive decline and reduce neuropsychiatric symptoms (such as delusions and depressed or anxious mood) in patients with mild cognitive impairment and dementia.228,231-233 A 2019 expert consensus paper found the evidence of efficacy and safety sufficient to recommend a standardized ginkgo extract, alone or in combination with conventional therapies, for treatment of mild cognitive impairment and dementia.234
Bacopa
Bacopa (Bacopa monnieri), a plant with religious, cultural, and medical importance in India, has been used in traditional Ayurvedic medicine for centuries.235 Bacopa extract has demonstrated effects such as reducing brain oxidative stress, modulating neurotransmitter activity, reducing β-amyloid deposition, strengthening neuronal connections, and increasing cerebral blood flow in preclinical research.236,237 Human research suggests bacopa may also improve the stress response.238 Furthermore, in mice, bacopa extract increased brain levels of brain-derived neurotrophic factor (BDNF) and production of new neurons.239
A meta-analysis that included data from 518 subjects in nine randomized controlled trials concluded bacopa has the potential to improve some aspects of cognition.240 In one randomized controlled trial in 54 participants aged 65 years and older, those who received 300 mg bacopa extract daily for 12 weeks had better cognitive performance and reduced symptoms of anxiety and depression after 12 weeks compared with placebo.241 Two clinical trials using different herb-nutrient combinations with bacopa reported cognitive benefits from treatment with these supplements in older adults with mild cognitive impairment.242,243
Huperzine A
Huperzine A is a biologically active compound from the Chinese medicinal herb Huperzia serrata, commonly known as Chinese club moss. Huperzine A has been shown to inhibit acetylcholinesterase, an enzyme that breaks down acetylcholine. Acetylcholine is a major neurotransmitter in the autonomic nervous system, and its accelerated breakdown by acetylcholinesterase is thought to contribute to age-related cognitive decline and dementia.244 Some anti-dementia drugs like donepezil (Aricept) also work by inhibiting acetylcholinesterase, and huperzine A has been proposed to have a disease-modifying effect in Alzheimer disease.245 In addition, preclinical evidence suggests huperzine A may reduce oxidative stress, prevent β-amyloid and phosphorylated tau accumulation, support mitochondrial function, and increase brain production of nerve growth factor.246
Numerous clinical trials have shown that huperzine A can improve cognitive function in people with dementia. One meta-analysis included 10 randomized controlled trials evaluating the effects of huperzine A, in doses ranging from 100–400 mcg daily, in a combined total of 825 patients with Alzheimer or vascular dementia. The results of the analysis indicated huperzine A can improve cognitive function in dementia patients, and longer use may result in greater benefits.247 Another meta-analysis of 20 randomized controlled trials in Alzheimer disease patients also noted likely benefits of huperzine A on cognitive function.248 One preliminary trial examined the effect of huperzine A on task switching, a higher-order cognitive function, in patients with Alzheimer disease. After eight weeks of treatment with 200 mcg huperzine A, cognitive function and performance on task switching tests improved.249 In another preliminary trial, a supplement containing huperzine A and curcumin improved cognitive performance after 6–12 and 22–28 weeks in people with dementia as well as those with mild cognitive impairment.250
It should be noted that mild adverse side effects such as digestive upset and constipation, dizziness, slow heart rate, and dry mouth have been reported by people taking huperzine A.247,248
Acetyl-L-carnitine
Acetyl-L-carnitine is a form of the amino acid, carnitine, produced in the mitochondria and involved in cellular energy production. It also is a precursor molecule for the formation of acetylcholine.551 One study reported progressively decreasing blood levels of acetyl-L-carnitine in subjects on the spectrum from no cognitive problems, to subjective memory complaints, to mild cognitive impairment, to dementia.251 Preclinical studies suggest acetyl-L-carnitine may preserve brain mitochondrial function, reduce oxidative stress, inhibit inflammatory activity by microglial cells, and improve dopamine signaling in the nervous system.252,253
A meta-analysis of randomized controlled trials that included data from 21 studies with over 1,200 subjects found that acetyl-L-carnitine supplementation for three months or longer was associated with clinical improvement in patients with mild cognitive impairment and early Alzheimer disease.254 One clinical trial in elderly participants found that treatment with acetyl-L-carnitine led to decreased physical and mental fatigue, and improved cognitive and physical function.255 A comparison trial found acetyl-L-carnitine worked slightly faster than fluoxetine (Prozac) and with similar efficacy in improving mild depressive symptoms in elderly individuals, an effect that may be associated with better cognitive function.256 A combination supplement containing acetyl-L-carnitine plus B vitamins, vitamin E, and other amino acid derivatives, taken for six months, improved cognitive function in participants with mild cognitive impairment relative to placebo.257
Polyphenols
Polyphenols are a family of strong oxidative-stress-reducing compounds found in plants. It is thought that the high polyphenol content of the traditional Mediterranean diet may be an important contributor to its cognitive benefits.265 In 652 dementia-free subjects aged 65 years and older, those with higher urinary polyphenols, indicating higher polyphenol intake, had less cognitive decline during three years of monitoring.266 Another study in 447 older adults with increased cardiovascular risk also noted better cognitive performance in those with greater urinary polyphenol concentrations. In addition, intake of specific polyphenol-rich foods (olive oil, coffee, walnuts, and wine) was independently linked to better performance on tests of certain aspects of cognitive function.265
Evidence suggests polyphenols can reduce brain oxidative stress and neuroinflammation and improve cerebrovascular function.267 In addition to quenching excess free radicals, polyphenols may affect signaling associated with aging, preserve neural stem cell activity, promote neuroplasticity, reduce protein accumulation, induce epigenetic changes in genes involved in synaptic plasticity, and support a healthy gut microbiome.267-270
Flavonoids are a class of polyphenolic compounds thought to support cognitive health. In an observational study following 49,493 women from the Nurses’ Health Study from 1984‒2006 and 27,842 men from the Health Professionals Follow-up Study from 1986‒2002, higher intake of total flavonoids was associated with lower odds of subjective cognitive decline. Among men and women whose total flavonoid intake was among the top 20% of combined participants (whose flavonoid intake averaged 600 mg per day), the risk of subjective cognitive impairment was 19% lower than those whose intake was among the lowest 20% (who had an average intake of 150 mg per day). The strongest associations were observed for flavones (38% lower risk), flavanones (36%), and anthocyanins (24%). In general, flavonoid-rich foods, such as strawberries, oranges, grapefruits, citrus juices, apples/pears, celery, peppers, and bananas, were significantly associated with lower odds of subjective cognitive decline.536
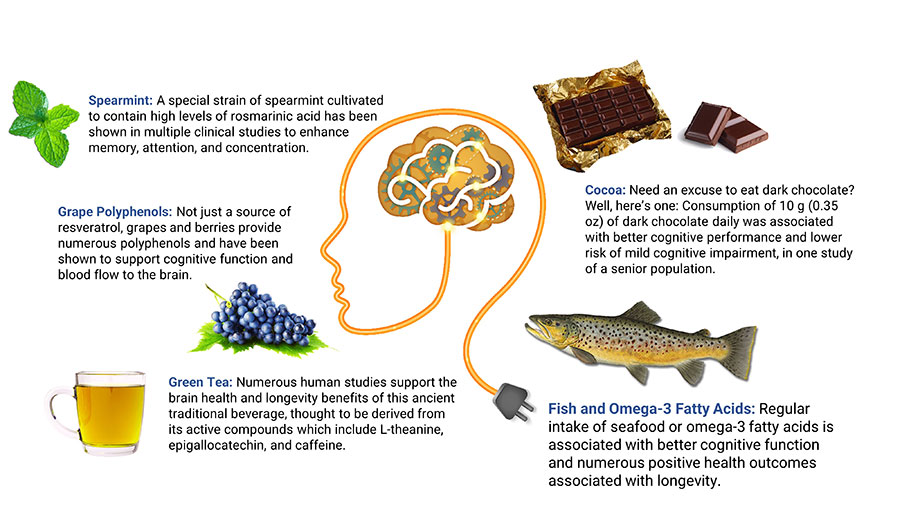
Berry polyphenols. Blueberries and their polyphenols, especially the anthocyanins that give them their color, may have preventive effects against chronic diseases including cognitive disorders.271 Studies in older adults have shown that blueberries can enhance cerebral blood flow and increase brain activity in regions associated with age-related cognitive decline.272,273 In a randomized controlled trial in 37 people age 60‒75 years, 24 grams of freeze dried blueberries (equivalent to one cup of fresh blueberries) daily for 90 days led to better cognitive performance compared with placebo.274 Another controlled trial found 24 weeks of treatment with whole-fruit blueberry powder improved cognitive function in elderly adults.275 In a placebo-controlled trial in 26 patients, 500 mL per day of blueberry juice at least 14 days before surgery resulted in reduced cognitive deficits associated with anesthesia.276 In a controlled trial in 40 healthy subjects aged 50–70 years old, taking a mixed berry drink for five weeks improved cardiovascular risk markers as well as performance on memory tests compared with placebo.277
Grape polyphenols. Grape polyphenols, such as quercetin, lycopene, resveratrol, and anthocyanins, have demonstrated neuroprotective actions.278 A randomized controlled trial in 111 healthy older subjects found 250 mg per day of grape extract improved scores on cognitive tests after 12 weeks.279 Older adults with cognitive decline and mild cognitive impairment experienced improved cognitive performance after drinking 15‒20 ounces (depending on weight) per day of Concord grape juice for 12–16 weeks in small controlled trials.280,281 In a small placebo-controlled trial of 10 participants with mild cognitive decline, those receiving placebo exhibited significant diminishment in metabolic activity in regions of the brain involved in dementia, but those receiving grape extract had no such decline.282 A randomized controlled trial in 215 healthy older adults identified a significant effect of a high-polyphenol grape plus blueberry extract, taken at a dose of 600 mg daily for six months, in those with the lowest cognitive test scores at baseline.283
Resveratrol. Resveratrol is a grape polyphenol found especially in red wine and shown in numerous studies to have powerful free radical-quenching capacity.284 Resveratrol appears to slow cognitive decline through regulating age-related signaling, improving cerebral blood flow, and increasing neuroplasticity.285 Findings from clinical trials have been mixed; however, a meta-analysis that included 10 randomized controlled trials found resveratrol may improve some aspects of cognitive function and mood in older individuals.286
In a randomized placebo-controlled trial in 46 healthy individuals aged 50‒75 years, 26 weeks of treatment with 200 mg resveratrol daily led to better performance on memory tasks, as well as improved glucose metabolism (indicated by lower hemoglobin A1C [HbA1c]), increased neuronal connectivity, and decreased body fat.287 Another trial in 40 patients with mild cognitive impairment found 26 weeks of treatment with 200 mg resveratrol daily resulted in better glucose metabolism and better preservation of brain structure, but no difference in cognitive function compared with placebo.288 In a controlled trial in sedentary, overweight, older adults, 1,000 mg resveratrol daily improved psychomotor speed, but not other aspects of cognitive function, after 90 days, while 300 mg daily had no effect.289 In a controlled trial in postmenopausal women, 150 mg resveratrol daily improved cerebrovascular function and cognitive performance after 14 weeks; the investigators proposed that some of resveratrol’s effects in this population were due to its phytoestrogenic actions.290
Green tea catechins. Green tea is a source of polyphenolic catechins. A growing body of evidence suggests green tea catechins may slow brain aging by modulating neural growth factors, regulating cell signaling involved in inflammation and neuronal survival, and reducing accumulation of abnormal proteins.291,292 A review of 21 studies found green tea may reduce anxiety, benefit memory and attention, and enhance brain function.293
Chlorogenic acids. Chlorogenic acids are polyphenols found in coffee, and many studies have linked chlorogenic acid consumption to better cognitive function and mood.294 In 38 healthy adults aged 50–69 years with subjective memory complaints, drinking a beverage providing 300 mg chlorogenic acid at bedtime for 16 weeks resulted in better cognitive performance and improved blood levels of proteins thought to be markers of early Alzheimer disease.295
Melatonin
Melatonin helps regulate the circadian control center of the brain and promotes sleep. Circadian patterns and nighttime melatonin production diminish with age, contributing to poor sleep and consequent neurodegeneration.296,297 In older individuals, lower nighttime melatonin levels have been correlated with mild cognitive impairment and dementia.298-300 Animal studies suggest melatonin can repair circadian and sleep disturbance and reduce associated cognitive problems.301-303 Melatonin’s potential to reduce neuroinflammation and neurodegeneration have also been noted in animal models of both Alzheimer and vascular dementia.304-306
In a retrospective analysis of patients with mild cognitive impairment, the effect of melatonin on sleep, mood, and several tests of cognitive function was reported. The analysis compared the effects of standard medication with or without the addition of melatonin in doses of 3‒24 mg at bedtime on a total of 96 outpatients. Participants were monitored for 15–60 months, and results showed the melatonin-treated group had improved sleep, mood, and performed better on all tests of cognitive function.307 In a similarly designed retrospective analysis that was previously conducted by these researchers, 9–18 months of treatment with 3–9 mg melatonin nightly improved performance on all but one cognitive test in patients with mild cognitive impairment.308
A randomized controlled trial in 139 elderly participants undergoing hip joint surgery found 1 mg melatonin taken before bedtime for six days beginning one day before surgery prevented postoperative cognitive decline; there were also improvements in sleep quality, fatigue, and general well-being compared with placebo.310 Clinical trials in Alzheimer disease patients indicate melatonin may improve sleep, reduce behavioral symptoms, and enhance some aspects of cognitive function.311,312
In a first-of-its-kind study, melatonin’s effect on memory was compared with two of its metabolites (N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK] and N1-acetyl-5-methoxykynuramine [AMK]).309 The study tested the interventions on mice using a novel object recognition task and an analysis of melatonin and its metabolites in specific brain regions involved in cognitive memory. It is important to note that the novel object recognition task is used on mice because they have an instinctive tendency to examine unfamiliar objects. The results showed melatonin and its metabolites produced notable improvements in object recognition compared with controls. The most potent effect was observed in old mice that received the metabolite AMK, as these mice indicated recognition of objects for up to four days. The study also showed melatonin can accumulate along with its metabolites in the hippocampus and the neighboring perirhinal cortex, which is involved in visual recognition.
Omega-3 Fatty Acids & Fish Oil
The long-chain omega-3 fatty acid docosahexaenoic acid (DHA) is a critical nutrient for brain health, and deficiency can cause symptoms such as poor mood and cognitive dysfunction. DHA is found in high concentrations in neuronal cell membranes where it plays an important structural role in maintaining membrane fluidity.313,314 Preclinical research showed DHA plus eicosapentaenoic acid (EPA), another omega-3 fatty acid from fish, may protect against amyloid plaques and neurofibrillary tangles,315 as well as prevent blockages and improve blood flow in small vessels in the brain.316 Adequate omega-3 fatty acid status may also be needed for proper use of B vitamins in the brain.314,317 In addition, DHA has anti-inflammatory effects and is a precursor for neuroprotectin D1, a signaling molecule involved in neuronal growth and survival.313
A number of studies have noted a strong association between higher seafood intake, or higher blood or dietary levels of omega-3 fatty acids, and better cognitive function.318-320 One study in 2,622 older adults found those with the highest blood levels of long-chain omega-3 fatty acids (including EPA and DHA) had an 18% lower risk of unhealthy aging, defined as chronic disease, physical or cognitive dysfunction, or death for any reason, over a 13-year period.321 Results from other research indicate the ratio of omega-6 to omega-3 fatty acids may be an important factor affecting brain structure and cognitive function.322,323
A meta-analysis of six randomized controlled trials using doses ranging from 400 to 1,800 mg daily of combined omega-3 fatty acids for periods of 3–40 months found that omega-3 fatty acid supplements can slow the rate of cognitive decline in the elderly.324 Similarly, a large review of 24 studies found evidence suggesting a beneficial effect of omega-3 fatty acid intake on cognitive aging.325 However, findings have been inconsistent. For example, 1,720 mg DHA and 600 mg EPA daily for 18 months had no effect on cognitive decline in 390 healthy older subjects326; and, in 99 participants with normal or mildly impaired cognitive function, 750 mg DHA plus 120 mg EPA daily for one year also showed no significant effects.327
In a randomized placebo-controlled trial, 1,680 participants aged 70 and over with subjective memory complaints received either 800 mg DHA plus 225 mg EPA daily or placebo for three years. Although DHA plus EPA supplementation did not affect cognitive function in the initial analysis,328 a secondary analysis including only those with a low baseline omega-3 index (a measure of omega-3 fatty acids in red blood cells) showed supplementation led to improved executive function in this group.329 Data from the same study suggest those with an omega-3 index of ≤ 5% have increased odds of cognitive decline and may benefit most from supplementation.330
Another factor that may influence clinical trial results is the presence of the apolipoprotein E4 (ApoE4) gene variant, which is associated with disrupted DHA metabolism.331 The ApoE4 variant is associated with an increased risk of dementia in individuals with cognitive impairment.552 One study in 915 elderly participants only noted a link between higher seafood consumption and reduced cognitive decline in ApoE4 carriers.332 In another study, an observed protective effect of seafood consumption against amyloid plaques and neurofibrillary tangles was found to be due solely to an effect in ApoE4 carriers.315 Because of such findings, it has been proposed that DHA supplementation may be more important in carriers of ApoE4.333
B Vitamins
B vitamins are needed for homocysteine metabolism, and lower levels of B vitamins, particularly folate, B12, and B6, have been correlated with high homocysteine levels and greater cognitive decline in the elderly.334-337 While B vitamin supplementation has been shown to effectively lower high homocysteine levels, so far, results from clinical trials have been mixed with regard to cognitive benefits.338-341
Researchers have been investigating factors that identify those most likely to benefit from treatment with B vitamins, such as omega-3 fatty acid status, homocysteine level, or degree of cognitive impairment. One randomized trial found the positive effect of B vitamin supplementation on cognitive function was dependent on sufficient omega-3 fatty acid status.317 A 2-year randomized controlled trial found supplementing with B6, B12, and folic acid slowed cognitive decline only in those whose baseline homocysteine levels were 11.3 mmol/L or higher.342 Supplementing with folic acid plus B12 was associated with reduced risk of dementia during five years of monitoring in older adults with mild cognitive impairment.343 In older adults diagnosed with mild cognitive impairment, 400 mcg folic acid daily reduced cognitive decline and decreased blood levels of inflammatory cytokines after six months344 and one year.345 Even after only 12 weeks, a supplement with B6, folic acid, and B12 decreased homocysteine levels and improved cognitive function and depression in a trial of participants with mild cognitive impairment.346
Another potentially important factor is the effect of the methylenetetrahydrofolate reductase (MTHFR) gene; carriers of a particular MTHFR variant have abnormal folate metabolism and require higher intake of folic acid to avoid deficiency.347 They may also benefit less from ordinary folic acid supplements. To overcome this obstacle, one preliminary study used a supplement with L-methylfolate (active form of folate) and B12 (methylcobalamin) in patients with high homocysteine levels and found this treatment reduced cognitive decline and was more effective in those with milder, versus more severe, cognitive dysfunction.348
Alpha-Glyceryl Phosphoryl Choline (Choline Alphoscerate)
Alpha-glyceryl phosphoryl choline (α-GPC) (also known as choline alphoscerate) is a semisynthetic derivative of the nutrient phosphatidylcholine and precursor to the neurotransmitter acetylcholine.349 Acetylcholine is a major neurotransmitter in the autonomic nervous system, and its accelerated breakdown is thought to contribute to age-related cognitive decline and dementia. In fact, reduced concentration of the acetylcholine-metabolizing enzyme, acetylcholinesterase, has been observed in brains of “super agers” (ie, elderly individuals with unusually youthful cognitive function).244
Acetylcholine precursors, alone or in conjunction with acetylcholinesterase inhibitor drugs, are a promising approach to dementia treatment.349-351 One randomized controlled trial compared the effects of 1,200 mg α-GPC daily for 180 days to placebo in 261 patients with mild-to-moderate Alzheimer disease. Those receiving α-GPC experienced improvements in cognitive function and behavioral assessments, while those receiving placebo experienced no change or worsening of clinical measures.352 In a preliminary trial in 50 subjects with mild cognitive impairment, 1,200 mg α-GPC per day for three months resulted in improved cognitive function. A follow-up evaluation performed seven to nine months after the end of treatment found cognitive function remained at a higher level than before treatment.353 Another pilot trial found α-GPC had beneficial effects on cognitive function after 15 days of treatment in patients who had experienced stroke.354
Reports from an ongoing randomized controlled trial showed combination treatment with the acetylcholinesterase inhibitor donepezil plus α-GPC was more effective than donepezil plus placebo in preserving cognitive and behavioral function in patients with Alzheimer disease and cerebrovascular injury after one355 and two years,356 and reduced apathy, the loss of motivation associated with progressive dementia, after three years.357 The most recent report from this trial showed co-treatment with these two agents reduced Alzheimer-related behavior and mood disorders.358
Mango Leaf Extract
Leaves from the mango plant (Mangifera indica) have been used in traditional medicine practices for general health and as a remedy for fatigue.553 Extracts from mango leaf have been shown to have anti-inflammatory and neuroprotective properties in preclinical studies; these positive effects are thought to be attributable largely to mangiferin, a major polyphenol in mango leaf extract.554-556 Mangiferin appears to act as an inhibitor of catechol-O-methyltransferase (COMT), an enzyme responsible for breaking down neurotransmitters such as dopamine, epinephrine, and norepinephrine.553,557 Animal studies have shown that mangiferin improves several aspects of cognition, including spatial recognition and short- and long-term memory.558
In two small, randomized, double-blind, placebo-controlled crossover trials, a single 500 mg dose of mango leaf extract standardized to 60% mangiferin was shown to improve reaction time by nearly 5% compared with placebo and lessen fatigue by nearly 50% compared to baseline.553 In another randomized, double-blind, placebo-controlled, crossover study with 70 healthy adults, a single 300 mg dose of the same mango leaf extract significantly improved performance accuracy (2.4%), episodic memory (2.8%), and visual information processing scores compared with placebo over the course of several hours.554
Peppermint
Extracts and essential oils of peppermint (Mentha piperita) are rich in monoterpenes, which are known for their cognitive benefits.559,560 Peppermint essential oil has been shown to inhibit acetylcholinesterase (AChE) and bind to GABAA and nicotinic receptors. In a small clinical study, 100 µL of peppermint essential oil improved performance on a cognitively demanding task and prevented cognitive fatigue up to several hours after the dose.560
Phosphatidylserine
The brain has a high concentration of phosphatidylserine, a phospholipid that incorporates two fatty acids and is part of cell membranes and myelin. Phosphatidylserine is necessary for all aspects of cognitive function, as well as nervous system control over motor function. Aging is associated with deterioration of brain structure and chemistry that can be affected by phosphatidylserine supplementation.359,360
Early clinical trials using phosphatidylserine extracted from bovine brain tissue showed promising cognitive benefits in elderly individuals361,362; however, safety issues concerning this source of phosphatidylserine led to its removal from the market. Phosphatidylserine can also be extracted from soybeans. Soybean phosphatidylserine, at a dose of 300 mg per day, has been shown in uncontrolled trials to improve cognitive performance in some older individuals with memory complaints.363,364
Bovine phosphatidylserine differs from soybean phosphatidylserine in its fatty acid profile: bovine-sourced contains the omega-3 fatty acid DHA, while soybean-sourced does not. A marine-sourced phosphatidylserine complexed with the omega-3 fatty acids EPA and DHA has been shown to be safe and may have positive effects on cognition in older adults.365 In an open trial in eight volunteers 60 years of age and older with subjective memory complaints, 300 mg per day phosphatidylserine with EPA and DHA for six weeks led to improved performance on a short-term memory test.366 A randomized controlled trial in 157 participants with subjective memory complaints compared the effects of 300 mg marine phosphatidylserine daily with placebo. At the end of 15 weeks, those receiving phosphatidylserine performed better on a test of short-term memory, and the effect was strongest in those with the best baseline cognitive function.367 The trial continued for another 15 weeks with all participants receiving 100 mg phosphatidylserine daily; those who had already been receiving the supplement maintained their cognitive gains and those who had been receiving placebo showed improved cognitive function.360
Colostrinin (Proline-rich Polypeptide Complex)
Colostrum, the first milk produced by the breasts after childbirth, is well known for its high levels of antibodies and other factors with immune-activating effects.368,369 Findings from preclinical and clinical studies suggest colostrinin, a proline-rich polypeptide complex found in colostrum, may help prevent the progression of cognitive decline, particularly in people with Alzheimer disease.370,371 A number of studies have found a range of possible mechanisms for colostrinin’s beneficial effects, including modulating immune activity, preventing oxidative stress and oxidative damage to DNA, reducing inflammation, inhibiting overproduction of nitric oxide, and decreasing age-related mitochondrial dysfunction.372-376
A randomized controlled trial compared colostrinin to placebo in 105 subjects with mild-to-moderate Alzheimer disease. The colostrinin group received 100 mcg colostrinin every other day for three weeks, followed by two weeks with no treatment, for three 5-week cycles. After the first 15-week period, all subjects received colostrinin for a second 15 weeks, following the same dosing regimen. Colostrinin treatment had a stabilizing effect on cognitive function and ability to perform activities of daily living, and participants with mild cognitive losses responded better to treatment than those with more advanced losses.377 Another trial used the same dosing schedule for 16 to 28 months in 33 Alzheimer disease patients and found it resulted in stabilization or improvement in health status.378 In an earlier clinical trial including 46 patients with mild-to-moderate Alzheimer disease, participants were assigned to receive either 100 mcg colostrinin, 100 mcg selenium, or placebo in 3-week cycles, followed by two weeks without treatment, for one year. Eight of the 15 patients treated with colostrinin experienced improvement, and the other seven had no change in their condition; in contrast, none of the patients in the selenium or placebo groups improved, and some worsened.379 Studies have reported mild side effects that resolve quickly in some patients treated with colostrinin.378,379
Vinpocetine
Vinpocetine, also known as Cavinton, is a synthetic derivative of an alkaloid from periwinkle (Vinca minor). Vinpocetine has demonstrated neuroprotective effects such as altering inflammatory signaling, reducing oxidative stress, improving cellular energy production, inhibiting thickening of blood vessel walls, dilating cerebral blood vessels, and possibly preventing atherosclerotic plaque formation.380-383
In a placebo-controlled trial in 26 patients who had experienced multiple strokes, vinpocetine prevented deterioration on one test of cognitive function after three months.384 Other studies using oral vinpocetine have noted its ability to improve cognitive performance in patients with mild cognitive impairment as well as cerebrovascular insufficiency.385,386 Note: women who are pregnant or could become pregnant should not use vinpocetine.
Lithium
Lithium is a mineral used in high doses as a mood stabilizer, primarily in patients with bipolar disorder.387,388 Lithium is naturally present in trace amounts in drinking water, and higher occurrence of lithium in drinking water has been correlated with lower rates of dementia and psychiatric disorders in population studies.389,390 A growing body of preclinical evidence suggests lithium may have neuroprotective effects through its abilities to prevent oxidative and inflammatory neuronal damage, enhance neuroplasticity, modulate protein metabolism, and regulate circadian rhythms and hypothalamic-pituitary-adrenal (HPA) axis activity.387,388,391-393 In addition, animal and laboratory research suggests chronic low-dose lithium treatment can increase neuronal production of BDNF.394-396
A meta-analysis of three clinical trials including a combined total of 222 subjects concluded lithium therapy may be beneficial in individuals with mild cognitive impairment and Alzheimer disease.397 Because of lithium’s substantial potential for toxicity in higher doses,388 microdose therapy is especially appealing. In one trial, Alzheimer disease patients treated with lithium, at a microdose of 300 mcg daily, had less cognitive decline than untreated patients. The difference in cognitive function was significant after three months and progressively widened during the course of the 15-month trial.398 Microdose lithium has been shown in rats predisposed to Alzheimer-like pathology to reduce oxidative stress, neuroinflammation, and abnormal protein accumulation, as well as promote neuronal regeneration and prevent memory loss.399,400 Preclinical research has shown lithium orotate, the form of lithium used in dietary supplements, has a high level of safety.561
Cocoa
Cocoa is made from the seeds of the cocoa tree, Theobroma cacao. Cocoa has high concentrations of free radical-quenching polyphenols called flavanols. Mounting evidence suggests cocoa and its flavanols can improve vascular function, promote cerebrovascular blood flow, and strengthen cognitive function.401,402 Findings from a laboratory study suggest cocoa may also inhibit aggregation of β-amyloid.403 In addition, cocoa’s caffeine, catechins, and other constituents may contribute to its benefits on brain health.404 In general, chocolate products contain polyphenols in proportion to their cocoa content; darker chocolate contains more beneficial compounds than lighter or “milk” chocolate.
Examining data from 2,056 participants in the Seniors-Study on Nutrition and Cardiovascular Risk in Spain, researchers noted daily consumption of 10 grams (0.35 ounces, roughly a one inch square piece) or more of dark chocolate in the previous year was associated with better cognitive performance and lower risk of mild cognitive impairment compared with not eating dark chocolate.405 Another study in 531 subjects, aged 65 years and older, found chocolate consumption was correlated with a reduced risk of cognitive decline during approximately four years of monitoring in those with low caffeine intake (less than 75 mg per day; roughly the amount in a 6-ounce cup of coffee or two cups of tea).406
In a randomized controlled trial in 40 healthy older individuals, taking a cocoa drink providing 494 mg flavanols once daily for 12 weeks increased blood levels of BDNF and improved cognitive function.407 An eight-week randomized controlled trial compared the effects of supplemental drinks providing different amounts of cocoa flavanols in 90 elderly subjects without clinical evidence of cognitive dysfunction. At the end of the trial, cognitive performance on some tests improved in those receiving 993 mg cocoa flavanols per day compared with lower amounts. In addition, those receiving 993 mg and 520 mg had improvements in insulin resistance, blood pressure, and lipid peroxidation (a measure of oxidative stress) compared with those receiving 48 mg per day.408 In a three-month randomized controlled trial in healthy older adults, eating a high-cocoa diet improved regional brain function as well as cognitive performance.409
Spearmint Extract
Spearmint (Mentha spicata) is an aromatic herb that is rich in water-soluble polyphenols, many of which have anti-inflammatory and free radical-reducing properties. Rosmarinic acid and its derivatives generally make up the greatest proportion of spearmint’s polyphenols.410
Rosmarinic acid has shown neuroprotective effects, such as reducing neuroinflammation and brain oxidative stress and preventing β-amyloid-induced cognitive decline, in laboratory models. 411,412 Rosmarinic acid also appears to inhibit an enzyme involved in tau protein pathology, which studies suggest plays a role in cognitive decline.413 In addition, spearmint extract has been found to inhibit the enzyme acetylcholinesterase, an action that may increase levels of acetylcholine and thereby support learning, memory, and mood.410
In a randomized placebo-controlled trial, 90 individuals with age-related memory impairment received either a high-rosmarinic acid spearmint extract (900 mg dose or 600 mg dose) or placebo daily for 90 days. Those receiving the 900 mg dose performed better on memory tests and reported improved ability to fall asleep compared with placebo.414 In a pilot trial in 11 subjects with self-reported mild memory impairment, 30 days of treatment with 900 mg of a high-rosmarinic acid spearmint extract daily resulted in improved performance on tests of reasoning, attention, and concentration. Even short-term administration resulted in improvements in attention and concentration that were noted within 2–4 hours.415
Green Oat Extract
Oat (Avena sativa) is a cereal grain with many active compounds.416,417 An extract from wild green oat has been shown to inhibit an enzyme called monoamine oxidase-B (MAO-B).416 The activity of MAO-B, which metabolizes dopamine, increases in older age, lowering dopamine levels and possibly driving oxidative stress and mitochondrial dysfunction and accelerating tissue aging.418,419 Blocking MAO-B helps normalize dopamine levels, which may reduce oxidative stress and improve aspects of cognition and memory.419,420 Wild green oat extract has also been found to dilate cerebral blood vessels and inhibit another enzyme called phosphodiesterase-4,421 an effect that may slow age-related cognitive decline.422,423
In healthy adults, 1,500 mg of wild green oat extract increased arterial blood flow by slightly more than 40% compared with placebo.424 In healthy middle-aged adults, a single 800 mg dose of wild green oat extract improved performance on tests of attention, delayed recall, memory, and executive function.425 In patients with mild age-related cognitive problems, 1,600 mg wild green oat extract improved performance on a test measuring attention, concentration, and ability to focus on a task.426
Lion’s Mane
Lion’s mane (Hericium erinaceus) is a mushroom with a long history of medicinal use in traditional Chinese medicine. Extracts have been shown to have anti-inflammatory and oxidative stress-reducing effects, and consumption of Lion’s mane has been reported to be associated with neuroprotective, pro-cognitive, anti-aging, and antidepressant properties, among other health benefits.427 In a randomized controlled trial in 30 older individuals with mild cognitive impairment, daily treatment with 3,000 mg powdered lion’s mane for 16 weeks resulted in improved cognitive function relative to placebo.428 In animal research, lion’s mane enhanced neuronal function and improved memory performance in healthy wild-type mice.429 Extracts of lion’s mane have also been found to stimulate neuronal growth factor and formation of new neurons, as well as decrease β-amyloid plaque and amyloid-induced inflammation, in mouse models of Alzheimer disease.430,431
Magnesium-L-threonate
Magnesium-L-threonate is a form of magnesium found to be particularly effective in raising brain magnesium levels.258,259 Increasing brain magnesium levels enhanced neuroplasticity and improved cognitive function in research animals.258,260 Supplementation with magnesium-L-threonate has been shown to prevent age-related decrease of a specific neurotransmitter receptor (NMDA receptor subunit NR2B), inhibit inflammatory signaling, reduce amyloid plaque formation, preserve neural connections, and protect against memory loss in animal models of aging and Alzheimer disease.259,261-263 Other animal research suggests magnesium-L-threonate may augment the cognitive-boosting effects of mentally and physically stimulating activity in mice with Alzheimer-like brain pathology.264
Pyrroloquinoline Quinone
Pyrroloquinoline quinone, or PQQ, is a vital compound that supports growth and development.432 PQQ plays a critical role in oxidation-reduction (or redox) biochemical reactions, in which electrons are given by donor molecules and taken up by recipient molecules. Redox reactions are fundamental to virtually all cellular processes.432,433 Preclinical research suggests boosting PQQ levels may improve mitochondrial numbers and function, reduce systemic and brain inflammation, increase cell longevity, protect against neurotoxins, and possibly improve neurological and cardiovascular health.433,434,435-439 A number of studies also show PQQ may prevent the accumulation of β-amyloid.440-443 Furthermore, PQQ has been found to stimulate the production of a protein called nerve growth factor,444-446 promote regeneration of nerve cells,447,448 and preserve cognitive function in laboratory animals.449
Research findings suggest PQQ may improve cognitive function by increasing regional brain blood flow and oxygen use. In a controlled trial in 41 healthy elderly subjects, 20 mg PQQ daily for 12 weeks resulted in increased cerebral blood flow and slower decline in cognitive performance compared with placebo. In addition, participants with the lowest cognitive function at the beginning of the trial exhibited improvement in one aspect of cognitive function at the end of the trial.450 Another study similarly noted that taking 20 mg PQQ daily for 12 weeks increased regional brain blood flow and oxygen utilization in healthy subjects.451
Nicotinamide Riboside
Nicotinamide riboside is a form of vitamin B3. Like other forms of B3 (nicotinamide and nicotinic acid), nicotinamide riboside is a precursor to nicotinamide adenine dinucleotide (NAD+) in the body.452,453 NAD+ is a universal cofactor that plays a critical role in redox biochemical reactions, through which it is converted to its reduced form, NADH. In addition, NAD+ appears to participate in regulating enzymes that govern an array of cell functions, including gene expression, metabolism, DNA repair, apoptosis (programmed cell death), and aging.454-456
Aging is associated with decreased NAD+ production, and a decreased NAD+/NADH ratio has been correlated with mitochondrial dysfunction and age-related and metabolic disorders such as cognitive decline and dementia, diabetes, obesity, non-alcoholic fatty liver disease, cardiovascular disease, and some cancers.452,457-459 It is thought that raising NAD+ availability may contribute to slowing the aging process and preventing age-related diseases.457,460
In healthy elderly volunteers, 250 mg per day and 500 mg per day of the NAD + precursor nicotinamide riboside safely and dose-dependently raised blood levels of NAD+ after four weeks.461 In mice, administering oral nicotinamide riboside has been found to increase cerebral NAD+ levels and improve cognitive function,462 enhance neuroplasticity, and reduce tau protein-induced neuronal damage.463 In a rat model of vascular dementia, intraperitoneal injection of NAD+ was shown to ameliorate cognitive deficits and inhibit neuroinflammation by protecting mitochondria from damage and decreasing reactive oxygen species production.562 Decreased gene expression of PPAR-γ co-activator 1α (PGC-1α), in addition to its upstream transcription factor Sirt1, is thought to contribute to this cognitive impairment, where NAD+ administration reversed this decrease. Further evidence from animal studies suggest NAD+ therapy could possibly stimulate mitochondrial activity, maintain the regenerative potential of stem cells, and extend lifespan.464,465
In an in vitro study employing a microglial cell line, NAD + displayed protective effects against inflammation, mitochondrial damage, and reactive oxygen species production. Further, Sirt1 overexpression mimicked the protective effects of NAD+.562
Coenzyme Q10
Coenzyme Q10 (CoQ10) plays an essential role in mitochondrial energy production. CoQ10 has demonstrated neuroprotective effects that may be mediated through enhanced mitochondrial function and modulation of microglial cells, the brain’s immune cells that mediate neuroinflammation.466,467 Lower blood levels of CoQ10 have been correlated with increased risk of disabling dementia in older individuals468 and worse cognitive function in heart failure patients.469 Preclinical evidence suggest CoQ10 may slow cognitive decline in patients with neurodegenerative diseases like Huntington, Parkinson, and Alzheimer disease.470-472 Long-term consumption (1 to 2 years) of 100–150 mg ubiquinol (the reduced form of CoQ10) daily has been shown to improve a marker of cognitive performance in Japanese individuals of a broad age range.563
Ashwagandha
Withania somnifera, more commonly known as ashwagandha, is a plant that has been used in Ayurvedic medicine for centuries. The plant and root extracts of ashwagandha contain several bioactive compounds with antioxidant, anti-inflammatory, and immune-stimulating properties.473 Cellular and animal models show that ashwagandha extracts and related compounds rescue neuronal cells from chemical damage and inflammation through multiple signaling pathways, protecting neurons from neurodegenerative processes that typify conditions including Alzheimer, Parkinson, and Huntington diseases.474
In human studies, ashwagandha extract has also been shown to improve memory and cognitive function. In a double-blind placebo-controlled study, 50 adults were randomly assigned to receive either ashwagandha root extract (300 mg twice daily) or placebo for eight weeks. Ashwagandha was associated with statistically significant improvements in short-term and general memory, executive function, sustained attention, and information-processing speed.475 Furthermore, in a randomized controlled trial of 53 people with bipolar disorder, adjunctive use of 500 mg per day of ashwagandha extract resulted in improvements in auditory-verbal working memory, reaction time, and social cognition.476
One of the active compounds found in ashwagandha extract is withanone. Administration of withanone to rats for three weeks was shown to significantly improve cognitive skills by decreasing levels of inflammatory molecules. In the same study, withanone was shown to inhibit β-amyloid, a protein implicated in the development of Alzheimer disease.479
Pregnenolone
Pregnenolone is a steroid hormone that is synthesized from cholesterol in the brain, adrenal glands, and other organs. Pregnenolone can either modulate signaling pathways itself or undergo further metabolism to form other steroid hormones, including progesterone, aldosterone, cortisol, and testosterone.480 In the brain, pregnenolone has been shown to modulate NMDA receptor-mediated neurotransmission, which supports learning and memory.481,564 Furthermore, pregnenolone has anti-inflammatory properties.482
In rats presented with learning and memory challenges, intranasal pregnenolone administration resulted in improvements in learning, long-term memory, and resistance to memory extinction.483 Intraperitoneal injection of 10 mg/kg pregnenolone was shown to ameliorate cognitive impairments induced by NMDA receptor antagonism in rats.565 In a preliminary study, aged rats were shown to have significantly reduced levels of pregnenolone in the hippocampus and other brain regions, and animals with less memory problems had higher concentrations of pregnenolone. Memory deficit in these animals was temporarily corrected by pregnenolone injection.484
Most human studies of the effects of pregnenolone on cognitive decline have been performed in the context of psychiatric disorders, such as schizophrenia and bipolar disorder. In an 8-week, randomized, placebo-controlled trial in patients with recent-onset schizophrenia or a related disorder, use of adjunctive pregnenolone, taken orally, at a dose of 50 mg/day was shown to result in a significant improvement in executive functions as well as visual and sustained attention.485 Another placebo-controlled study in patients with schizophrenia found 30 mg/day pregnenolone improved attention and working memory performance.486
Sage
Sage (Salvia officinalis), a perennial bush native to the Mediterranean region, has been used for its medicinal (and culinary) properties since ancient times. More recently, sage has drawn attention for its cognitive-enhancing abilities.566 Sage extracts have been shown in animal models of Alzheimer to inhibit acetylcholinesterase activity and prevent decline in peripheral BDNF levels,567,568 which tend to be reduced in people with Alzheimer or mild cognitive impairment.569 Extracts and bioactive compounds from sage have also been shown to have some anti-inflammatory effects.566
In a placebo-controlled trial in patients with mild-to-moderate Alzheimer disease, those treated with sage extract for 16 weeks scored better on two different standardized assessments of cognitive function compared with those treated with placebo.570 In another randomized placebo-controlled trial, a standardized extract of sage at doses ranging from 167 mg to 1,332 mg was given to 20 participants aged 65 and older. The participants took single doses separated by 7-day washout periods. The 333 mg dose improved accuracy of attention and certain aspects of memory for up to six hours post-treatment compared with placebo.571 In an acute, double-blind, placebo-controlled study in healthy young adults aged 18‒25, 150 and 300 mg of a commercial sage extract improved memory performance compared with placebo.572
Dried sage leaf was also shown to improve mood and cognitive performance in healthy young volunteers.573 A systematic review concluded that sage preparations may help enhance cognitive performance in healthy subjects and in patients with dementia; however, further rigorous trials are needed.574
Gotu kola
Gotu kola (Centella asiatica), a leafy green plant historically used in traditional medicine in Southeast Asia, is valued for its high concentrations of several beneficial nutrients, including triterpenoids, carotenoids, vitamins B and C, minerals, and other phytonutrients.488 One key active ingredient found in gotu kola is asiatic acid, a triterpene that preclinical studies suggest may prevent cognitive decline caused by some drugs and also modulate neurotransmission, among other beneficial cognitive and neural effects.489-492 Many gotu kola preparations are standardized to asiaticosides, which are metabolized to asiatic acid in the body.493
Preclinical studies have shown gotu kola can reduce markers of oxidative stress, which may in turn improve neuronal health.494-496 Furthermore, gotu kola has been shown to decrease acetylcholinesterase levels in the hippocampus and cerebral cortex of rats at a rate comparable with donepezil, a pharmacologic acetylcholinesterase inhibitor.495,497
In a mouse model of Alzheimer disease, gotu kola water extract was shown to improve memory in a dose-dependent manner. Furthermore, markers of neural density were increased in brain regions that influence information processing (ie, the hippocampus and prefrontal cortex).494 Among older mice, gotu kola water extract was shown to improve performance on a test that measures spatial learning and memory.498
In a study of 28 healthy elderly volunteers, 250–750 mg gotu kola extract daily for two months improved working memory and self-rated mood.499 In a systematic review and meta-analysis of randomized controlled trials in humans, researchers reported that the gotu kola products did not significantly improve cognitive function domains compared with placebo; however, gotu kola did improve mood and alertness, and no adverse events were reported.500
Carotenoids
Carotenoids are organic, strongly pigmented compounds found in algae, plants, fungi, and many bacteria. Carotenoids can be classified as xanthophylls (eg, lutein and zeaxanthin) or carotenes (eg, β-carotene and lycopene). About 50 different types of carotenoids have been found in fruits and vegetables consumed by humans, and about 20 carotenoids are found in human tissues and blood.501
Higher serum and retinal concentrations of lutein and zeaxanthin have been associated with improvements in several biomarkers of inflammation and measures of cognition and neurotransmission.501 Neuroimaging studies revealed that older adults with higher concentrations of lutein and zeaxanthin had increased white matter integrity, particularly in brain regions that are vulnerable to age-related changes.502 Carotenoids have also been shown to reduce inflammatory signaling by decreasing circulating levels of cytokines and other proinflammatory molecules. Furthermore, carotenoids protect cells from oxidative stress, thereby possibly slowing cognitive decline by preventing neuronal cell damage.501
There is extensive evidence supporting the role of carotenoids in cognitive performance. Based on results of the 2011‒2014 National Health and Nutrition Examination Survey (NHANES), higher dietary intake of lutein and zeaxanthin was associated with better scores on all learning and memory tests in participants 60 years and older, suggesting lutein and zeaxanthin may help prevent or slow age-related cognitive decline.503 In contrast, low intake of carotene, among other nutrients, was significantly correlated with an increased risk for cognitive decline in an elderly population.504 Another observational study followed over 7,000 participants who were at least 45 years old at baseline for an average of 16 years. Total serum lutein and zeaxanthin concentration at baseline was associated with a reduced risk of developing all-cause dementia—each 15.4 μg/dL increase in lutein and zeaxanthin corresponded with a 7% decrease in risk in those 65 years or older. Serum β-cryptoxanthin at baseline was associated with a 14% decreased risk of dementia for each 8.6 μg/dL increase in both age groups (45+ and 65+).609 Furthermore, higher serum and retinal concentrations of lutein and zeaxanthin have been associated with improved visual-spatial processing and decision making. During processing and decision-making tasks, functional MRI (fMRI) showed improved neural efficiency in participants who performed better on tasks and who had higher levels of lutein and zeaxanthin.505 Finally, the effects of dietary carotenoids were assessed in nearly 50,000 female nurses. Self-reported subjective cognitive function, assessed in 2012 through 2014, was significantly associated with dietary carotenoid consumption during the prior three decades (1984 through 2006). Nurses who consumed the lowest amounts of carotenoids had 33% higher risk of poor cognitive function than those who consumed the highest amounts.506
In a randomized controlled trial, 80 adults aged 65 to 92 years received either 12 mg lutein and zeaxanthin or placebo for 12 months. Neurons in the cortex, which is critical for higher-order cognition, were significantly more responsive to visual stimuli in participants with higher levels of lutein and zeaxanthin compared to those with low levels, suggesting improved visual memory and processing speeds.507 Furthermore, in a placebo-controlled trial of 91 participants (mean age 45 years) with low levels of macular pigment, a biomarker for levels of these carotenoids in the brain, daily supplementation with 10 mg lutein, 10 mg meso-zeaxanthin, and 2 mg zeaxanthin for 12 months significantly improved memory.508 Finally, in a placebo-controlled study of 62 older adults (mean age 73.7 years), supplementation with 12 mg lutein and zeaxanthin for 12 months was associated with a significant increase in both complex attention and cognitive flexibility.509
Pycnogenol
Pycnogenol is an extract from French maritime pine bark with potent anti-inflammatory and antioxidant properties that supports cognitive function and mental performance. Pycnogenol has been shown to combat oxidative stress, promote sustained attention, and improve memory.537,538 Both preclinical and clinical research supports the use of Pycnogenol for cognitive support.539-541 For instance, Pycnogenol has been shown to attenuate the progression of cognitive impairment in patients with Parkinson disease.542 Furthermore, in a mouse model of Alzheimer disease, Pycnogenol supplementation decreased the number of β-amyloid plaques and improved some aspects of memory.543
A 12-month clinical trial that enrolled 44 healthy subjects aged 55‒70 with high oxidative stress found 100 mg Pycnogenol daily appeared to improve cognitive function.544 A separate 12-week trial involving 30 healthy individuals and 29 matched controls aged 35‒55 found supplementation with 150 mg Pycongenol daily promoted cognitive function and reduced oxidative stress.545 As of mid-2021, a randomized controlled trial was underway in Australia to investigate the efficacy of 150 mg Pycnogenol daily in improving cognitive performance and reducing cognitive decline in elderly participants aged 60‒75.546
Multivitamin/Multi-nutrient Formulas
The effect of multivitamins on cognitive trajectory has been examined in several relatively small studies over the years. When considered collectively, the results of these studies have suggested that multivitamins may provide small benefits for some aspects of cognitive function.575 However, most studies have been short-term, and the multivitamin formulations used have differed.
Two studies of multivitamins and cognitive function stand out for their methodological strengths and sizes. These trials occurred in the context of the Physician’s Health Study II and Cocoa Supplement and Multivitamin Outcomes Study for the Mind (COSMOS-Mind) trial. The first trial enrolled over 5,900 men aged 65 years or older. Participants took a multivitamin and underwent periodic cognitive assessments. The mean follow-up time was about 8.5 years. Multivitamins did not provide cognitive benefits in this study.576 On the other hand, in the COSMOS-Mind trial, over 2,200 participants aged 65 and older were randomized to take a multivitamin and/or cocoa flavanols or a placebo. They were followed-up with cognitive assessments annually for three years. Subjects taking the cocoa flavanols did not exhibit slowing of cognitive impairment. However, subjects taking the multivitamin showed improvements (relative to placebo) in global cognitive function, episodic memory, and executive function.577 Further long-term, randomized, controlled trials are needed to clarify the role of multivitamin/multi-nutrient supplementation on cognitive function.
Vitamin K2
“Vitamin K2” refers to a group of at least 15 related compounds known as menaquinones (MKs).610 Among the menaquinones, MK-4 and MK-7 have been associated with age-related changes in cognitive function.611,612 For example, in a study examining 365 elderly participants, a higher brain concentration of MK-4 was associated with a 17-20% lower risk of MCI.613
Several in vitro and animal model studies have investigated the mechanisms by which menaquinones may support brain health and cognitive function. These investigations have shown that menaquinones are involved in the synthesis of sphingolipids, which are lipids in neural cell membranes that help facilitate cellular signaling, synaptic plasticity, and myelin formation.614 Menaquinones may also help preserve the expression of tyrosine hydroxylase, an enzyme involved in the production of dopamine, norepinephrine, and epinephrine.611,612 MK-4 and MK-7 specifically have been shown to inhibit the upregulation of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), and to protect against oxidative stress.615-618
In a preclinical study examining the effect of MK-7 on cognitive aging, 3-month-old rats were given high-dose vitamin K2 (MK-7) over 17 months. Their cognitive function was compared with that of control rats given a placebo. Vitamin K2 administration improved memory performance, reduced social anxiety and depressive-like behavior, and decreased inflammation, suggesting an attenuation of age-related changes in the brain.619
Although low levels of vitamin K2 have been associated with progression of neurodegenerative diseases, such as Parkinson disease, clinical studies are required to examine the association between vitamin K2 and cognitive decline.611,620
Vitamin D
Although vitamin D is often thought of in the context of bone and immune health, it also exerts substantial activity within the central nervous system.The vitamin D receptor, which influences cellular function and gene expression, can be found on nearly every cell in the body, including the cells of the central nervous system (eg, neurons, astrocytes, and microglial cells). And some of these cells can synthesize the biologically active form of vitamin D (1,25-dihydroxyvitamin D), which suggests the active form is important in the central nervous system.621,626
An observational study published in 2022 evaluated data on vitamin D levels and brain aging in 1,865 subjects with an average age of 52 years. Structural MRI data was obtained on whole brain volumes, gray matter volumes, and hippocampal volumes. Among the men in this study, vitamin D deficiency, defined as 25-hydroxyvitamin D levels under 16.08 ng/mL, was significantly associated with neuroimaging patterns of advanced brain aging. In addition, vitamin D levels were positively associated with total brain and gray matter volumes.622
A prospective cohort study published in 2023 assessed vitamin D exposure and dementia occurrence across 12,388 dementia-free persons. Vitamin D exposure compared with no exposure was associated with significantly longer dementia-free survival and a 40% lower dementia incidence rate. Dementia incidence rates across groups remained significantly lower when adjusted for age, sex, education, race, cognitive diagnosis, depression, and apolipoprotein E4 (APOE4) status.623
In a randomized-controlled trial, 183 participants aged 65 years and older with mild cognitive impairment received 20 mcg (800 IU) vitamin D daily or placebo for 12 months. At 12 months, those in the vitamin D group had significantly greater improvements in overall cognitive function, as measured with a variety of tests assessing memory, attention, and processing; increased telomere length; and reduced oxidative stress compared with the placebo group.624
In a larger randomized controlled trial, 3,424 participants aged 60 years and over received either 50 mcg (2,000 IU) vitamin D or placebo daily for 2–3 years. Changes in scores on several cognitive assessments and a composite of these cognitive assessments were evaluated at baseline and at the end of follow-up. The results showed that vitamin D supplementation only provided better cognitive maintenance over placebo in the Black participants. Among possible explanations for this finding is that Black participants had lower 25-hydroxyvitamin D status at baseline.625
Ergothioneine
Ergothioneine is an amino acid with potent antioxidant and anti-inflammatory activity found chiefly in mushrooms. It accumulates in the mitochondria, which are especially susceptible to oxidative damage.674 Ergothioneine has demonstrated neuroprotective, cardioprotective, and other anti-aging effects.674-676 Animal research suggests ergothioneine can cross the blood–brain barrier, and clinical and preclinical studies have indicated ergothioneine has positive effects on cognitive function and memory.677
In a randomized controlled trial of 52 volunteers that included both healthy people and those with mild cognitive impairment, once daily administration of a mushroom extract providing 5 mg ergothioneine over 12 weeks resulted in significant improvements in psychomotor speed, verbal memory, and simple attention compared with placebo.640 In another small randomized controlled trial, 19 subjects aged 60 years and older with cognitive impairment received either 25 mg ergothioneine or placebo three times weekly for one year. Neurofilament light chain levels stabilized (indicating a slowing of neuronal damage) and cognitive performance improved in the ergothioneine group, while neurofilament light chain levels increased and cognitive function did not improve in the placebo group.678 However, a randomized controlled trial in 147 subjects aged 55–79 years with self-reported memory problems found neither 10 mg nor 25 mg of ergothioneine daily for 16 weeks improved cognitive function significantly more than placebo, although some subjective improvements in memory and sleep were reported by those receiving 25 mg per day.679
Multiple observational studies have identified an association between ergothioneine levels and cognitive function. One study followed 1,344 elderly participants in Japan for a median of 11.2 years; participants were aged 65 years and older and were dementia-free at the beginning of the study. The study found increasing levels of ergothioneine were correlated with decreasing risks of Alzheimer disease-related dementia, non–Alzheimer disease-related dementia, and all-cause dementia. In fact, those with the highest ergothioneine levels had a 44% lower rate of all-cause dementia, 39% lower rate of Alzheimer dementia, and 57% lower rate of non–Alzheimer disease-related dementia than those with the lowest levels.680 Another study conducted in Singapore found ergothioneine levels declined significantly beyond 60 years of age. Furthermore, a subset of subjects with mild cognitive impairment was found to have significantly lower plasma levels of ergothioneine compared with age-matched controls.642 In an observational study that followed 496 elderly subjects attending memory clinics in Singapore, lower plasma levels of ergothioneine were associated with poorer baseline cognitive function and faster rates of cognitive decline in domains including memory, executive function, attention, visuomotor speed, and language.681
An observational study that analyzed 18 years of data from 5,091 participants in the European Investigation of Cancer-Norfolk (EPIC-Norfolk) found people who regularly consumed mushrooms performed better on a battery of cognitive tests than non-consumers.644 Another study that used data from 2,840 adults in the United States aged 60 years and older participating in the National Health and Nutrition Examination Survey (NHANES) found those who reported the highest level of mushroom consumption performed significantly better on certain cognitive tests assessing memory, attention, and processing speed than those who reported the lowest mushroom consumption.682
5 Dietary & Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment (MCI)
An approach that includes healthy diet, physical activity, adequate sleep, and mentally and socially stimulating activities is more important for preserving cognitive health with age than any individual intervention.1,4,143
Physical Activity
Staying physically active throughout life is critical to maintaining healthy cognitive function. Exercise has been linked to slower rates of age-related brain atrophy in key areas of the brain involved in cognitive decline, and being physically active in midlife is associated with slower cognitive decline and reduced risk of cognitive impairment and dementia later in life.144
Preclinical research suggests exercise can stimulate production of growth factors, such as BDNF, which promote neuroplasticity. Exercise also improves cerebrovascular function, supports formation of new blood vessels in the brain, and increases blood flow.144
A large body of evidence indicates that any type and amount of physical activity is better than none when it comes to cognitive health. It is clear that both aerobic exercise and strength training can prevent or delay cognitive decline in older adults, regardless of their current cognitive status.145 Even seated exercises have cognitive benefits for older patients who are unable to exercise upright.146 Exercise therapy has been found to improve the functional connectivity of various brain networks, which is thought to underlie the improvements in cognitive function. In a clinical trial of 33 older adults, roughly half of whom had mild cognitive impairment and the other half had intact cognition, cognitive function was assessed before and after a 12-week exercise therapy intervention. The intervention included walking 30 minutes a day, four days per week for the first four weeks. During the remaining weeks, participants performed 10 minutes of warm-up and cool-down in addition to 30 minutes of walking (50 minutes per session) four days per week. Exercise therapy resulted in an improvement in cardiorespiratory fitness, measures of cognitive function, and functional connectivity between brain networks.638
Diet
Healthy eating patterns help preserve cognitive function and reduce the risk of neurodegenerative disease and age-related cognitive decline.520-522 Diet may also influence the body’s inflammatory environment, which becomes proinflammatory with age and can contribute to cognitive decline. In fact, an observational study of over 1,000 individuals found that higher intake of fruits, vegetables, beans, and tea or coffee was correlated with a stronger anti-inflammatory diet, and those who consumed less of these foods (ie, a less anti-inflammatory diet) were at a 21% higher risk of developing dementia.578
Three diets in particular have demonstrated an ability to protect against cognitive decline:
- a diet based on traditional Mediterranean dietary patterns
- DASH, or Dietary Approaches to Stop Hypertension
- the MIND diet, or Mediterranean-DASH Intervention for Neurodegenerative Delay
Mediterranean diet. Numerous studies have shown that a Mediterranean dietary pattern is associated with less cognitive decline and reduced risk of dementia.199,522 With its emphasis on extra virgin cold-pressed olive oil; vegetables including leafy greens; fruits; grains; nuts and legumes; moderate amounts of seafood, milk products, eggs, and red wine; and low intake of sweets and meat,523 the traditional Mediterranean diet provides ample amounts of critical nutrients such as mono- and polyunsaturated fatty acids, antioxidants, vitamins, minerals, and phytonutrients. It can be used as a template that can be adapted to include local and seasonal availability of specific foods.143,198
The Mediterranean diet has been the subject of PREDIMED, a large, randomized, controlled, multi-center, ongoing trial in Spain. Multiple studies within the PREDIMED cohort found that the Mediterranean diet improved cognitive and memory performance,522 while another found those following a Mediterranean dietary pattern tended to have higher plasma BDNF levels.524 A study including 832 subjects examined participants every two to three years for up to 18 years and found that those whose diets most closely resembled a Mediterranean dietary pattern experienced significantly less cognitive decline than those whose diets least resembled a Mediterranean diet.201
A study based on data collected over 16 years from nearly 28,000 men participating in the Health Professionals’ Follow-up Study found that those whose diets were most Mediterranean-like were 36% less likely to report poor subjective cognitive function than those whose diets were least Mediterranean-like.202 Examining the brains of cognitively normal older-age subjects has revealed that adherence to a Mediterranean-like dietary pattern is associated with reduced β-amyloid accumulation. The components of the diet most closely linked with this effect were high fruit and vegetable consumption and moderate wine consumption.203,204
- Extra virgin olive oil. While it appears that the combination of eating habits comprising the Mediterranean diet is the key to its efficacy, the high consumption of extra virgin olive oil (EVOO) featured in the diet is thought to be an important reason for its protective effect on cognition. Several preclinical studies indicate polyphenols in EVOO can reduce β-amyloid and tau accumulation and toxicity, and may modulate microRNA profiles.205-207 One clinical trial in 285 older adults compared three diets: Mediterranean diet supplemented with as much as 1 liter per week (more than ¼ cup per day) of EVOO; Mediterranean diet including 30 grams per day of mixed nuts; and a low-fat diet. After 6.5 years, the olive-oil-supplemented group had better cognitive performance than the other two groups.208
DASH diet. The DASH diet, developed based on research sponsored by the National Institutes of Health (NIH), shares many important characteristics with the Mediterranean diet. It focuses on vegetables, fruits, and whole grains, while including fat-free or low-fat dairy products, fish, poultry, beans, nuts, and vegetable oils. Foods high in salt (sodium), including processed foods, restaurant foods, olives, and some cheeses and seafoods, are restricted. Sugars from sweets, sweetened foods, and sugar-sweetened beverages are to be limited, as are foods high in saturated fat. These include red meat and fatty meats, full-fat dairy products, and tropical oils such as coconut, palm kernel, and palm oils. A DASH diet is high in magnesium, potassium, and calcium, as well as protein and fiber.525,526
The DASH diet has been demonstrated to reduce high blood pressure, improve lipid levels, and increase circulating levels of antioxidants including glutathione.525-529 An observational study in over 16,000 women over the age of 70 years found that long-term adherence to DASH diet principles was associated with better average cognitive function and verbal memory. This difference was comparable to being one year younger in age.530
In a randomized controlled trial, 160 sedentary men and women over age 55 who had cognitive impairment but no dementia, and cardiovascular risk factors, undertook either aerobic exercise, a DASH diet, both, or a health education program. There was some improvement in executive function in the DASH diet group. The largest and most significant improvements compared to health education alone were in subjects who combined aerobic exercise and DASH diet.531
MIND diet. The MIND diet was developed as a hybrid of Mediterranean and DASH diets specifically targeted to improve brain health and retard loss of brain function. Like the Mediterranean and DASH diets, the MIND diet emphasizes plant foods and limits intake of animal foods and foods high in saturated fat. However, the MIND diet encourages high consumption of berries and green leafy vegetables, but de-emphasizes fruit overall, dairy, potatoes, and fish—all departures from DASH and Mediterranean dietary patterns.532
In an observational study, the MIND diet adherence score compared favorably to DASH or Mediterranean diet scores for predicting cognitive decline. Subjects who most closely adhered to the MIND diet had a rate of cognitive decline similar to that of people 7.5 years younger. A prospective study cohort found that the highest adherence scores for all three diets were associated with a substantially lower risk of developing Alzheimer disease, compared to the lowest adherence score.532 In an Australian cohort of 1,200 individuals followed-up for 12 years, a MIND dietary pattern lowered the risk of cognitive decline by 53%.533
Continued study of the MIND diet found it is associated with significant protection against neurodegenerative disease and cognitive decline. These effects have been comparable, or even superior, to the DASH and Mediterranean diets for these purposes.534 Furthermore, a high level of adherence to the MIND diet has been shown to slow the rate of cognitive decline after stroke.533 Finally, a 2021 study of recently deceased individuals found that greater adherence to the MIND diet was associated with better cognition regardless of underlying brain pathologies such as Alzheimer disease. In this study, researchers analyzed dietary, medical, and autopsy records of 569 people who had recently died. The link between better cognition and MIND diet scores held even when the investigators restricted their analysis to people with autopsy evidence of Alzheimer disease or those without a history of cognitive impairment. These findings suggest greater MIND diet adherence supports healthy cognition regardless of underlying neurocognitive pathology.579
On the other hand, some evidence suggests that a calorie-restricted MIND diet is no more effective at improving cognition than a standard calorie-restricted diet. In a controlled trial, 604 cognitively unimpaired people (65–84 years of age) with overweight or obesity and a history of familial Alzheimer disease were randomized to a control diet (mild calorie restriction only) or MIND diet plus mild calorie restriction for three years. The control diet group focused on portion control whereas the MIND diet group increased consumption of foods found in the MIND diet. Both groups displayed a similar increase in cognitive function, measured by a composite score obtained from a battery of 12 tests, and no significant difference in physical brain characteristics measured with MRI.627
Sleep
Sleep is a critical time for brain rest and repair, and both acute and chronic sleep disturbances have measurable negative impacts on physical, emotional, and cognitive health. Long-term sleep deprivation and chronic sleep restriction or fragmentation damages neuronal function and contributes to stress, mood symptoms, and cognitive dysfunction.178 In healthy older adults, subjective cognitive symptoms are reported more often in those also reporting sleep disturbances.179
Aging is naturally associated with diminished sleep quantity and quality,180,181 which may influence cognitive function by disrupting circadian regulation and stress hormone signaling, and promoting systemic inflammation, metabolic disturbance, and fat deposition.182,183 Furthermore, poor sleep may influence cognition through epigenetic changes affecting neuroplasticity.183
According to large meta-analyses of research into this relationship, the optimum amount of sleep for healthy cognitive function appears to be 7–9 hours per night, with both shorter and longer sleep durations associated with increasing risks of cognitive decline, mild cognitive impairment, and dementia.184,185580,581
Sufficient daily activity and a nighttime environment that promotes sleep may help older people struggling with sleep disorders. Strategies that have demonstrated some effectiveness include181,186,187:
- Daytime activities. Because too little or too much activity during the day can contribute to sleep problems at night, maintaining a balanced schedule of daily activities and engagements may help prevent daytime napping and facilitate better nighttime sleep.
- Meditation. Mind-body therapies and meditations promote relaxation and may increase time asleep.
- Cognitive behavioral therapy. Cognitive behavioral interventions for insomnia focus on reframing negative thought patterns around sleep.
- Sleep-enhancing devices. Use of devices that improve the sleep environment (eg, earplugs, eye masks, white noise machines, weighted blankets, and devices that play sleep-inducing music) have all demonstrated some effectiveness in reducing sleep disturbance.
- Daytime bright-light therapy. Bright light therapy may help increase daytime activity, reduce daytime napping, and reset the circadian clock, thereby assisting in restoring normal sleep patterns.
A variety of other important considerations for healthy sleep are reviewed in Life Extension’s Insomnia protocol.
Essential Oils/Odorants
The olfactory system is responsible for the sense of smell and is highly integrated with various regions of the brain such as the hippocampus, a structure that plays a significant role in learning and memory. As a result, aromatherapy and essential oils are emerging as safe, non-nutritional interventions to support brain and cognitive health. Animal studies suggest that aromatherapy helps improve cognitive dysfunction by preserving synaptic density, supporting neurotransmission, and reducing the levels of beta [ß]-amyloid and abnormally phosphorylated tau.628-630 Several clinical studies have shown that olfactory training, sometimes called olfactory enrichment, can improve mood, enhance memory and cognition, and positively modify brain structure in healthy individuals as well as those experiencing cognitive decline.631
A study including 36 healthy young individuals found that six weeks of olfactory training for 20 minutes per day resulted in increased volume of various brain regions as measured with MRI.632 A separate study including 43 adults aged 60–85 not diagnosed with cognitive decline or dementia exposed participants to seven different odorants (rose, orange, eucalyptus, lemon, peppermint, rosemary, and lavender) per week nightly for two hours; trace amount of odorant was used as control. Those in the odorant group exhibited a significant improvement on a test of learning and memory, as well as improved functioning of a brain circuit involved in learning and memory.633 Olfactory training twice daily for five months with odors of lime, cloves, eucalyptus, and rose was found to increase scores on a test of cognitive function, improve subjective well-being, decrease depressive symptoms, and reduce cognitive age in healthy adults aged 50–84 years.634
In a randomized controlled trial, 37 patients with mild cognitive impairment were randomized to olfactory training or placebo twice daily for four months. Olfactory training was associated with improved global cognition that correlated with increased thickness of the hippocampus and other regions involved in cognitive function.635 Aromatherapy with rosemary and lemon oils in the morning and lavender and orange in the evening was also found to improve cognitive function in patients with Alzheimer disease after 28 days.636 A systematic review of seven studies also found that olfactory training can act as a simple and convenient intervention to help improve and maintain cognitive function.637
Mental Activity
Engaging in mentally stimulating activities has been shown in numerous studies to be important for cognitive health during all life stages. It is thought that education and other forms of mental stimulation build cognitive reserve (ie, the brain’s ability to use alternative neuronal pathways to accomplish cognitive tasks) over a lifetime. Having greater cognitive reserve may delay the onset of symptoms related to aging or pathological changes in the brain.6,147
Higher education level and bilingualism over a lifetime have protective effects on cognitive function, and early research suggests educational pursuits and language acquisition at older ages may still benefit some aspects of cognition.6,148,149 A meta-analysis of studies found that musical practice is associated with preservation of a variety of aspects of cognitive function, including those affected by aging. The effect is strongest in those with long-term musical training, but is also observed after short-term musical training later in life.150
Mentally stimulating leisure activities have been linked to cognitive benefits in multiple studies. For example, in one study in 100 cognitively healthy older adults, long-term jigsaw puzzlers were found to have higher function in all aspects of cognitive ability examined.151 There is some evidence that doing Sudoku and crossword puzzles can benefit the aging brain.152,153 A study in 16,572 participants aged 65–100 years found that higher frequency of engagement in word or number games was associated with better cognitive performance on tests of memory, numeracy, and verbal fluency both at baseline and after two years. Even those who began playing word or number games after the beginning of the study demonstrated better cognitive function at the end than those who did not play word or number games regularly. The effect was equally strong in participants of all ages and more pronounced in those with a lower level of education.154 Another study found that activities such as computer use, crossword puzzles, handicrafts, and educational courses were each associated with reduced cognitive decline over one year.155 Early research suggests mentally stimulating computerized programs may be useful for improving cognitive performance in patients with mild cognitive impairment and dementia.156
Stress Reduction
Chronic stress, anxiety, depression, and sleep disturbance are often inter-related. These conditions have well-established detrimental effects on brain structure and function, and are associated with increased risk of dementia.157 Stress reduction techniques, such as meditation and yoga, may have a role in slowing age-related cognitive decline and preventing cognitive impairment.157,158 Mindfulness meditation, for example, has consistently been found to positively affect brain structure, function, and plasticity, including in regions of the brain associated with cognitive dysfunction.6 Long-term yoga practitioners have been found to be less likely to have age-related brain atrophy and perform better on some cognitive tests compared with those not practicing yoga.159,160 Other research found similar effects in long-term meditators.161 Looking at cognitive function in subjects with a wide age range, one study determined that long-term yoga practitioners and meditators had slower cognitive decline and more resilient neuronal networks than those with neither practice.162
Preliminary studies have indicated that meditation practice can improve memory, attention, and executive function in older adults,158,163 and may mitigate age-related cognitive decline.164 Mindful movement therapies such as tai chi, yoga, and walking meditation also have positive effects on quality of life, mood, and cognitive function.165 In one randomized controlled clinical trial in 118 participants with an average age of 62, an eight-week Hatha yoga program involving postures, breathing, and meditation exercises improved performance on tests of attention and information processing speed.166
Social Engagement
Social engagement, like physical and mental activity, may be an important mediator of healthy brain function throughout life.167 A growing number of studies indicate high social engagement and strong social networks are correlated with reduced age-related cognitive decline, mild cognitive impairment, and dementia.167,168 On the other hand, progressive loss of cognitive function, as well as physical function, can lead to diminished strength of social networks and increased isolation, contributing to further cognitive losses.169-171
Strong social networks have been correlated with better cognitive function in older adults and reduced likelihood of experiencing anxiety and depression symptoms in those with mild cognitive impairment.172 One study found that cognitive decline in older men and women over an eight-year period was mitigated by regular engagement in social activities, regardless of cognitive status at the beginning of the study.173 Similarly, social engagement was correlated with slower cognitive decline in 543 cognitively healthy participants, initially 67-years of age, who were monitored for eight years.174 Volunteering was identified in one study as a particular predictor of cognitive resiliency with aging, which may be due in part to the combined social and cognitive aspects of many volunteer activities.175
Participating in group social activities may become more important to cognitive health at older ages.176 Even in the very old, social engagement appears to be beneficial; participation in arts, crafts, and social activities were all noted as protective against mild cognitive impairment in a group of 256 people aged 85 years and older who were cognitively normal at the beginning of the study and were monitored for approximately four years.177
Coffee
Numerous preclinical and clinical studies have examined the potential for drinking coffee to help prevent cognitive decline. Caffeine and coffee are recognized to improve short-term memory and cognition, and some research indicates long-term coffee consumption could protect against dementia and cognitive decline. Furthermore, preclinical models have demonstrated plausible biological mechanisms for bioactive components in coffee to be neuroprotective.209 For instance, in a three-year study in 145 cognitively healthy elderly participants, moderate-to-heavy coffee drinking was linked to reduced cognitive decline, as well as better preservation of brain white matter and cerebral blood flow.210 Similarly, an analysis of 11 prospective studies found the highest levels of coffee consumption were associated with a 27% lower risk of Alzheimer disease.211 However, some studies have found that smaller amounts also appear to protect cognitive function. An analysis of nine prospective studies concluded that optimal protection resulted from 1‒2 cups of coffee per day,212 while other studies suggest coffee’s effects on cognition and the brain are complex and require further study.213-215
Caloric Restriction
Caloric restriction, a dietary intervention in which calorie intake is reduced but adequate nutrient intake is preserved, has been shown to delay the onset of age-related diseases and extend lifespan in many organisms.216 This effect is thought to be due to a triggering of resilience mechanisms that enhance cellular resistance to stress.217,218 This effect is known as hormesis.
In rodent models, caloric restriction was associated with decreased neural stem cell senescence, increased neuroplasticity, and better cognitive performance.218 Specifically, caloric restriction has been found in animal models to stimulate neural stem cell activity,219 promote normal metabolism of phospholipids needed for myelin production,220 lower stress reactivity and stress-related changes in brain structure,221 and induce epigenetic changes that support youthful gene expression in aging brains.222 Some of the same metabolic and molecular changes and health benefits associated with caloric restriction in animals have been demonstrated in humans engaging in 25% caloric restriction or through an intermittent fasting strategy, combined with physical activity.216
More information is available in Life Extension’s Caloric Restriction protocol.
Hearing Loss
Hearing loss can negatively affect cognitive function and increase the risk of cognitive decline, in part by impairing verbal communication and social connections.645 An umbrella review of 11 systematic reviews and meta-analyses identified a 30% increased risk for cognitive impairment and 59% increased risk for dementia in individuals with age-related hearing loss compared to those without.646 A separate meta-analysis of 18 studies totaling approximately 20,000 individuals identified that hearing loss was associated with cognitive decline in the elderly.647
The use of hearing restorative devices, such as hearing aids and cochlear implants, can help mitigate or delay cognitive decline associated with hearing impairment. A trial of 98 individuals with hearing loss (aged 62–82 years of age) assessed the effect of hearing aids on cognitive decline. Participants were assessed before and 18 months after hearing aid use. Those who used hearing aids had clinically significant improvements in cognition compared with those who did not, suggesting that treatment with hearing aids can delay cognitive decline.648 A meta-analysis of eight studies with nearly 127,000 participants followed-up for two to 25 years found that the use of hearing restorative devices was associated with a 19% decreased risk of long-term cognitive decline.649
Vision Correction
Untreated vision loss and blindness affects over 7 million Americans, including more than 1.6 million individuals under 40 years of age.663 Evidence suggests that vision loss, particularly when left untreated, is a risk factor for cognitive decline and dementia.664,665 A meta-analysis of 14 cohort studies totaling over six million individuals who did not have cognitive problems at baseline found that visual impairment was associated with 47% increased risk of dementia. An accompanying meta-analysis of 12 studies including 45,313 individuals found that vision loss was associated with a 35% increased risk of developing cognitive impairment.666
Correcting vision loss appears to reduce the impact on cognitive decline and dementia. A study examining 3,038 adults aged 65 years or older with cataracts and healthy cognition at baseline found that cataract extraction was associated with a 29% reduced dementia risk compared with those whose cataracts were not removed.667 In a separate study, data from over 300,000 individuals in the UK Biobank was assessed—those with cataracts had a 21% increased risk of dementia, but those who underwent cataract extraction had no difference in dementia risk compared with healthy controls.668 It is unclear if vision loss is a direct cause of cognitive decline, or if it is only associated with cognitive decline due to other underlying health conditions that contribute to cognitive decline.664
Avoid Tobacco Smoke
Smoking, including exposure to secondhand smoke and maternal smoking, increases the risk for dementia and cognitive impairment, but cessation of smoking can lower the risk of dementia in former smokers.650,651 In a study analyzing health data from nearly 800,000 Korean individuals, cessation of smoking was associated with a lower risk of dementia compared to a sustained smoking intensity or reduction in smoking without cessation.652 A population-based study examining over 350,000 individuals from the UK biobank found an 11% and 31% increased risk of developing dementia in individuals exposed to secondhand smoke for up to four hours a week or greater than four hours a week, respectively, compared to those who live in an environment without smokers.651
The Neurological Disorders in Central Spain (NEDICES) cohort study examined 2,624 adults (mean age = 72.7 years) at two time points separated by three years and found that cumulative smoking frequency was associated with cognitive decline in adults without dementia. The effect was more pronounced for individuals who smoked more frequently, and former smokers were found to have less decline in cognitive function than current smokers.653 Smoking is also associated with health problems that increase the risk of dementia and cognitive impairment, such as stroke and cardiovascular disease.654
Avoid Excessive Alcohol Consumption
Excessive alcohol consumption of 12 or more standard drinks (21 units or 168 grams of ethanol) weekly has been associated with brain changes and increased risk of cognitive impairment and dementia. A study including 97 patients with alcohol use disorder found that age, particularly those aged 53 years or older, and increased total bilirubin level (a measure of alcohol use) were associated with lower scores on a test of cognitive function.655 A meta-analysis of 76 studies found that alcohol use disorder was associated with increased risk of cognitive impairments as well as Alzheimer disease and vascular dementia.656 Men may be at greater risk of alcohol-related cognitive impairment and dementia than women. A review of seven publications revealed that 3.2% and 7.8% of incident dementia cases were attributable to excess alcohol use (at least 24 grams of ethanol daily) in 45–64 year old women and men, respectively.657 In a French longitudinal study of over 31.5 million adults, excessive alcohol consumption was the strongest modifiable risk factor for dementia onset.658 This association appears most frequently with heavy alcohol consumption, and the effect of light-to-moderate drinking remains unclear. For instance, a meta-analysis of three studies found that consuming more than 12 standard drinks per week was associated with an 18% higher risk of dementia compared with drinking fewer than 12 standard drinks per week.650
Air Pollution
Air pollutants are associated with poor health outcomes and may accelerate cognitive decline, particularly among the elderly.650,659 An assessment of 460,872 individuals (mean age 56–58 years) from the UK biobank found that those with high exposure to various pollutants, such as particulate matter and nitrogen oxides, had an increased risk of all-cause dementia, Alzheimer disease, and mild cognitive impairment of 7%, 8%, and 19%, respectively, compared to those with low exposure.660 A separate study examining approximately 9,000 individuals from the English Longitudinal Study of Ageing (ELSA) cohort also identified an association between increased exposure to air pollutants and cognitive decline.661
Improving air quality may help mitigate this risk. A study including 2,239 women aged 74–92 years monitored air quality for 10 years and found that a reduction in air pollutants was associated with reduced risk of incident dementia.662 More research is needed to identify the impact of pollution mitigation strategies on cognitive decline and dementia.
6 Risk Factors Associated with Cognitive Decline & Mild Cognitive Impairment (MCI)
Older age is the number one risk factor for age-related cognitive decline, as well as mild cognitive impairment and dementia. Women have a higher risk of dementia than men. Furthermore, a number of risk factors for late-life dementia have been identified, many of which have their strongest impact on late-life cognitive function when they occur in midlife.21-23 These risk factors include:
- Sedentary lifestyle21,22
- Low educational attainment21,22
- Smoking21,22
- Obesity21,22
- Insulin resistance and type 2 diabetes21,22,582
- Chronic inflammation582
- Hypertension21,22
- High total and LDL cholesterol664
- Chronic kidney disease21
- Atrial fibrillation (a type of arrhythmia)21
- Cardiovascular disease24
- Cerebrovascular disease512
- Traumatic brain injury58-60
- Depression21
- Sleep disorders25
- Sleep apnea26
- Hearing loss583
- High homocysteine levels27
- Heavy metal toxicity28
- Excessive alcohol consumption650
- Air pollution650
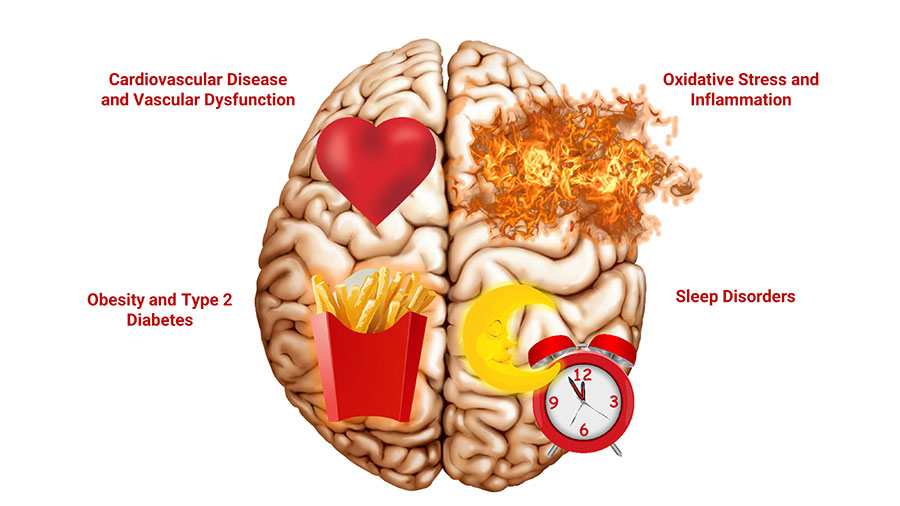
Anticholinergic medications block the effects of acetylcholine and are used to treat a wide variety of illnesses, including asthma, chronic obstructive pulmonary disease (COPD), Parkinson disease, depression, and allergies, among other conditions.510 In a recent study of 688 cognitively healthy older adults, the effect of anticholinergic medication on cognitive function was assessed over a 10-year period. Individuals who used anticholinergic medications at least once per week for more than six months at baseline had a significant, 1.5-fold increased risk of progression to mild cognitive impairment. The increase in risk associated with anticholinergic medication use was particularly pronounced among individuals with the APOE ε4 genotype, who had a 2.7-fold risk increase compared with those not using anticholinergic medications who did not have the APOE ε4 genotype. Furthermore, anticholinergic medication use in individuals with biomarkers of Alzheimer disease in their cerebrospinal fluid was associated with a nearly 5-fold increase in risk for development of mild cognitive impairment. Anticholinergic use was also associated with a more rapid decline in memory and language, particularly among those with the APOE ε4 genotype or with cerebrospinal fluid markers for Alzheimer disease.511
7 Mechanisms Involved in Cognitive Decline & Mild Cognitive Impairment (MCI)
Cognitive decline is a complex process with multiple overlapping mechanisms that are not fully understood. Below is a discussion of current understandings regarding some of the processes that contribute to cognitive decline in older age.
Stem Cell Senescence
Groundbreaking studies in the 1990s revealed specialized regions of the human brain harbor stem cells, known as neural stem cells, that may continue to repair and regenerate brain tissue throughout life.8,32 Growth factors such as BDNF and other signaling factors in the brain environment appear to stimulate neural stem cell proliferation and the formation of neurons and neuronal connections.33,34 The ability of the brain to form new neurons and connections and rearrange neural networks in response to signals from the environment is known as brain plasticity, or neuroplasticity.32
With age, neural stem cells become less responsive to stimulation and stem cell signaling can become dysregulated.35 This condition, known as stem cell senescence, is thought to be a major contributing factor in the diminishing plasticity that characterizes the aging brain.36-38
Circadian Rhythm Disturbance
The circadian rhythm is a natural cycle that affects the brain and the rest of the body in many important ways. Circadian clocks synchronize metabolic, physiologic, and behavioral rhythms with environmental cycles, such as light-dark cycles and daily eating patterns.39,40 Among the many bodily functions regulated by circadian signaling are acquisition of learning and consolidation and recall of memories.40,41 Desynchronization of the circadian clock, such as through shift work, chronic stress, and sleep disorders, can contribute to cognitive decline.40 Circadian rhythm disruption is thought to interfere with neurogenesis and reduce neuroplasticity.42 Melatonin is one hormone that plays a role in the circadian rhythm and its decline with increasing age may be a contributing factor.584
Cerebrovascular Dysfunction
The term “cerebrovascular” refers to the blood vessels supplying the brain. Cerebrovascular dysfunction refers to various (often age-related) pathologies of the brain’s circulatory system, including atherosclerosis, hypertension, and amyloid deposition in blood vessels.512,585 Another age-related cerebral blood vessel defect is arterial stiffness, in which vessels become less responsive to changes in blood pressure, and to oxygen and nutrient demands.513 These and other changes can eventually result in an interruption of cerebral blood flow, depriving the brain of oxygen and fuel.512,514,515 Stroke, the most serious manifestation of cerebrovascular problems, occurs when blood clots or hemorrhages (uncontrolled bleeding) arise in cerebral blood vessels. Cognitive function is often impaired after a stroke.516-518 Another possible outcome of cerebrovascular dysfunction is transient ischemic attack (sometimes called a mini-stroke), which is a temporary decrease in blood supply that can negatively affect cognition.519
The blood vessels that supply the brain have a unique structural feature called the blood‒brain barrier, made up of specialized junctions between endothelial cells (ie, the cells that form the inner lining of blood vessels). In healthy individuals, these junctions exert tight control over the movement of compounds between the blood and the brain. The blood‒brain barrier has been observed to lose integrity with age, becoming increasingly permeable to potential toxins and pro-inflammatory factors.14
Orthostatic hypotension—low blood pressure upon standing after sitting or lying down—has been associated with cognitive decline. In a population-based cohort study including 2,532 dementia-free participants aged 60 or older, orthostatic hypotension was associated with an increased risk of dementia and cognitive decline. Participants with orthostatic hypotension (defined as a decrease of ≥20/10 mmHg in systolic/diastolic blood pressure upon standing) with no cognitive impairment at baseline had a 40% increased risk of developing dementia during 12 years of follow-up, and those exhibiting cognitive impairment had a 54% increased risk of progression to dementia.586 These results suggest orthostatic hypotension, even when asymptomatic, is associated with increased risk of dementia and accelerated progression from cognitive impairment to dementia in older adults.
Blood pressure variability (BPV) is also being investigated as a risk factor for cognitive impairment and dementia. While it is known that BPV in midlife can increase the risk of late-life dementia, the effect of BPV on cognition in older adults without cognitive impairment remains unclear. An assessment of data from the ASPREE clinical trial, which includes nearly 20,000 participants who were free of dementia and significant cognitive impairment at time of enrollment, suggests a link between higher BPV and cognitive deficits in older adults (aged 70 years or older or 65 and older if a United States minority) without previous major cognitive impairment. Individuals displaying the highest degree of BPV had an increased risk of dementia and cognitive decline compared with those in the lowest tertile of BPV. Interestingly, there was a sex-association with increased risk of dementia in men, but not women.587
Neuroinflammation
Aging is associated with elevated inflammatory signaling involving activated microglia, astrocytes, blood vessel endothelial cells, and other cell types, causing neuroinflammation. This leads to increased production of free radicals and other neurotoxins that damage neurons and trigger neuronal degeneration.11,47 Neuroinflammation also degrades the blood‒brain barrier, exposing neurons to more potential toxins.9 Conditions associated with systemic inflammation, such as lack of physical activity, poor diet, obesity, and type 2 diabetes, have all been associated with age-related cognitive decline and dementia.21,48 An unhealthy gut microbiome is another possible source of inflammatory signaling that may contribute to deterioration of the blood‒brain barrier and neuroinflammation.49
Mitochondrial Dysfunction & Oxidative Stress
The brain uses about 20% of the resting body’s oxygen, and roughly 85% of that oxygen is consumed by brain cell mitochondria. The brain is particularly sensitive to mitochondrial dysfunction, and free radical production in the brain is exceptionally high. Free radical production in a healthy brain is balanced by powerful antioxidant defenses; however, in an aging brain, antioxidant enzymes like glutathione reductase and superoxide dismutase (SOD) are less active, leading to an imbalance that favors free radical production and creates an environment of high oxidative stress.47,50
Oxidative stress damages cellular and mitochondrial DNA, membranes, and proteins, contributing to decreased brain cell activity and increased mitochondrial dysfunction. Mitochondrial dysfunction results in lower ATP production and even more free radical generation,50 and contributes to depletion of neural stem cells.51 Reduced energy for metabolic activity within brain cells leads to their diminished ability to engage in normal neuronal activities, including maintenance of cell membranes and production of myelin,13 as well as activities related to learning, memory, and cognition.47 In addition, oxidative stress increases inflammatory signaling, exacerbating neuronal damage and loss.48
Metabolic Disturbance
Metabolic disturbances like insulin resistance and obesity are implicated as contributors to cognitive impairment and dementia. It is thought that systemic inflammation caused by insulin resistance and obesity may drive neuroinflammation, brain insulin resistance, brain mitochondrial dysfunction, and brain oxidative stress. These conditions eventually lead to neuronal damage and cognitive decline.52,53
Disordered blood lipid levels and high blood glucose levels have consistently been found to correlate with cognitive dysfunction,52-54 and type 2 diabetes has been correlated with increased risk of mild cognitive impairment, as well as its progression to dementia.55 In addition, Alzheimer disease increases the risk of developing type 2 diabetes, suggesting a bidirectional relationship.56 Although the mechanism underlying the connection is not fully understood, the relationship between insulin resistance and Alzheimer dementia in particular is so compelling that it is sometimes referred to as “type 3 diabetes.”57
Abnormal Protein Accumulation
Beta [β]-amyloid and tau proteins occur normally in the brain, but when high levels of these proteins accumulate, they can trigger structural changes that disrupt neuronal function and signal transmission.2,64 In the aging brain, β-amyloid proteins accumulate in the spaces between neurons due to increased production, reduced clearance, or both.1,14 At high concentrations, β-amyloid proteins coalesce and form plaques around neurons.64 Tau proteins become damaged through a chemical process called phosphorylation. Aggregates of phosphorylated tau inside neurons trigger neurofibrillary tangle formation.64 Such plaques and tangles interfere with normal neuron-to-neuron communication, and are the hallmarks of Alzheimer disease, but recent studies revealed β-amyloid and phosphorylated tau may begin to accumulate decades before the onset of clinical dementia.65-68
High levels of β-amyloid and tau in the brain, as well as higher tau levels in the blood, have each been independently correlated with cognitive decline in the elderly and disease progression in those with mild cognitive impairment.65,69,70 While the nature of the relationship between abnormal protein accumulation and cognitive decline is not fully understood, phosphorylated tau proteins in particular appear to interfere with synapse function and induce a neuroinflammatory process leading to neuronal dysfunction even before tangles develop.65,71
Epigenetics
“Epigenetics” is a term used to describe biological phenomena that affect how cells use the information stored in the genetic code. Epigenetic processes emphasize or de-emphasize the information contained in sections of the genome. Sections emphasized by epigenetic processes are said to be “expressed,” and de-emphasized sections are “silenced.” Epigenetic processes do not change the genetic code, but rather how cells “read” the code. Factors such as lifestyle habits, nutrition, and the environment (eg, exposure to air pollution) can influence epigenetic gene expression and silencing.
There is increasing evidence that epigenetics play a crucial role in learning, memory, and cognition in older adults and influence development of cognitive impairment and dementia.72 For example, epigenetic alterations affecting the brain’s circadian clock have been noted to impact function in key brain regions associated with cognitive decline.73 Factors that may trigger epigenetic changes associated with cognitive decline and dementia include disordered breathing patterns (eg, hyperventilation syndrome), poor diet, alcohol overconsumption, and sleep deprivation.74
Disrupted Homocysteine Metabolism
Homocysteine is an amino acid derivative that has detrimental effects on blood vessels, contributing to vascular inflammation, thickening of the vessel walls, and endothelial dysfunction. It is a contributing factor in atherosclerosis and increases the risk of stroke. Homocysteine’s effects on brain function may be related to its impact on cerebral blood vessels,75 but some evidence also suggests homocysteine increases oxidative stress and neuroinflammation, and may have direct neurotoxic effects.76
A consensus statement by a panel of experts published in the Journal of Alzheimer’s Disease in 2018 stated that moderately elevated blood homocysteine levels (>11 mmol/L) are a contributing cause of age-related cognitive decline.27 High homocysteine levels have been linked to brain atrophy,77 and have consistently been associated with an increased risk of cognitive impairment, dementia, and Alzheimer disease.27 Homocysteine is converted into cysteine via a pathway requiring pyridoxine (B6), or into methionine through a chemical process called methylation that is dependent on the B vitamins folate (B9) and cobalamin (B12). Folate and B12 deficiencies are common in the elderly and are the main cause of hyperhomocysteinemia.76 Treatment with these B vitamins has been shown to reduce homocysteine levels, slow brain atrophy, inhibit cognitive decline, and improve memory.27,77
Fibrinogen & Cognitive Decline
In a paper published in 2019, scientists at the Gladstone Institutes in San Francisco demonstrated that the blood-clotting protein fibrinogen, which can enter the brain after vascular damage weakens the blood‒brain barrier, may contribute to cognitive decline.78 Once fibrinogen enters the brain, the researchers found, it forms deposits that activate certain types of microglial cells (the immune cells of the central nervous system). Microglial activation generates reactive oxygen species and damages the dendritic spines, destroying the synaptic connections between neurons and causing cognitive decline. The scientists demonstrated that even very small quantities of fibrinogen in healthy brains caused a loss of synapses as seen in Alzheimer disease—and even in the absence of amyloid plaques. Blocking fibrinogen from binding microglia reduced synaptic deficits and cognitive decline in a mouse model of Alzheimer disease. The vascular component of Alzheimer pathology could be a reason why clinical trials aimed solely at reducing amyloid plaque have been unsuccessful. Combination therapies that address vascular changes as well as amyloid deposits may prove to be more successful in the future.
Several earlier studies established the connection between fibrinogen levels and cognitive decline. In a study of over 2,300 middle-aged to elderly subjects, higher plasma fibrinogen levels at baseline were predictive of cognitive decline after five years.79 Another study by the same research group linked a specific genotype associated with cognitive decline to higher fibrinogen levels.80 Higher fibrinogen levels after ischemic stroke have also been associated with poorer cognitive outcomes.81
Hormone Imbalance
The brain is an integral part of the body’s hormonal network, regulating hormone production and responding to hormone signals. The hypothalamic-pituitary-adrenal (HPA) axis and the hypothalamic-pituitary-gonadal (HPG) axis demonstrate the integrated relationship between the brain and hormone-producing glands. Hormones such as cortisol, pregnenolone, dehydroepiandrosterone (DHEA), estrogen, and testosterone affect brain structure and function. Age-related diminishment in levels of these hormones and dampening of the brain’s responsiveness to hormone signaling may impact susceptibility to cognitive decline and dementia.82
Estrogen. Estrogen modulates brain function by enhancing cerebral blood flow, activating nerve growth factors, and preventing neuronal damage. It also appears to have a critical impact on mitochondrial energy production. In women, the drop in estrogen levels that occurs during perimenopause may be a contributing factor in age-related cognitive decline.83,84 Indeed, many women report changes in cognitive function around the time of menopause, although objective measures suggest this may be more profound with surgical menopause than natural menopause. Clinical trials in women suggest initiating estrogen therapy soon after menopause may have the greatest benefit in lowering dementia risk later in life. In fact, initiating estrogen therapy at an older age has been associated with no effects or detrimental effects on cognitive function.84,85 One complicating factor in clinical trials is the use of progesterone in combination with estrogen: while natural progesterone may augment the neuroprotective effects of estrogen, synthetic progestins such as medroxyprogesterone acetate (MPA) (which are typically used with estrogen in postmenopausal hormone therapy) appear to have the opposite effect.84 More information is available in Life Extension’s Female Hormone Restoration protocol.
Testosterone. Testosterone is an important regulator of cognition and mood, and lower levels in middle-aged and elderly men have been associated with depressive symptoms, worse cognitive performance, and increased risk of dementia in some studies.86 In men 70 years and older, greater reductions in testosterone levels have been correlated with increased cognitive decline.87 Some research suggests testosterone therapy may improve mental health, quality of life, and aspects of cognitive function in men with low levels.82,86 In women, however, higher testosterone levels in older age appear to be associated with more rapid cognitive decline,88 but this finding is complicated by other data suggesting testosterone replacement therapy may benefit cognitive function in the short-term in women whose ovaries have been surgically removed.89 More research is needed to clarify the potential role of testosterone replacement on cognitive function in women.
Cortisol. Cortisol, a glucocorticoid hormone produced by the adrenal glands in response to HPA axis activation, is a critical moderator of the stress response. Cortisol also affects mood, attention, and memory, as well as immune, metabolic, and other physiologic functions.82 The HPA axis and resulting cortisol release are normally regulated by circadian signals; under healthy conditions, cortisol levels show a clear diurnal cycle, peaking in the morning and dipping at night.90
In older adults, average cortisol levels are higher and the circadian rhythm of cortisol release is blunted. This may be partly related to diminished negative-feedback control over adrenal stimulation. Chronic or repetitive stress can add to persistent dysregulation of HPA axis signaling and cortisol release, and is linked to depression and anxiety, brain atrophy, cognitive impairment, and dementia.82,90 Exercise and mindfulness training may help reduce stress, repair cortisol regulation, and slow cognitive decline.90,91
Dehydroepiandrosterone (DHEA). DHEA is a precursor to other steroidal hormones such as estrogen, progesterone, and testosterone. In addition, DHEA has a range of direct hormonal actions. Most DHEA in blood is in the form of DHEA-sulfate (DHEA-S). DHEA is produced mainly in the adrenal glands, but smaller amounts are produced in the ovaries and testes. Levels of both DHEA and DHEA-S drop with age, such that levels in elderly individuals may be 80–90% lower than in younger individuals.92,93 A large research review concluded higher blood levels of DHEA-S are associated with better cognitive performance in men and women.94 Brain DHEA and DHEA-S concentrations are substantially higher than blood concentrations, and it has been proposed that DHEA may also be synthesized in the brain.93,95 Preclinical evidence suggests DHEA may contribute to cortisol regulation, reduce neuroinflammation and brain oxidative stress, and promote neuronal growth.93,95,96
Pregnenolone. Pregnenolone is upstream of DHEA; it gives rise not only to DHEA but also progesterone, cortisol, and other intermediary metabolites. The synthesis and metabolism of pregnenolone primarily takes place in the adrenals.588 Smaller amounts of pregnenolone are made in the gonads as well as the brain.589-591 Similar to DHEA, pregnenolone declines with increasing age after reaching its peak around 20 years of age.592 As compared with healthy controls, patients with Alzheimer disease tend to have lower levels of pregnenolone and its primary products in their brain.593,594
Numerous studies have investigated the effects of pregnenolone in the brain.591 Pregnenolone and its derivatives may enhance learning and memory, reduce dementia risk, and promote the growth and survival of brain cells.595,596 Peripherally and in the brain, pregnenolone is metabolized to progesterone and further converted to allopregnanolone (ALLO-P).597 Pregnenolone, progesterone, and ALLO-P have been demonstrated to have neuroprotective effects.598 The action of ALLO-P as a gamma-aminobutyric acid (GABA) receptor agonist may contribute to pregnenolone’s central nervous system calming effects.599,600 Pregnenolone and its metabolites have thus been considered as therapies for a variety of conditions associated with neuroinflammation and central nervous system hyperexcitability.601,602
Memory formation and cognitive function rely on an appropriate balance of excitation and inhibition. Like DHEA, pregnenolone also exists in its sulfated form, pregnenolone-S, throughout the body, including in the brain.603 In contrast to ALLO-P, pregnenolone-S has GABA-inhibitory effects and as such may enhance processes such as memory formation.604 Pregnenolone-S mediates many aspects of synaptic plasticity; that is, the connections between neurons that are responsible for their communication, the learning of new skills, and formation of memory.605
8 Nootropic Drugs & Novel Approaches to Cognitive Decline & Mild Cognitive Impairment (MCI)
Although there are currently no pharmacologic interventions specifically for age-related cognitive decline, medical approaches to underlying issues such as vascular disease and systemic inflammation may help prevent progressive cognitive loss. For example, the use of certain antihypertensive medications to lower high blood pressure may slow cognitive decline and prevent dementia; however, they also pose a risk of reducing cerebrovascular blood flow and causing more cognitive harm.105,106
A number of observational studies have noted that patients using cholesterol-lowering drugs called statins (such as simvastatin [Zocor] and atorvastatin [Lipitor]) have a lower risk of mild cognitive impairment and dementia than those not using statins107; however, other studies and case reports suggest statins may impair cognitive function in some individuals108 and other studies have found no benefit.109 Large randomized controlled trials have found statins had no impact on cognitive decline or dementia risk.108,110-114
While once thought to hold promise for patients with mild cognitive impairment, it now seems that anti-dementia drugs, like the acetylcholinesterase inhibitors donepezil, galantamine (Razadyne), tacrine (Cognex), and rivastigmine (Exelon), have neither been shown to restore cognitive function nor protect against dementia in this population.115,116 The use of biological and genetic markers in the future may help researchers identify those most likely to benefit from certain drug therapies.115
Several other medications with potential brain-protective effects are:
- Piracetam (Nootropil) and levetiracetam (Keppra) are anti-seizure medications sometimes used as cognitive enhancers. These and several related drugs (the “racetams”) are often colloquially referred to as “nootropics.” Preclinical evidence suggests these medications may reduce neuroinflammation, improve mitochondrial function, and prevent β-amyloid-induced neuronal dysfunction.117-119 In early research, levetiracetam was found to improve cognitive performance on a memory test.120 Findings from a clinical trial in cognitive impairment and dementia patients suggest piracetam may be most effective in those with depressive symptoms.121 Whether these medications hold benefits for patients with age-related cognitive decline and mild cognitive impairment has not yet been established. Also, the regulatory status of piracetam and related compounds is vague, and legal status varies between countries.
- Zileuton (Zyflo) is an inhibitor of the pro-inflammatory enzyme 5-lipoxygenase. While its main use is as an anti-asthma medication, zileuton has demonstrated some intriguing effects on brain function in preclinical trials. In animal research, zileuton has been found to reduce brain levels of β-amyloid and tau, as well as amyloid- and tau-related neuroinflammation, neuronal dysfunction, and cognitive impairment.122-125 Other animal research suggests zileuton may reduce brain damage and cognitive losses after stroke.126-128 The possible usefulness of zileuton in age-related cognitive decline, mild cognitive impairment, and dementia awaits future investigation.
- Hydergine, a mixture of ergot alkaloids (also called ergoloid mesylates), has been found to improve cognitive function and mood in preliminary trials in elderly subjects with age-related cognitive dysfunction.129,130 Its mechanism of action is not completely understood; however, it appears to modulate neurotransmitter activity, improve cerebral metabolism, and increase antioxidant enzyme activity in the brain.131,132 Despite interesting findings, there have been no clinical trials investigating hydergine’s usefulness in age-related cognitive decline and mild cognitive impairment for decades.
- Selegiline or deprenyl (Eldepryl) is a monoamine oxidase-B inhibitor that blocks the enzymatic breakdown of certain neurotransmitters, such as dopamine and serotonin. It is used to treat Parkinson disease, Alzheimer disease, and major depressive disorder, and is thought to have anti-aging effects.133 Selegiline was found to improve cognitive performance in a six-month preliminary trial in human subjects with mild-to-moderate brain atrophy.134 Studies in animals suggest it can reduce oxidative stress, protect against brain damage due to loss of blood flow, and preserve neurotransmission and memory.135-138 More clinical trials are needed to ascertain the potential benefits of this medication in age-related cognitive decline.
- Centrophenoxine or meclofenoxate (Lucidril) enhances activation of cholinergic pathways in the central nervous system. An early trial in elderly subjects found centrophenoxine improved formation of new memory and increased subjective reports of mental alertness.139 Animal research has found this medication may protect against cognitive losses due to aluminum toxicity,140 drug toxicity,141 and lack of blood flow.142
- Angiotensin receptor blockers (ARBs) (eg, candesartan [Atacand], telmisartan [Micardis]) are being examined for their ability to provide protection against cognitive impairment. The protective effect of candesartan on cognition was assessed in a randomized clinical trial of 176 participants aged 55 years or older with mild cognitive impairment and hypertension. Compared with the ACE inhibitor lisinopril (Zestril), one year of treatment with candesartan (up to 32 mg orally once daily) displayed superior outcomes on an executive function assessment.606 Both groups achieved similar blood pressures suggesting these results are independent of candesartan’s antihypertensive effects.
- Metformin is a widely used medication for type 2 diabetes that may reduce the risk of cognitive impairment. A retrospective study of 234 participants with type 2 diabetes found that long-term use of metformin (>6 years) was associated with a 55% reduced risk of cognitive impairment.607 Although clinical studies suggest metformin may maintain cognitive function and reduce the risk of developing dementia and Alzheimer disease in aged diabetic patients, its efficacy in nondiabetics remains unclear. However, animal studies suggest metformin treatment can improve cognitive function in old age by decreasing neuroinflammation, inhibiting mammalian target of rapamycin (mTOR) signaling, activating adenosine monophosphate-activated protein kinase (MAPK), and augmenting hippocampal autophagy to help maintain cognitive function.608 Additional studies in humans are required to clarify the effect of metformin on cognitive decline in nondiabetics.
2026
- Jan: Updated section on ergothioneine in Nutrients
2025
- Aug: Added section on multidomain lifestyle interventions to Dietary & Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment (MCI)
2024
- Aug: Added section on vision correction to Dietary & Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment (MCI)
- Jun: Added section on ergothioneine to Nutrients
- Jun: Added sections on hearing loss, tobacco smoke, alcohol consumption, and air pollution to Dietary and Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment
- Jan: Updated section on physical activity in Dietary & Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment (MCI)
2023
- Nov: Added section on essential oils/odorants to Dietary & Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment (MCI)
- Aug: Updated section on MIND diet in Dietary & Lifestyle Considerations for Cognitive Decline & Mild Cognitive Impairment
- Mar: Added section on vitamin D to Nutrients
2022
- Jun: Added section on vitamin K2 to Nutrients
- May: Updated section on carotenoids in Nutrients
- Feb: Comprehensive update & review
2021
- Jan: Updated section on melatonin in Nutrients
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Miquel S, Champ C, Day J, et al. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res Rev. 2018;42:40-55.
- Knight JN, Y. . Anatomy and physiology of ageing 5: the nervous system. Nursing Times [online]. 2017;113(6):55-58.
- Park DC, Festini SB. Theories of Memory and Aging: A Look at the Past and a Glimpse of the Future. The journals of gerontology Series B, Psychological sciences and social sciences. 2017;72(1):82-90.
- Anastasiou CA, Yannakoulia M, Kontogianni MD, et al. Mediterranean Lifestyle in Relation to Cognitive Health: Results from the HELIAD Study. Nutrients. 2018;10(10).
- Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180-186.
- Phillips C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural plasticity. 2017;2017:3589271.
- Steindler DA, Reynolds BA. Perspective: Neuroregenerative Nutrition. Adv Nutr. 2017;8(4):546-557.
- Poulose SM, Miller MG, Scott T, Shukitt-Hale B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv Nutr. 2017;8(6):804-811.
- Palmer AL, Ousman SS. Astrocytes and Aging. Frontiers in aging neuroscience. 2018;10:337.
- Liu H, Yang Y, Xia Y, et al. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64-76.
- Lopez-Valdes HE, Martinez-Coria H. The Role of Neuroinflammation in Age-Related Dementias. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 2016;68(1):40-48.
- Smith LK, White CW, 3rd, Villeda SA. The systemic environment: at the interface of aging and adult neurogenesis. Cell and tissue research. 2018;371(1):105-113.
- Raz N, Daugherty AM. Pathways to Brain Aging and Their Modifiers: Free-Radical-Induced Energetic and Neural Decline in Senescence (FRIENDS) Model - A Mini-Review. Gerontology. 2018;64(1):49-57.
- Yang T, Sun Y, Lu Z, Leak RK, Zhang F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev. 2017;34:15-29.
- Safaiyan S, Kannaiyan N, Snaidero N, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19(8):995-998.
- Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. Jama. 2014;312(23):2551-2561.
- Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30(3):421-442.
- Allan CL, Behrman S, Ebmeier KP, Valkanova V. Diagnosing early cognitive decline-When, how and for whom? Maturitas. 2017;96:103-108.
- Langa KM, Larson EB, Crimmins EM, et al. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA internal medicine. 2017;177(1):51-58.
- Cheng YW, Chen TF, Chiu MJ. From mild cognitive impairment to subjective cognitive decline: conceptual and methodological evolution. Neuropsychiatr Dis Treat. 2017;13:491-498.
- Michel JP. Is It Possible to Delay or Prevent Age-Related Cognitive Decline? Korean journal of family medicine. 2016;37(5):263-266.
- Vicario A, Cerezo GH. At the Heart of Brain Disorders - Preventing Cognitive Decline and Dementia. European cardiology. 2015;10(1):60-63.
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer's & Dementia. 2015;11(6):718-726.
- Deckers K, Schievink SHJ, Rodriquez MMF, et al. Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS One. 2017;12(9):e0184244.
- Zhao C, Noble JM, Marder K, Hartman JS, Gu Y, Scarmeas N. Dietary Patterns, Physical Activity, Sleep, and Risk for Dementia and Cognitive Decline. Current nutrition reports. 2018;7(4):335-345.
- Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurol. 2017;74(10):1237-1245.
- Smith AD, Refsum H, Bottiglieri T, et al. Homocysteine and Dementia: An International Consensus Statement. J Alzheimers Dis. 2018;62(2):561-570.
- Karri V, Schuhmacher M, Kumar V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environmental toxicology and pharmacology. 2016;48:203-213.
- Brandt J, Leong C. Benzodiazepines and Z-Drugs: An Updated Review of Major Adverse Outcomes Reported on in Epidemiologic Research. Drugs in R&D. 2017;17(4):493-507.
- Picton JD, Marino AB, Nealy KL. Benzodiazepine use and cognitive decline in the elderly. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2018;75(1):e6-e12.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Therapeutic advances in drug safety. 2016;7(5):217-224.
- Colangelo A, Cirillo G, Alberghina L, Papa M, Westerhoff H. Neural plasticity and adult neurogenesis: the deep biology perspective. Neural Regeneration Research. 2019;14(2):201-205.
- de Lucia C, Murphy T, Thuret S. Emerging Molecular Pathways Governing Dietary Regulation of Neural Stem Cells during Aging. Front Physiol. 2017;8:17.
- Numakawa T, Odaka H, Adachi N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. International journal of molecular sciences. 2018;19(11).
- Rivera A, Vanzuli I, Arellano JJ, Butt A. Decreased Regenerative Capacity of Oligodendrocyte Progenitor Cells (NG2-Glia) in the Ageing Brain: A Vicious Cycle of Synaptic Dysfunction, Myelin Loss and Neuronal Disruption? Current Alzheimer research. 2016;13(4):413-418.
- Apple DM, Solano-Fonseca R, Kokovay E. Neurogenesis in the aging brain. Biochemical pharmacology. 2017;141:77-85.
- Wang Y, Ji X, Leak RK, Chen F, Cao G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res Rev. 2017;34:39-50.
- Nivet E. Modifiers of Neural Stem Cells and Aging: Pulling the Trigger of a Neurogenic Decline. Current Stem Cell Reports. 2016;2(3):273-281.
- Brainard J, Gobel M, Bartels K, Scott B, Koeppen M, Eckle T. Circadian rhythms in anesthesia and critical care medicine: potential importance of circadian disruptions. Seminars in cardiothoracic and vascular anesthesia. 2015;19(1):49-60.
- Krishnan HC, Lyons LC. Synchrony and desynchrony in circadian clocks: impacts on learning and memory. Learning & memory (Cold Spring Harbor, NY). 2015;22(9):426-437.
- Pace-Schott EF, Spencer RM. Sleep-dependent memory consolidation in healthy aging and mild cognitive impairment. Curr Top Behav Neurosci. 2015;25:307-330.
- Terzibasi-Tozzini E, Martinez-Nicolas A, Lucas-Sanchez A. The clock is ticking. Ageing of the circadian system: From physiology to cell cycle. Seminars in cell & developmental biology. 2017;70:164-176.
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. American journal of physiology Heart and circulatory physiology. 2017;312(1):H1-h20.
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52-58.
- Martinez-Ramirez S, Greenberg SM, Viswanathan A. Cerebral microbleeds: overview and implications in cognitive impairment. Alzheimer's Research & Therapy. 2014;6(3):33.
- De Luca C, Colangelo AM, Alberghina L, Papa M. Neuro-Immune Hemostasis: Homeostasis and Diseases in the Central Nervous System. Frontiers in cellular neuroscience. 2018;12:459.
- Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer's disease. Free radical biology & medicine. 2016;100:108-122.
- Grizzanti J, Lee HG, Camins A, Pallas M, Casadesus G. The therapeutic potential of metabolic hormones in the treatment of age-related cognitive decline and Alzheimer's disease. Nutr Res. 2016;36(12):1305-1315.
- Noble EE, Hsu TM, Kanoski SE. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Frontiers in behavioral neuroscience. 2017;11:9.
- Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. Journal of neurochemistry. 2017;143(4):418-431.
- Khacho M, Clark A, Svoboda DS, et al. Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis. Human molecular genetics. 2017;26(17):3327-3341.
- Sripetchwandee J, Chattipakorn N, Chattipakorn SC. Links Between Obesity-Induced Brain Insulin Resistance, Brain Mitochondrial Dysfunction, and Dementia. Frontiers in endocrinology. 2018;9:496.
- Salameh TS, Rhea EM, Banks WA, Hanson AJ. Insulin resistance, dyslipidemia, and apolipoprotein E interactions as mechanisms in cognitive impairment and Alzheimer's disease. Experimental biology and medicine (Maywood, NJ). 2016;241(15):1676-1683.
- Assuncao N, Sudo FK, Drummond C, de Felice FG, Mattos P. Metabolic Syndrome and cognitive decline in the elderly: A systematic review. PLoS One. 2018;13(3):e0194990.
- Moon JH. Endocrine Risk Factors for Cognitive Impairment. Endocrinology and metabolism (Seoul, Korea). 2016;31(2):185-192.
- Walker JM, Harrison FE. Shared Neuropathological Characteristics of Obesity, Type 2 Diabetes and Alzheimer's Disease: Impacts on Cognitive Decline. Nutrients. 2015;7(9):7332-7357.
- Bae CS, Song J. The Role of Glucagon-Like Peptide 1 (GLP1) in Type 3 Diabetes: GLP-1 Controls Insulin Resistance, Neuroinflammation and Neurogenesis in the Brain. International journal of molecular sciences. 2017;18(11).
- McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS One. 2017;12(4):e0174847.
- McAllister T, McCrea M. Long-Term Cognitive and Neuropsychiatric Consequences of Repetitive Concussion and Head-Impact Exposure. Journal of athletic training. 2017;52(3):309-317.
- Fehily B, Fitzgerald M. Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell transplantation. 2017;26(7):1131-1155.
- Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75 Suppl 4:S24-33.
- Oehr L, Anderson J. Diffusion-Tensor Imaging Findings and Cognitive Function Following Hospitalized Mixed-Mechanism Mild Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Archives of physical medicine and rehabilitation. 2017;98(11):2308-2319.
- Broglio SP, Eckner JT, Paulson HL, Kutcher JS. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exercise and sport sciences reviews. 2012;40(3):138-144.
- NIH. National Institues of Health: National Institute on Aging: What happens to the brain in Alzheimer's disease? Available at https://www.nia.nih.gov/health/what-happens-brain-alzheimers-disease. Last updated 05/16/2017. Accessed 01/09/2019. 2017.
- Laurent C, Buee L, Blum D. Tau and neuroinflammation: What impact for Alzheimer's Disease and Tauopathies? Biomedical journal. 2018;41(1):21-33.
- Chalermpalanupap T, Weinshenker D, Rorabaugh JM. Down but Not Out: The Consequences of Pretangle Tau in the Locus Coeruleus. Neural plasticity. 2017;2017:7829507.
- Mufson EJ, Ikonomovic MD, Counts SE, et al. Molecular and cellular pathophysiology of preclinical Alzheimer's disease. Behavioural brain research. 2016;311:54-69.
- Colijn MA, Grossberg GT. Amyloid and Tau Biomarkers in Subjective Cognitive Impairment. J Alzheimers Dis. 2015;47(1):1-8.
- Villemagne VL, Dore V, Bourgeat P, et al. Abeta-amyloid and Tau Imaging in Dementia. Seminars in nuclear medicine. 2017;47(1):75-88.
- Mielke MM, Hagen CE, Wennberg AMV, et al. Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017;74(9):1073-1080.
- Guerrero-Munoz MJ, Gerson J, Castillo-Carranza DL. Tau Oligomers: The Toxic Player at Synapses in Alzheimer's Disease. Frontiers in cellular neuroscience. 2015;9:464.
- Cui D, Xu X. DNA Methyltransferases, DNA Methylation, and Age-Associated Cognitive Function. International journal of molecular sciences. 2018;19(5).
- Deibel SH, Zelinski EL, Keeley RJ, Kovalchuk O, McDonald RJ. Epigenetic alterations in the suprachiasmatic nucleus and hippocampus contribute to age-related cognitive decline. Oncotarget. 2015;6(27):23181-23203.
- Daulatzai MA. "Boomerang Neuropathology" of Late-Onset Alzheimer's Disease is Shrouded in Harmful "BDDS": Breathing, Diet, Drinking, and Sleep During Aging. Neurotoxicity research. 2015;28(1):55-93.
- Moretti R, Caruso P. The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. International journal of molecular sciences. 2019;20(1).
- Price BR, Wilcock DM, Weekman EM. Hyperhomocysteinemia as a Risk Factor for Vascular Contributions to Cognitive Impairment and Dementia. Frontiers in aging neuroscience. 2018;10:350.
- Mikkelsen K, Stojanovska L, Tangalakis K, Bosevski M, Apostolopoulos V. Cognitive decline: A vitamin B perspective. Maturitas. 2016;93:108-113.
- Merlini M, Rafalski VA, Rios Coronado PE, et al. Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer's Disease Model. Neuron. 2019.
- Marioni RE, Stewart MC, Murray GD, et al. Peripheral levels of fibrinogen, C-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosomatic medicine. 2009;71(8):901-906.
- Marioni RE, Deary IJ, Murray GD, et al. Genetic associations between fibrinogen and cognitive performance in three Scottish cohorts. Behav Genet. 2011;41(5):691-699.
- Pedersen A, Stanne TM, Redfors P, et al. Fibrinogen concentrations predict long-term cognitive outcome in young ischemic stroke patients. Research and practice in thrombosis and haemostasis. 2018;2(2):339-346.
- Ebner NC, Kamin H, Diaz V, Cohen RA, MacDonald K. Hormones as "difference makers" in cognitive and socioemotional aging processes. Frontiers in psychology. 2014;5:1595.
- Lejri I, Grimm A, Eckert A. Mitochondria, Estrogen and Female Brain Aging. Frontiers in aging neuroscience. 2018;10:124.
- Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol Rev. 2015;95(3):785-807.
- Dumas JA. Strategies for Preventing Cognitive Decline in Healthy Older Adults. Can J Psychiatry. 2017;62(11):754-760.
- Yeap BB. Hormonal changes and their impact on cognition and mental health of ageing men. Maturitas. 2014;79(2):227-235.
- Hsu B, Cumming RG, Waite LM, et al. Longitudinal Relationships between Reproductive Hormones and Cognitive Decline in Older Men: The Concord Health and Ageing in Men Project. J Clin Endocrinol Metab. 2015;100(6):2223-2230.
- Hogervorst E. Effects of gonadal hormones on cognitive behaviour in elderly men and women. J Neuroendocrinol. 2013;25(11):1182-1195.
- Hogervorst E. Prevention of dementia with sex hormones: a focus on testosterone and cognition in women. Minerva Med. 2012;103(5):353-359.
- Tortosa-Martinez J, Manchado C, Cortell-Tormo JM, Chulvi-Medrano I. Exercise, the diurnal cycle of cortisol and cognitive impairment in older adults. Neurobiology of stress. 2018;9:40-47.
- Russell-Williams J, Jaroudi W, Perich T, Hoscheidt S, El Haj M, Moustafa AA. Mindfulness and meditation: treating cognitive impairment and reducing stress in dementia. Rev Neurosci. 2018;29(7):791-804.
- Samaras N, Papadopoulou MA, Samaras D, Ongaro F. Off-label use of hormones as an antiaging strategy: a review. Clinical interventions in aging. 2014;9:1175-1186.
- Maggio M, De Vita F, Fisichella A, et al. DHEA and cognitive function in the elderly. J Steroid Biochem Mol Biol. 2015;145:281-292.
- de Menezes KJ, Peixoto C, Nardi AE, Carta MG, Machado S, Veras AB. Dehydroepiandrosterone, Its Sulfate and Cognitive Functions. Clinical practice and epidemiology in mental health : CP & EMH. 2016;12:24-37.
- Powrie YSL, Smith C. Central intracrine DHEA synthesis in ageing-related neuroinflammation and neurodegeneration: therapeutic potential? Journal of neuroinflammation. 2018;15(1):289.
- Jimenez-Rubio G, Herrera-Perez JJ, Hernandez-Hernandez OT, Martinez-Mota L. Relationship between androgen deficiency and memory impairment in aging and Alzheimer’s disease. Actas espanolas de psiquiatria. 2017;45(5):227-247.
- Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction - current preventive strategies. Clin Interv Aging. 2018;13:2267-2273.
- Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. British journal of anaesthesia. 2017;119(suppl_1):i115-i125.
- Kant IMJ, de Bresser J, van Montfort SJT, Slooter AJC, Hendrikse J. MRI Markers of Neurodegenerative and Neurovascular Changes in Relation to Postoperative Delirium and Postoperative Cognitive Decline. Am J Geriatr Psychiatry. 2017;25(10):1048-1061.
- Liu Y, Yin Y. Emerging Roles of Immune Cells in Postoperative Cognitive Dysfunction. Mediators of inflammation. 2018;2018:6215350.
- Cascella M, Bimonte S. The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction. Neural Regen Res. 2017;12(11):1780-1785.
- Luo A, Yan J, Tang X, Zhao Y, Zhou B, Li S. Postoperative cognitive dysfunction in the aged: the collision of neuroinflammaging with perioperative neuroinflammation. Inflammopharmacol. 2019.
- Safavynia SA, Goldstein PA. The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Frontiers in psychiatry. 2018;9:752.
- Brown Ct, Deiner S. Perioperative cognitive protection. British journal of anaesthesia. 2016;117(suppl 3):iii52-iii61.
- Hernandorena I, Duron E, Vidal JS, Hanon O. Treatment options and considerations for hypertensive patients to prevent dementia. Expert opinion on pharmacotherapy. 2017;18(10):989-1000.
- Tadic M, Cuspidi C, Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC cardiovascular disorders. 2016;16(1):208.
- Chu CS, Tseng PT, Stubbs B, et al. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci Rep. 2018;8(1):5804.
- Schultz BG, Patten DK, Berlau DJ. The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Translational neurodegeneration. 2018;7:5.
- Bosch J, O'Donnell M, Swaminathan B, et al. Effects of blood pressure and lipid lowering on cognition. Results from the HOPE-3 study. 2019:10.1212/WNL.0000000000007174.
- Ong KL, Morris MJ, McClelland RL, et al. Relationship of Lipids and Lipid-Lowering Medications With Cognitive Function: The Multi-Ethnic Study of Atherosclerosis. American journal of epidemiology. 2018;187(4):767-776.
- McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. The Cochrane database of systematic reviews. 2016(1):Cd003160.
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630.
- Group HPSC. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
- Mora S, Ridker PM. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)--can C-reactive protein be used to target statin therapy in primary prevention? The American journal of cardiology. 2006;97(2a):33a-41a.
- Karakaya T, Fußer F, Schröder J, Pantel J. Pharmacological Treatment of Mild Cognitive Impairment as a Prodromal Syndrome of Alzheimer´s Disease. Curr Neuropharmacol. 2013;11(1):102-108.
- Farlow MR. Treatment of mild cognitive impairment (MCI). Current Alzheimer research. 2009;6(4):362-367.
- Tripathi A, Paliwal P, Krishnamurthy S. Piracetam Attenuates LPS-Induced Neuroinflammation and Cognitive Impairment in Rats. Cellular and molecular neurobiology. 2017;37(8):1373-1386.
- Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc Natl Acad Sci U S A. 2012;109(42):E2895-2903.
- Stockburger C, Kurz C, Koch KA, Eckert SH, Leuner K, Muller WE. Improvement of mitochondrial function and dynamics by the metabolic enhancer piracetam. Biochem Soc Trans. 2013;41(5):1331-1334.
- Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467-474.
- Tariska P, Paksy A. [Cognitive enhancement effect of piracetam in patients with mild cognitive impairment and dementia]. Orvosi hetilap. 2000;141(22):1189-1193.
- Giannopoulos PF, Chiu J, Pratico D. Learning Impairments, Memory Deficits, and Neuropathology in Aged Tau Transgenic Mice Are Dependent on Leukotrienes Biosynthesis: Role of the cdk5 Kinase Pathway. Molecular neurobiology. 2018.
- Giannopoulos PF, Chiu J, Pratico D. Antileukotriene therapy by reducing tau phosphorylation improves synaptic integrity and cognition of P301S transgenic mice. Aging Cell. 2018;17(3):e12759.
- Chu J, Li JG, Pratico D. Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer's disease with plaques and tangles. PLoS One. 2013;8(8):e70991.
- Di Meco A, Lauretti E, Vagnozzi AN, Pratico D. Zileuton restores memory impairments and reverses amyloid and tau pathology in aged Alzheimer's disease mice. Neurobiol Aging. 2014;35(11):2458-2464.
- Shi SS, Yang WZ, Tu XK, Wang CH, Chen CM, Chen Y. 5-Lipoxygenase inhibitor zileuton inhibits neuronal apoptosis following focal cerebral ischemia. Inflammation. 2013;36(6):1209-1217.
- Silva BC, de Miranda AS, Rodrigues FG, et al. The 5-lipoxygenase (5-LOX) Inhibitor Zileuton Reduces Inflammation and Infarct Size with Improvement in Neurological Outcome Following Cerebral Ischemia. Curr Neurovasc Res. 2015;12(4):398-403.
- Tu XK, Zhang HB, Shi SS, et al. 5-LOX Inhibitor Zileuton Reduces Inflammatory Reaction and Ischemic Brain Damage Through the Activation of PI3K/Akt Signaling Pathway. Neurochem Res. 2016;41(10):2779-2787.
- Rouy JM, Douillon AM, Compan B, Wolmark Y. Ergoloid mesylates ('Hydergine') in the treatment of mental deterioration in the elderly: a 6-month double-blind, placebo-controlled trial. Current medical research and opinion. 1989;11(6):380-389.
- van Loveren-Huyben CM, Engelaar HF, Hermans MB, van der Bom JA, Leering C, Munnichs JM. Double-blind clinical and psychologic study of ergoloid mesylates (Hydergine) in subjects with senile mental deterioration. J Am Geriatr Soc. 1984;32(8):584-588.
- Wadworth AN, Chrisp P. Co-dergocrine mesylate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in age-related cognitive decline. Drugs Aging. 1992;2(3):153-173.
- Sozmen EY, Kanit L, Kutay FZ, Hariri NI. Possible supportive effects of co-dergocrine mesylate on antioxidant enzyme systems in aged rat brain. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1998;8(1):13-16.
- Miklya I. The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015). Molecular psychiatry. 2016;21(11):1499-1503.
- Bettini R, Gorini M. [Effectiveness and tolerability of selegiline in the treatment of pathological cerebral involutions]. Clin Ter. 2002;153(6):377-380.
- Goverdhan P, Sravanthi A, Mamatha T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. International journal of Alzheimer's disease. 2012;2012:974013.
- Unal I, Gursoy-Ozdemir Y, Bolay H, Soylemezoglu F, Saribas O, Dalkara T. Chronic daily administration of selegiline and EGb 761 increases brain's resistance to ischemia in mice. Brain research. 2001;917(2):174-181.
- Kiray M, Bagriyanik HA, Pekcetin C, Ergur BU, Uysal N. Protective effects of deprenyl in transient cerebral ischemia in rats. The Chinese journal of physiology. 2008;51(5):275-281.
- Kiray M, Bagriyanik HA, Pekcetin C, et al. Deprenyl and the relationship between its effects on spatial memory, oxidant stress and hippocampal neurons in aged male rats. Physiological research / Academia Scientiarum Bohemoslovaca. 2006;55(2):205-212.
- Marcer D, Hopkins SM. The differential effects of meclofenoxate on memory loss in the elderly. Age Ageing. 1977;6(2):123-131.
- Nehru B, Bhalla P, Garg A. Evidence for centrophenoxine as a protective drug in aluminium induced behavioral and biochemical alteration in rat brain. Molecular and cellular biochemistry. 2006;290(1-2):33-42.
- Voronina TA, Garibova TL, Trofimov SS, Sopyev Zh A, Petkov VD, Lazarova MB. Comparative studies on the influence of ONK (N(5-hydroxynicotinoil) glutamic acid), piracetam and meclofenoxate on the learning- and memory-impairing effect of scopolamine, clonidine, and methergoline. Acta physiologica et pharmacologica Bulgarica. 1991;17(4):8-16.
- Liao Y, Wang R, Tang XC. Centrophenoxine improves chronic cerebral ischemia induced cognitive deficit and neuronal degeneration in rats. Acta Pharmacol Sin. 2004;25(12):1590-1596.
- Klimova B, Valis M. Nutritional Interventions as Beneficial Strategies to Delay Cognitive Decline in Healthy Older Individuals. Nutrients. 2018;10(7).
- Chieffi S, Messina G, Villano I, et al. Neuroprotective Effects of Physical Activity: Evidence from Human and Animal Studies. Frontiers in neurology. 2017;8:188.
- Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. British journal of sports medicine. 2018;52(3):154-160.
- Sexton BP, Taylor NF. To sit or not to sit? A systematic review and meta-analysis of seated exercise for older adults. Australasian journal on ageing. 2018.
- Cheng ST. Cognitive Reserve and the Prevention of Dementia: the Role of Physical and Cognitive Activities. Curr Psychiatry Rep. 2016;18(9):85.
- Thow ME, Summers MJ, Saunders NL, Summers JJ, Ritchie K, Vickers JC. Further education improves cognitive reserve and triggers improvement in selective cognitive functions in older adults: The Tasmanian Healthy Brain Project. Alzheimer's & dementia (Amsterdam, Netherlands). 2018;10:22-30.
- Antoniou M, Wright SM. Uncovering the Mechanisms Responsible for Why Language Learning May Promote Healthy Cognitive Aging. Frontiers in psychology. 2017;8:2217.
- Roman-Caballero R, Arnedo M, Trivino M, Lupianez J. Musical practice as an enhancer of cognitive function in healthy aging - A systematic review and meta-analysis. PLoS One. 2018;13(11):e0207957.
- Fissler P, Kuster OC, Laptinskaya D, Loy LS, von Arnim CAF, Kolassa IT. Jigsaw Puzzling Taps Multiple Cognitive Abilities and Is a Potential Protective Factor for Cognitive Aging. Frontiers in aging neuroscience. 2018;10:299.
- Pillai JA, Hall CB, Dickson DW, Buschke H, Lipton RB, Verghese J. Association of crossword puzzle participation with memory decline in persons who develop dementia. Journal of the International Neuropsychological Society : JINS. 2011;17(6):1006-1013.
- Ferreira N, Owen A, Mohan A, Corbett A, Ballard C. Associations between cognitively stimulating leisure activities, cognitive function and age-related cognitive decline. International journal of geriatric psychiatry. 2015;30(4):422-430.
- Litwin H, Schwartz E, Damri N. Cognitively Stimulating Leisure Activity and Subsequent Cognitive Function: A SHARE-based Analysis. The Gerontologist. 2017;57(5):940-948.
- Howard EP, Morris JN, Steel K, et al. Short-Term Lifestyle Strategies for Sustaining Cognitive Status. Biomed Res Int. 2016;2016:7405748.
- Lee GJ, Bang HJ, Lee KM, et al. A comparison of the effects between 2 computerized cognitive training programs, Bettercog and COMCOG, on elderly patients with MCI and mild dementia: A single-blind randomized controlled study. Medicine. 2018;97(45):e13007.
- Chetelat G, Lutz A, Arenaza-Urquijo E, Collette F, Klimecki O, Marchant N. Why could meditation practice help promote mental health and well-being in aging? Alzheimers Res Ther. 2018;10(1):57.
- Sperduti M, Makowski D, Blonde P, Piolino P. Meditation and successful aging: can meditative practices counteract age-related cognitive decline? Geriatrie et psychologie neuropsychiatrie du vieillissement. 2017;15(2):205-213.
- Villemure C, Ceko M, Cotton VA, Bushnell MC. Neuroprotective effects of yoga practice: age-, experience-, and frequency-dependent plasticity. Frontiers in human neuroscience. 2015;9:281.
- Prakash R, Rastogi P, Dubey I, Abhishek P, Chaudhury S, Small BJ. Long-term concentrative meditation and cognitive performance among older adults. Neuropsychology, development, and cognition Section B, Aging, neuropsychology and cognition. 2012;19(4):479-494.
- Luders E, Cherbuin N, Kurth F. Forever Young(er): potential age-defying effects of long-term meditation on gray matter atrophy. Frontiers in psychology. 2014;5:1551.
- Gard T, Taquet M, Dixit R, et al. Fluid intelligence and brain functional organization in aging yoga and meditation practitioners. Frontiers in aging neuroscience. 2014;6:76.
- Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging & mental health. 2017;21(11):1113-1120.
- Gard T, Holzel BK, Lazar SW. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann N Y Acad Sci. 2014;1307:89-103.
- Laird KT, Paholpak P, Roman M, Rahi B, Lavretsky H. Mind-Body Therapies for Late-Life Mental and Cognitive Health. Curr Psychiatry Rep. 2018;20(1):2.
- Gothe NP, Kramer AF, McAuley E. Hatha Yoga Practice Improves Attention and Processing Speed in Older Adults: Results from an 8-Week Randomized Control Trial. Journal of alternative and complementary medicine (New York, NY). 2017;23(1):35-40.
- Dause TJ, Kirby ED. Aging gracefully: social engagement joins exercise and enrichment as a key lifestyle factor in resistance to age-related cognitive decline. Neural Regen Res. 2019;14(1):39-42.
- Pillemer SC, Holtzer R. The differential relationships of dimensions of perceived social support with cognitive function among older adults. Aging & mental health. 2016;20(7):727-735.
- Parisi JM, Roberts L, Szanton SL, Hodgson NA, Gitlin LN. Valued Activities among Individuals with and without Cognitive Impairments: Findings from the National Health and Aging Trends Study. The Gerontologist. 2017;57(2):309-318.
- Hughes TF, Flatt JD, Fu B, Chang CC, Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: the MYHAT study. International psychogeriatrics. 2013;25(4):587-595.
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26(2):144-155.
- Yates JA, Clare L, Woods RT. "You've got a friend in me": can social networks mediate the relationship between mood and MCI? BMC geriatrics. 2017;17(1):144.
- Lee Y, Jean Yeung WJ. Gender matters: Productive social engagement and the subsequent cognitive changes among older adults. Social science & medicine (1982). 2018.
- Saint Martin M, Sforza E, Barthelemy JC, et al. Long-lasting active lifestyle and successful cognitive aging in a healthy elderly population: The PROOF cohort. Revue neurologique. 2017;173(10):637-644.
- Park S, Kwon E, Lee H. Life Course Trajectories of Later-Life Cognitive Functions: Does Social Engagement in Old Age Matter? International journal of environmental research and public health. 2017;14(4).
- Haslam C, Cruwys T, Haslam SA. "The we's have it": evidence for the distinctive benefits of group engagement in enhancing cognitive health in aging. Social science & medicine (1982). 2014;120:57-66.
- Roberts RO, Cha RH, Mielke MM, et al. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84(18):1854-1861.
- Trost Bobic T, Secic A, Zavoreo I, et al. The Impact of Sleep Deprivation on the Brain. Acta Clin Croat. 2016;55(3):469-473.
- Tsapanou A, Vlachos GS, Cosentino S, et al. Sleep and subjective cognitive decline in cognitively healthy elderly: Results from two cohorts. J Sleep Res. 2018.
- Dzierzewski JM, Dautovich N, Ravyts S. Sleep and Cognition in Older Adults. Sleep medicine clinics. 2018;13(1):93-106.
- Wennberg AMV, Wu MN, Rosenberg PB, Spira AP. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Seminars in neurology. 2017;37(4):395-406.
- Atienza M, Ziontz J, Cantero JL. Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging. Sleep medicine reviews. 2018;42:171-183.
- Akers KG, Cherasse Y, Fujita Y, Srinivasan S, Sakurai T, Sakaguchi M. Concise Review: Regulatory Influence of Sleep and Epigenetics on Adult Hippocampal Neurogenesis and Cognitive and Emotional Function. Stem cells (Dayton, Ohio). 2018;36(7):969-976.
- Wu L, Sun D, Tan Y. A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders. Sleep & breathing = Schlaf & Atmung. 2018;22(3):805-814.
- Liang Y, Qu LB, Liu H. Non-linear associations between sleep duration and the risks of mild cognitive impairment/dementia and cognitive decline: a dose-response meta-analysis of observational studies. Aging clinical and experimental research. 2018.
- Ho ECM, Siu AMH. Occupational Therapy Practice in Sleep Management: A Review of Conceptual Models and Research Evidence. Occupational therapy international. 2018;2018:8637498.
- Gutman SA, Gregory KA, Sadlier-Brown MM, et al. Comparative Effectiveness of Three Occupational Therapy Sleep Interventions: A Randomized Controlled Study. OTJR : occupation, participation and health. 2017;37(1):5-13.
- Bucks RS, Olaithe M, Rosenzweig I, Morrell MJ. Reviewing the relationship between OSA and cognition: Where do we go from here? Respirology (Carlton, Vic). 2017;22(7):1253-1261.
- Ayas NT, Taylor CM, Laher I. Cardiovascular consequences of obstructive sleep apnea. Current opinion in cardiology. 2016;31(6):599-605.
- Floras JS. Sleep Apnea and Cardiovascular Disease: An Enigmatic Risk Factor. Circ Res. 2018;122(12):1741-1764.
- Chowdhuri S, Patel P, Badr MS. Apnea in Older Adults. Sleep medicine clinics. 2018;13(1):21-37.
- Zhu X, Zhao Y. Sleep-disordered breathing and the risk of cognitive decline: a meta-analysis of 19,940 participants. Sleep & breathing = Schlaf & Atmung. 2018;22(1):165-173.
- Hobzova M, Hubackova L, Vanek J, et al. Cognitive function and depressivity before and after cpap treatment in obstructive sleep apnea patients. Neuro endocrinology letters. 2017;38(3):145-153.
- Yan B, Jin Y, Hu Y, Li S. Effects of continuous positive airway pressure on elderly patients with obstructive sleep apnea: a meta-analysis. Medecine sciences : M/S. 2018;34 Focus issue F1:66-73.
- Devita M, Zangrossi A, Marvisi M, Merlo P, Rusconi ML, Mondini S. Global cognitive profile and different components of reaction times in obstructive sleep apnea syndrome: Effects of continuous positive airway pressure over time. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2018;123:121-126.
- Eleftheriou D, Benetou V, Trichopoulou A, La Vecchia C, Bamia C. Mediterranean diet and its components in relation to all-cause mortality: meta-analysis. The British journal of nutrition. 2018;120(10):1081-1097.
- Carlos S, De La Fuente-Arrillaga C, Bes-Rastrollo M, et al. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients. 2018;10(4).
- Romagnolo DF, Selmin OI. Mediterranean Diet and Prevention of Chronic Diseases. Nutrition today. 2017;52(5):208-222.
- Aridi YS, Walker JL, Wright ORL. The Association between the Mediterranean Dietary Pattern and Cognitive Health: A Systematic Review. Nutrients. 2017;9(7).
- Martinez-Gonzalez MA, Hershey MS, Zazpe I, Trichopoulou A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. What Is and What Is Not the Mediterranean Diet. Nutrients. 2017;9(11).
- Tanaka T, Talegawkar SA, Jin Y, Colpo M, Ferrucci L, Bandinelli S. Adherence to a Mediterranean Diet Protects from Cognitive Decline in the Invecchiare in Chianti Study of Aging. Nutrients. 2018;10(12).
- Bhushan A, Fondell E, Ascherio A, Yuan C, Grodstein F, Willett W. Adherence to Mediterranean diet and subjective cognitive function in men. European journal of epidemiology. 2018;33(2):223-234.
- Vassilaki M, Aakre JA, Syrjanen JA, et al. Mediterranean Diet, Its Components, and Amyloid Imaging Biomarkers. J Alzheimers Dis. 2018;64(1):281-290.
- Rainey-Smith SR, Gu Y, Gardener SL, et al. Mediterranean diet adherence and rate of cerebral Abeta-amyloid accumulation: Data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Translational psychiatry. 2018;8(1):238.
- Qosa H, Mohamed LA, Batarseh YS, et al. Extra-virgin olive oil attenuates amyloid-beta and tau pathologies in the brains of TgSwDI mice. J Nutr Biochem. 2015;26(12):1479-1490.
- Batarseh YS, Mohamed LA, Al Rihani SB, et al. Oleocanthal ameliorates amyloid-beta oligomers' toxicity on astrocytes and neuronal cells: In vitro studies. Neuroscience. 2017;352:204-215.
- Luceri C, Bigagli E, Pitozzi V, Giovannelli L. A nutrigenomics approach for the study of anti-aging interventions: olive oil phenols and the modulation of gene and microRNA expression profiles in mouse brain. European journal of nutrition. 2017;56(2):865-877.
- Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. The journal of nutrition, health & aging. 2013;17(6):544-552.
- Carman AJ, Dacks PA, Lane RF, Shineman DW, Fillit HM. Current evidence for the use of coffee and caffeine to prevent age-related cognitive decline and Alzheimer's disease. The journal of nutrition, health & aging. 2014;18(4):383-392.
- Haller S, Montandon ML, Rodriguez C, Herrmann FR, Giannakopoulos P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients. 2018;10(10).
- Liu QP, Wu YF, Cheng HY, et al. Habitual coffee consumption and risk of cognitive decline/dementia: A systematic review and meta-analysis of prospective cohort studies. Nutrition (Burbank, Los Angeles County, Calif). 2016;32(6):628-636.
- Wu L, Sun D, He Y. Coffee intake and the incident risk of cognitive disorders: A dose-response meta-analysis of nine prospective cohort studies. Clin Nutr. 2017;36(3):730-736.
- Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee Consumption Habits and the Risk of Mild Cognitive Impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47(4):889-899.
- Araujo LF, Giatti L, Reis RC, et al. Inconsistency of Association between Coffee Consumption and Cognitive Function in Adults and Elderly in a Cross-Sectional Study (ELSA-Brasil). Nutrients. 2015;7(11):9590-9601.
- Araujo LF, Mirza SS, Bos D, et al. Association of Coffee Consumption with MRI Markers and Cognitive Function: A Population-Based Study. J Alzheimers Dis. 2016;53(2):451-461.
- Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: An update. Ageing research reviews. 2017;39:36-45.
- Mattson MP. The impact of dietary energy intake on cognitive aging. Frontiers in aging neuroscience. 2010;2:5.
- Fusco S, Pani G. Brain response to calorie restriction. Cellular and molecular life sciences : CMLS. 2013;70(17):3157-3170.
- Park JH, Glass Z, Sayed K, et al. Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur J Neurosci. 2013;37(12):1987-1993.
- Babenko NA, Shakhova EG. Long-term food restriction prevents aging-associated sphingolipid turnover dysregulation in the brain. Arch Gerontol Geriatr. 2014;58(3):420-426.
- Willette AA, Coe CL, Colman RJ, et al. Calorie restriction reduces psychological stress reactivity and its association with brain volume and microstructure in aged rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):903-916.
- Hadad N, Unnikrishnan A, Jackson JA, et al. Caloric restriction mitigates age-associated hippocampal differential CG and non-CG methylation. Neurobiol Aging. 2018;67:53-66.
- Campagna G, Pesce M, Tatangelo R, Rizzuto A, La Fratta I, Grilli A. The progression of coeliac disease: its neurological and psychiatric implications. Nutr Res Rev. 2017;30(1):25-35.
- Casella G, Bordo BM, Schalling R, et al. Neurological disorders and celiac disease. Minerva gastroenterologica e dietologica. 2016;62(2):197-206.
- Makhlouf S, Messelmani M, Zaouali J, Mrissa R. Cognitive impairment in celiac disease and non-celiac gluten sensitivity: review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet. Acta neurologica Belgica. 2018;118(1):21-27.
- Daulatzai MA. Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. CNS & neurological disorders drug targets. 2015;14(1):110-131.
- Lurie Y, Landau DA, Pfeffer J, Oren R. Celiac disease diagnosed in the elderly. Journal of clinical gastroenterology. 2008;42(1):59-61.
- Zhang HF, Huang LB, Zhong YB, et al. An Overview of Systematic Reviews of Ginkgo biloba Extracts for Mild Cognitive Impairment and Dementia. Frontiers in aging neuroscience. 2016;8:276.
- Gargouri B, Carstensen J, Bhatia HS, Huell M, Dietz GPH, Fiebich BL. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine. 2018;44:45-55.
- Osman NM, Amer AS, Abdelwahab S. Effects of Ginko biloba leaf extract on the neurogenesis of the hippocampal dentate gyrus in the elderly mice. Anatomical science international. 2016;91(3):280-289.
- Tan MS, Yu JT, Tan CC, et al. Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2015;43(2):589-603.
- Hashiguchi M, Ohta Y, Shimizu M, Maruyama J, Mochizuki M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. Journal of pharmaceutical health care and sciences. 2015;1:14.
- Gauthier S, Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761(R) in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging. 2014;9:2065-2077.
- Kandiah N, Ong PA, Yuda T, et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS neuroscience & therapeutics. 2019;25(2):288-298.
- Stough C, Singh H, Zangara A. Mechanisms, Efficacy, and Safety of Bacopa monnieri (Brahmi) for Cognitive and Brain Enhancement. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:717605.
- Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer's Disease. Annals of neurosciences. 2017;24(2):111-122.
- Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013;16(4):313-326.
- Benson S, Downey LA, Stough C, Wetherell M, Zangara A, Scholey A. An acute, double-blind, placebo-controlled cross-over study of 320 mg and 640 mg doses of Bacopa monnieri (CDRI 08) on multitasking stress reactivity and mood. Phytotherapy research : PTR. 2014;28(4):551-559.
- Kwon HJ, Jung HY, Hahn KR, et al. Bacopa monnieri extract improves novel object recognition, cell proliferation, neuroblast differentiation, brain-derived neurotrophic factor, and phosphorylation of cAMP response element-binding protein in the dentate gyrus. Laboratory animal research. 2018;34(4):239-247.
- Kongkeaw C, Dilokthornsakul P, Thanarangsarit P, Limpeanchob N, Norman Scholfield C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. Journal of ethnopharmacology. 2014;151(1):528-535.
- Calabrese C, Gregory WL, Leo M, Kraemer D, Bone K, Oken B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. Journal of alternative and complementary medicine (New York, NY). 2008;14(6):707-713.
- Zanotta D, Puricelli S, Bonoldi G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: a noncomparative, exploratory clinical study. Neuropsychiatric disease and treatment. 2014;10:225-230.
- Cicero AF, Bove M, Colletti A, et al. Short-Term Impact of a Combined Nutraceutical on Cognitive Function, Perceived Stress and Depression in Young Elderly with Cognitive Impairment: A Pilot, Double-Blind, Randomized Clinical Trial. The journal of prevention of Alzheimer's disease. 2017;4(1):12-15.
- Janeczek M, Gefen T, Samimi M, et al. Variations in Acetylcholinesterase Activity within Human Cortical Pyramidal Neurons Across Age and Cognitive Trajectories. Cerebral cortex (New York, NY : 1991). 2018;28(4):1329-1337.
- Damar U, Gersner R, Johnstone JT, Schachter S, Rotenberg A. Huperzine A: A promising anticonvulsant, disease modifying, and memory enhancing treatment option in Alzheimer's disease. Med Hypotheses. 2017;99:57-62.
- Qian ZM, Ke Y. Huperzine A: Is it an Effective Disease-Modifying Drug for Alzheimer's Disease? Frontiers in aging neuroscience. 2014;6:216.
- Xing SH, Zhu CX, Zhang R, An L. Huperzine a in the treatment of Alzheimer's disease and vascular dementia: a meta-analysis. Evidence-based complementary and alternative medicine : eCAM. 2014;2014:363985.
- Yang G, Wang Y, Tian J, Liu JP. Huperzine A for Alzheimer's disease: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2013;8(9):e74916.
- Gul A, Bakht J, Mehmood F. Huperzine-A response to cognitive impairment and task switching deficits in patients with Alzheimer's disease. Journal of the Chinese Medical Association : JCMA. 2018.
- Tabira T, Kawamura N. A Study of a Supplement Containing Huperzine A and Curcumin in Dementia Patients and Individuals with Mild Cognitive Impairment. J Alzheimers Dis. 2018;63(1):75-78.
- Cristofano A, Sapere N, La Marca G, et al. Serum Levels of Acyl-Carnitines along the Continuum from Normal to Alzheimer's Dementia. PLoS One. 2016;11(5):e0155694.
- Mehrotra A, Kanwal A, Banerjee SK, Sandhir R. Mitochondrial modulators in experimental Huntington's disease: reversal of mitochondrial dysfunctions and cognitive deficits. Neurobiol Aging. 2015;36(6):2186-2200.
- Singh S, Mishra A, Srivastava N, Shukla R, Shukla S. Acetyl-L-Carnitine via Upegulating Dopamine D1 Receptor and Attenuating Microglial Activation Prevents Neuronal Loss and Improves Memory Functions in Parkinsonian Rats. Molecular neurobiology. 2018;55(1):583-602.
- Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. International clinical psychopharmacology. 2003;18(2):61-71.
- Malaguarnera M, Gargante MP, Cristaldi E, et al. Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Archives of gerontology and geriatrics. 2008;46(2):181-190.
- Bersani G, Meco G, Denaro A, et al. L-Acetylcarnitine in dysthymic disorder in elderly patients: a double-blind, multicenter, controlled randomized study vs. fluoxetine. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23(10):1219-1225.
- Remington R, Lortie JJ, Hoffmann H, Page R, Morrell C, Shea TB. A Nutritional Formulation for Cognitive Performance in Mild Cognitive Impairment: A Placebo-Controlled Trial with an Open-Label Extension. J Alzheimers Dis. 2015;48(3):591-595.
- Slutsky I, Abumaria N, Wu LJ, et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65(2):165-177.
- Wang D, Jacobs SA, Tsien JZ. Targeting the NMDA receptor subunit NR2B for treating or preventing age-related memory decline. Expert opinion on therapeutic targets. 2014;18(10):1121-1130.
- Abumaria N, Yin B, Zhang L, et al. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J Neurosci. 2011;31(42):14871-14881.
- Yu X, Guan PP, Zhu D, et al. Magnesium Ions Inhibit the Expression of Tumor Necrosis Factor alpha and the Activity of gamma-Secretase in a beta-Amyloid Protein-Dependent Mechanism in APP/PS1 Transgenic Mice. Frontiers in molecular neuroscience. 2018;11:172.
- Yu X, Guan PP, Guo JW, et al. By suppressing the expression of anterior pharynx-defective-1alpha and -1beta and inhibiting the aggregation of beta-amyloid protein, magnesium ions inhibit the cognitive decline of amyloid precursor protein/presenilin 1 transgenic mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(12):5044-5058.
- Li W, Yu J, Liu Y, et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer's disease mouse model. Molecular brain. 2014;7:65.
- Huang Y, Huang X, Zhang L, et al. Magnesium boosts the memory restorative effect of environmental enrichment in Alzheimer's disease mice. CNS neuroscience & therapeutics. 2018;24(1):70-79.
- Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29(4):773-782.
- Rabassa M, Cherubini A, Zamora-Ros R, et al. Low Levels of a Urinary Biomarker of Dietary Polyphenol Are Associated with Substantial Cognitive Decline over a 3-Year Period in Older Adults: The Invecchiare in Chianti Study. J Am Geriatr Soc. 2015;63(5):938-946.
- Flanagan E, Muller M, Hornberger M, Vauzour D. Impact of Flavonoids on Cellular and Molecular Mechanisms Underlying Age-Related Cognitive Decline and Neurodegeneration. Current nutrition reports. 2018;7(2):49-57.
- Sarubbo F, Moranta D, Pani G. Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition. Neuroscience and biobehavioral reviews. 2018;90:456-470.
- Caracciolo B, Xu W, Collins S, Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mech Ageing Dev. 2014;136-137:59-69.
- Frolinger T, Herman F, Sharma A, Sims S, Wang J, Pasinetti GM. Epigenetic modifications by polyphenolic compounds alter gene expression in the hippocampus. Biology open. 2018;7(10).
- Ma L, Sun Z, Zeng Y, Luo M, Yang J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. International journal of molecular sciences. 2018;19(9).
- Boespflug EL, Eliassen JC, Dudley JA, et al. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutritional neuroscience. 2018;21(4):297-305.
- Bowtell JL, Aboo-Bakkar Z, Conway ME, Adlam AR, Fulford J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2017;42(7):773-779.
- Miller MG, Hamilton DA, Joseph JA, Shukitt-Hale B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. European journal of nutrition. 2018;57(3):1169-1180.
- McNamara RK, Kalt W, Shidler MD, et al. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging. 2018;64:147-156.
- Traupe I, Giacalone M, Agrimi J, et al. Postoperative cognitive dysfunction and short-term neuroprotection from blueberries: a pilot study. Minerva Anestesiol. 2018;84(12):1352-1360.
- Nilsson A, Salo I, Plaza M, Bjorck I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PloS one. 2017;12(11):e0188173.
- Herman F, Westfall S, Brathwaite J, Pasinetti GM. Suppression of Presymptomatic Oxidative Stress and Inflammation in Neurodegeneration by Grape-Derived Polyphenols. Frontiers in pharmacology. 2018;9:867.
- Calapai G, Bonina F, Bonina A, et al. A Randomized, Double-Blinded, Clinical Trial on Effects of a Vitis vinifera Extract on Cognitive Function in Healthy Older Adults. Frontiers in pharmacology. 2017;8:776.
- Krikorian R, Boespflug EL, Fleck DE, et al. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem. 2012;60(23):5736-5742.
- Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. The British journal of nutrition. 2010;103(5):730-734.
- Lee J, Torosyan N, Silverman DH. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp Gerontol. 2017;87(Pt A):121-128.
- Bensalem J, Dudonne S, Etchamendy N, et al. Polyphenols from grape and blueberry improve episodic memory in healthy elderly with lower level of memory performance: a bicentric double-blind, randomized, placebo-controlled clinical study. The journals of gerontology Series A, Biological sciences and medical sciences. 2018.
- Ramirez-Garza SL, Laveriano-Santos EP, Marhuenda-Munoz M, et al. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients. 2018;10(12).
- Cao W, Dou Y, Li A. Resveratrol Boosts Cognitive Function by Targeting SIRT1. Neurochem Res. 2018;43(9):1705-1713.
- Marx W, Kelly JT, Marshall S, et al. Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta-analysis of randomized controlled trials. Nutrition reviews. 2018;76(6):432-443.
- Witte AV, Kerti L, Margulies DS, Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34(23):7862-7870.
- Kobe T, Witte AV, Schnelle A, et al. Impact of Resveratrol on Glucose Control, Hippocampal Structure and Connectivity, and Memory Performance in Patients with Mild Cognitive Impairment. Front Neurosci. 2017;11:105.
- Anton SD, Ebner N, Dzierzewski JM, et al. Effects of 90 Days of Resveratrol Supplementation on Cognitive Function in Elders: A Pilot Study. Journal of alternative and complementary medicine (New York, NY). 2018;24(7):725-732.
- Evans HM, Howe PR, Wong RH. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients. 2017;9(1).
- Farzaei MH, Bahramsoltani R, Abbasabadi Z, Braidy N, Nabavi SM. Role of green tea catechins in prevention of age-related cognitive decline: Pharmacological targets and clinical perspective. Journal of cellular physiology. 2019;234(3):2447-2459.
- Pervin M, Unno K, Ohishi T, Tanabe H, Miyoshi N, Nakamura Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules (Basel, Switzerland). 2018;23(6).
- Mancini E, Beglinger C, Drewe J, Zanchi D, Lang UE, Borgwardt S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2017;34:26-37.
- Heitman E, Ingram DK. Cognitive and neuroprotective effects of chlorogenic acid. Nutritional neuroscience. 2017;20(1):32-39.
- Saitou K, Ochiai R, Kozuma K, et al. Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2018;10(10).
- Spinedi E, Cardinali DP. Neuroendocrine-Metabolic Dysfunction and Sleep Disturbances in Neurodegenerative Disorders: Focus on Alzheimer s Disease and Melatonin. <br>. Neuroendocrinology. 2018.
- Sarlak G, Jenwitheesuk A, Chetsawang B, Govitrapong P. Effects of melatonin on nervous system aging: neurogenesis and neurodegeneration. Journal of pharmacological sciences. 2013;123(1):9-24.
- Waller KL, Mortensen EL, Avlund K, et al. Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nature and science of sleep. 2016;8:47-53.
- Sirin FB, Kumbul Doguc D, Vural H, et al. Plasma 8-isoPGF2alpha and serum melatonin levels in patients with minimal cognitive impairment and Alzheimer disease. Turkish journal of medical sciences. 2015;45(5):1073-1077.
- Obayashi K, Saeki K, Iwamoto J, et al. Physiological Levels of Melatonin Relate to Cognitive Function and Depressive Symptoms: The HEIJO-KYO Cohort. J Clin Endocrinol Metab. 2015;100(8):3090-3096.
- Xia T, Cui Y, Chu S, et al. Melatonin pretreatment prevents isoflurane-induced cognitive dysfunction by modulating sleep-wake rhythm in mice. Brain research. 2016;1634:12-20.
- Kwon KJ, Lee EJ, Kim MK, et al. The potential role of melatonin on sleep deprivation-induced cognitive impairments: implication of FMRP on cognitive function. Neuroscience. 2015;301:403-414.
- Song J, Chu S, Cui Y, et al. Circadian rhythm resynchronization improved isoflurane-induced cognitive dysfunction in aged mice. Experimental neurology. 2018;306:45-54.
- Shen D, Tian X, Sang W, Song R. Effect of Melatonin and Resveratrol against Memory Impairment and Hippocampal Damage in a Rat Model of Vascular Dementia. Neuroimmunomodulation. 2016;23(5-6):318-331.
- Corpas R, Grinan-Ferre C, Palomera-Avalos V, et al. Melatonin induces mechanisms of brain resilience against neurodegeneration. Journal of pineal research. 2018;65(4):e12515.
- Permpoonputtana K, Tangweerasing P, Mukda S, Boontem P, Nopparat C, Govitrapong P. Long-term administration of melatonin attenuates neuroinflammation in the aged mouse brain. EXCLI journal. 2018;17:634-646.
- Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, Brusco LI. Therapeutic application of melatonin in mild cognitive impairment. American journal of neurodegenerative disease. 2012;1(3):280-291.
- Furio AM, Brusco LI, Cardinali DP. Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study. Journal of pineal research. 2007;43(4):404-409.
- Iwashita H, Matsumoto Y, Maruyama Y, Watanabe K, Chiba A, Hattori A. The melatonin metabolite N1-acetyl-5-methoxykynuramine facilitates long-term object memory in young and aging mice. Journal of pineal research. 2021;70(1):e12703.
- Fan Y, Yuan L, Ji M, Yang J, Gao D. The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty: A randomized controlled trial. Journal of clinical anesthesia. 2017;39:77-81.
- Srinivasan V, Pandi-Perumal SR, Cardinali DP, Poeggeler B, Hardeland R. Melatonin in Alzheimer's disease and other neurodegenerative disorders. Behavioral and brain functions : BBF. 2006;2:15.
- Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI. Melatonin Therapy in Patients with Alzheimer's Disease. Antioxidants (Basel, Switzerland). 2014;3(2):245-277.
- Cardoso C, Afonso C, Bandarra NM. Dietary DHA and health: cognitive function ageing. Nutrition research reviews. 2016;29(2):281-294.
- Rathod R, Kale A, Joshi S. Novel insights into the effect of vitamin B(1)(2) and omega-3 fatty acids on brain function. J Biomed Sci. 2016;23:17.
- Morris MC, Brockman J, Schneider JA, et al. Association of Seafood Consumption, Brain Mercury Level, and APOE epsilon4 Status With Brain Neuropathology in Older Adults. Jama. 2016;315(5):489-497.
- Luo C, Ren H, Yao X, et al. Enriched Brain Omega-3 Polyunsaturated Fatty Acids Confer Neuroprotection against Microinfarction. EBioMedicine. 2018;32:50-61.
- Oulhaj A, Jerneren F, Refsum H, Smith AD, de Jager CA. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J Alzheimers Dis. 2016;50(2):547-557.
- Nishihira J, Tokashiki T, Higashiuesato Y, et al. Associations between Serum Omega-3 Fatty Acid Levels and Cognitive Functions among Community-Dwelling Octogenarians in Okinawa, Japan: The KOCOA Study. J Alzheimers Dis. 2016;51(3):857-866.
- Lukaschek K, von Schacky C, Kruse J, Ladwig KH. Cognitive Impairment Is Associated with a Low Omega-3 Index in the Elderly: Results from the KORA-Age Study. Dement Geriatr Cogn Disord. 2016;42(3-4):236-245.
- D'Ascoli TA, Mursu J, Voutilainen S, Kauhanen J, Tuomainen TP, Virtanen JK. Association between serum long-chain omega-3 polyunsaturated fatty acids and cognitive performance in elderly men and women: The Kuopio Ischaemic Heart Disease Risk Factor Study. European journal of clinical nutrition. 2016;70(8):970-975.
- Lai HT, de Oliveira Otto MC, Lemaitre RN, et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ (Clinical research ed). 2018;363:k4067.
- Zamroziewicz MK, Paul EJ, Zwilling CE, Barbey AK. Predictors of Memory in Healthy Aging: Polyunsaturated Fatty Acid Balance and Fornix White Matter Integrity. Aging Dis. 2017;8(4):372-383.
- Andruchow ND, Konishi K, Shatenstein B, Bohbot VD. A lower ratio of omega-6 to omega-3 fatty acids predicts better hippocampus-dependent spatial memory and cognitive status in older adults. Neuropsychology. 2017;31(7):724-734.
- Zhang XW, Hou WS, Li M, Tang ZY. Omega-3 fatty acids and risk of cognitive decline in the elderly: a meta-analysis of randomized controlled trials. Aging clinical and experimental research. 2016;28(1):165-166.
- Masana MF, Koyanagi A, Haro JM, Tyrovolas S. n-3 Fatty acids, Mediterranean diet and cognitive function in normal aging: A systematic review. Exp Gerontol. 2017;91:39-50.
- Danthiir V, Hosking DE, Nettelbeck T, et al. An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. Am J Clin Nutr. 2018;107(5):754-762.
- Baleztena J, Ruiz-Canela M, Sayon-Orea C, et al. Association between cognitive function and supplementation with omega-3 PUFAs and other nutrients in >/= 75 years old patients: A randomized multicenter study. PLoS One. 2018;13(3):e0193568.
- Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377-389.
- Hooper C, De Souto Barreto P, Coley N, et al. Cognitive Changes with Omega-3 Polyunsaturated Fatty Acids in Non-Demented Older Adults with Low Omega-3 Index. The journal of nutrition, health & aging. 2017;21(9):988-993.
- Coley N, Raman R, Donohue MC, Aisen PS, Vellas B, Andrieu S. Defining the Optimal Target Population for Trials of Polyunsaturated Fatty Acid Supplementation Using the Erythrocyte Omega-3 Index: A Step Towards Personalized Prevention of Cognitive Decline? The journal of nutrition, health & aging. 2018;22(8):982-998.
- Yassine HN, Croteau E, Rawat V, et al. DHA brain uptake and APOE4 status: a PET study with [1-(11)C]-DHA. Alzheimers Res Ther. 2017;9(1):23.
- van de Rest O, Wang Y, Barnes LL, Tangney C, Bennett DA, Morris MC. APOE epsilon4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology. 2016;86(22):2063-2070.
- Nock TG, Chouinard-Watkins R, Plourde M. Carriers of an apolipoprotein E epsilon 4 allele are more vulnerable to a dietary deficiency in omega-3 fatty acids and cognitive decline. Biochimica et biophysica acta Molecular and cell biology of lipids. 2017;1862(10 Pt A):1068-1078.
- Porter K, Hoey L, Hughes CF, Ward M, McNulty H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients. 2016;8(11).
- Mendonca N, Granic A, Mathers JC, et al. One-Carbon Metabolism Biomarkers and Cognitive Decline in the Very Old: The Newcastle 85+ Study. Journal of the American Medical Directors Association. 2017;18(9):806.e819-806.e827.
- Mizrahi EH, Lubart E, Leibovitz A. Low Borderline Levels of Serum Vitamin B12 May Predict Cognitive Decline in Elderly Hip Fracture Patients. The Israel Medical Association journal : IMAJ. 2017;19(5):305-308.
- Hughes CF, Ward M, Tracey F, et al. B-Vitamin Intake and Biomarker Status in Relation to Cognitive Decline in Healthy Older Adults in a 4-Year Follow-Up Study. Nutrients. 2017;9(1).
- Zhang DM, Ye JX, Mu JS, Cui XP. Efficacy of Vitamin B Supplementation on Cognition in Elderly Patients With Cognitive-Related Diseases. Journal of geriatric psychiatry and neurology. 2017;30(1):50-59.
- Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. The Cochrane database of systematic reviews. 2018;12:Cd011906.
- D'Cunha NM, Georgousopoulou EN, Dadigamuwage L, et al. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: a 10-year systematic review of randomised controlled trials. The British journal of nutrition. 2018;119(3):280-298.
- Butler M, Nelson VA, Davila H, et al. Over-the-Counter Supplement Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia: A Systematic Review. Ann Intern Med. 2018;168(1):52-62.
- de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. International journal of geriatric psychiatry. 2012;27(6):592-600.
- Blasko I, Hinterberger M, Kemmler G, et al. Conversion from mild cognitive impairment to dementia: influence of folic acid and vitamin B12 use in the VITA cohort. The journal of nutrition, health & aging. 2012;16(8):687-694.
- Ma F, Wu T, Zhao J, et al. Effects of 6-Month Folic Acid Supplementation on Cognitive Function and Blood Biomarkers in Mild Cognitive Impairment: A Randomized Controlled Trial in China. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71(10):1376-1383.
- Ma F, Wu T, Zhao J, et al. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci Rep. 2016;6:37486.
- Lee HK, Kim SY, Sok SR. Effects of Multivitamin Supplements on Cognitive Function, Serum Homocysteine Level, and Depression of Korean Older Adults With Mild Cognitive Impairment in Care Facilities. Journal of nursing scholarship : an official publication of Sigma Theta Tau International Honor Society of Nursing. 2016;48(3):223-231.
- Mitchell ES, Conus N, Kaput J. B vitamin polymorphisms and behavior: evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neuroscience and biobehavioral reviews. 2014;47:307-320.
- Hara J, Shankle WR, Barrentine LW, Curole MV. Novel Therapy of Hyperhomocysteinemia in Mild Cognitive Impairment, Alzheimer's Disease, and Other Dementing Disorders. The journal of nutrition, health & aging. 2016;20(8):825-834.
- Scapicchio PL. Revisiting choline alphoscerate profile: a new, perspective, role in dementia? Int J Neurosci. 2013;123(7):444-449.
- Traini E, Bramanti V, Amenta F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent. Current Alzheimer research. 2013;10(10):1070-1079.
- Tayebati SK, Amenta F. Choline-containing phospholipids: relevance to brain functional pathways. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2013;51(3):513-521.
- De Jesus Moreno Moreno M. Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial. Clinical therapeutics. 2003;25(1):178-193.
- Gavrilova SI, Kolykhalov IV, Ponomareva EV, Fedorova YB, Selezneva ND. [Clinical efficacy and safety of choline alfoscerate in the treatment of late-onset cognitive impairment]. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118(5):45-53.
- Pizova NV. [The use of cereton in patients with chronic brain ischemia and moderate cognitive impairment]. Zh Nevrol Psikhiatr Im S S Korsakova. 2014;114(12):78-83.
- Amenta F, Carotenuto A, Fasanaro AM, Rea R, Traini E. The ASCOMALVA trial: association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in Alzheimer's disease with cerebrovascular injury: interim results. J Neurol Sci. 2012;322(1-2):96-101.
- Amenta F, Carotenuto A, Fasanaro AM, Rea R, Traini E. The ASCOMALVA (Association between the Cholinesterase Inhibitor Donepezil and the Cholinergic Precursor Choline Alphoscerate in Alzheimer's Disease) Trial: interim results after two years of treatment. J Alzheimers Dis. 2014;42 Suppl 3:S281-288.
- Rea R, Carotenuto A, Traini E, Fasanaro AM, Manzo V, Amenta F. Apathy Treatment in Alzheimer's Disease: Interim Results of the ASCOMALVA Trial. J Alzheimers Dis. 2015;48(2):377-383.
- Carotenuto A, Rea R, Traini E, et al. The Effect of the Association between Donepezil and Choline Alphoscerate on Behavioral Disturbances in Alzheimer's Disease: Interim Results of the ASCOMALVA Trial. J Alzheimers Dis. 2017;56(2):805-815.
- Glade MJ, Smith K. Phosphatidylserine and the human brain. Nutrition (Burbank, Los Angeles County, Calif). 2015;31(6):781-786.
- Vakhapova V, Cohen T, Richter Y, Herzog Y, Kam Y, Korczyn AD. Phosphatidylserine containing omega-3 Fatty acids may improve memory abilities in nondemented elderly individuals with memory complaints: results from an open-label extension study. Dementia and geriatric cognitive disorders. 2014;38(1-2):39-45.
- Maggioni M, Picotti GB, Bondiolotti GP, et al. Effects of phosphatidylserine therapy in geriatric patients with depressive disorders. Acta psychiatrica Scandinavica. 1990;81(3):265-270.
- Cenacchi T, Bertoldin T, Farina C, Fiori MG, Crepaldi G. Cognitive decline in the elderly: a double-blind, placebo-controlled multicenter study on efficacy of phosphatidylserine administration. Aging (Milan, Italy). 1993;5(2):123-133.
- Kato-Kataoka A, Sakai M, Ebina R, Nonaka C, Asano T, Miyamori T. Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. Journal of clinical biochemistry and nutrition. 2010;47(3):246-255.
- Richter Y, Herzog Y, Lifshitz Y, Hayun R, Zchut S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: a pilot study. Clin Interv Aging. 2013;8:557-563.
- Vakhapova V, Richter Y, Cohen T, Herzog Y, Korczyn AD. Safety of phosphatidylserine containing omega-3 fatty acids in non-demented elderly: a double-blind placebo-controlled trial followed by an open-label extension. BMC Neurol. 2011;11:79.
- Richter Y, Herzog Y, Cohen T, Steinhart Y. The effect of phosphatidylserine-containing omega-3 fatty acids on memory abilities in subjects with subjective memory complaints: a pilot study. Clinical interventions in aging. 2010;5:313-316.
- Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD. Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement Geriatr Cogn Disord. 2010;29(5):467-474.
- Rathe M, Muller K, Sangild PT, Husby S. Clinical applications of bovine colostrum therapy: a systematic review. Nutrition reviews. 2014;72(4):237-254.
- Bagwe S, Tharappel LJ, Kaur G, Buttar HS. Bovine colostrum: an emerging nutraceutical. Journal of complementary & integrative medicine. 2015;12(3):175-185.
- Camfield DA, Owen L, Scholey AB, Pipingas A, Stough C. Dairy constituents and neurocognitive health in ageing. The British journal of nutrition. 2011;106(2):159-174.
- Janusz M, Zablocka A. Colostrinin: a proline-rich polypeptide complex of potential therapeutic interest. Cellular and molecular biology (Noisy-le-Grand, France). 2013;59(1):4-11.
- Bacsi A, Aguilera-Aguirre L, German P, Kruzel ML, Boldogh I. Colostrinin decreases spontaneous and induced mutation frequencies at the hprt locus in Chinese hamster V79 cells. Journal of experimental therapeutics & oncology. 2006;5(4):249-259.
- Bacsi A, Woodberry M, Kruzel ML, Boldogh I. Colostrinin delays the onset of proliferative senescence of diploid murine fibroblast cells. Neuropeptides. 2007;41(2):93-101.
- Zablocka A, Ogorzalek A, Macala J, Janusz M. A proline-rich polypeptide complex (PRP) influences inducible nitric oxide synthase in mice at the protein level. Nitric oxide : biology and chemistry. 2010;23(1):20-25.
- Zablocka A, Janusz M. Effect of the proline-rich polypeptide complex/colostrinin on the enzymatic antioxidant system. Arch Immunol Ther Exp (Warsz). 2012;60(5):383-390.
- Janusz M, Zablocka A. Colostral proline-rich polypeptides--immunoregulatory properties and prospects of therapeutic use in Alzheimer's disease. Current Alzheimer research. 2010;7(4):323-333.
- Bilikiewicz A, Gaus W. Colostrinin (a naturally occurring, proline-rich, polypeptide mixture) in the treatment of Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2004;6(1):17-26.
- Leszek J, Inglot AD, Janusz M, et al. Colostrinin proline-rich polypeptide complex from ovine colostrum--a long-term study of its efficacy in Alzheimer's disease. Medical science monitor : international medical journal of experimental and clinical research. 2002;8(10):Pi93-96.
- Leszek J, Inglot AD, Janusz M, Lisowski J, Krukowska K, Georgiades JA. Colostrinin: a proline-rich polypeptide (PRP) complex isolated from ovine colostrum for treatment of Alzheimer's disease. A double-blind, placebo-controlled study. Archivum immunologiae et therapiae experimentalis. 1999;47(6):377-385.
- Jeon KI, Xu X, Aizawa T, et al. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9795-9800.
- Hadjiev D. Asymptomatic ischemic cerebrovascular disorders and neuroprotection with vinpocetine. Ideggyogyaszati szemle. 2003;56(5-6):166-172.
- Bagoly E, Feher G, Szapary L. [The role of vinpocetine in the treatment of cerebrovascular diseases based in human studies]. Orvosi hetilap. 2007;148(29):1353-1358.
- Cai Y, Li JD, Yan C. Vinpocetine attenuates lipid accumulation and atherosclerosis formation. Biochemical and biophysical research communications. 2013;434(3):439-443.
- Kemeny V, Molnar S, Andrejkovics M, Makai A, Csiba L. Acute and chronic effects of vinpocetine on cerebral hemodynamics and neuropsychological performance in multi-infarct patients. J Clin Pharmacol. 2005;45(9):1048-1054.
- Valikovics A, Csanyi A, Nemeth L. [Study of the effects of vinpocetin on cognitive functions]. Ideggyogyaszati szemle. 2012;65(3-4):115-120.
- Valikovics A. [Investigation of the effect of vinpocetine on cerebral blood flow and cognitive functions]. Ideggyogyaszati szemle. 2007;60(7-8):301-310.
- Rybakowski JK. Challenging the Negative Perception of Lithium and Optimizing Its Long-Term Administration. Frontiers in molecular neuroscience. 2018;11:349.
- Won E, Kim YK. An Oldie but Goodie: Lithium in the Treatment of Bipolar Disorder through Neuroprotective and Neurotrophic Mechanisms. International journal of molecular sciences. 2017;18(12).
- Kessing LV, Gerds TA, Knudsen NN, et al. Association of Lithium in Drinking Water With the Incidence of DementiaAssociation of Lithium in Drinking Water With Dementia IncidenceAssociation of Lithium in Drinking Water With Dementia Incidence. JAMA Psychiatry. 2017;74(10):1005-1010.
- Brown EE, Gerretsen P, Pollock B, Graff-Guerrero A. Psychiatric benefits of lithium in water supplies may be due to protection from the neurotoxicity of lead exposure. Med Hypotheses. 2018;115:94-102.
- Morris G, Berk M. The Putative Use of Lithium in Alzheimer's Disease. Current Alzheimer research. 2016;13(8):853-861.
- Brzozka MM, Havemann-Reinecke U, Wichert SP, Falkai P, Rossner MJ. Molecular Signatures of Psychosocial Stress and Cognition Are Modulated by Chronic Lithium Treatment. Schizophrenia bulletin. 2016;42 Suppl 1:S22-33.
- Kerr F, Bjedov I, Sofola-Adesakin O. Molecular Mechanisms of Lithium Action: Switching the Light on Multiple Targets for Dementia Using Animal Models. Frontiers in molecular neuroscience. 2018;11:297.
- De-Paula VJ, Gattaz WF, Forlenza OV. Long-term lithium treatment increases intracellular and extracellular brain-derived neurotrophic factor (BDNF) in cortical and hippocampal neurons at subtherapeutic concentrations. Bipolar disorders. 2016;18(8):692-695.
- Gideons ES, Lin PY, Mahgoub M, Kavalali ET, Monteggia LM. Chronic lithium treatment elicits its antimanic effects via BDNF-TrkB dependent synaptic downscaling. Elife. 2017;6.
- Valvassori SS, Borges CP, Varela RB, et al. The different effects of lithium and tamoxifen on memory formation and the levels of neurotrophic factors in the brain of male and female rats. Brain research bulletin. 2017;134:228-235.
- Matsunaga S, Kishi T, Annas P, Basun H, Hampel H, Iwata N. Lithium as a Treatment for Alzheimer's Disease: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2015;48(2):403-410.
- Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's disease. Current Alzheimer research. 2013;10(1):104-107.
- Wilson EN, Do Carmo S, Iulita MF, et al. Microdose Lithium NP03 Diminishes Pre-Plaque Oxidative Damage and Neuroinflammation in a Rat Model of Alzheimer's-like Amyloidosis. Current Alzheimer research. 2018;15(13):1220-1230.
- Wilson EN, Do Carmo S, Iulita MF, et al. BACE1 inhibition by microdose lithium formulation NP03 rescues memory loss and early stage amyloid neuropathology. Translational psychiatry. 2017;7(8):e1190.
- Rees A, Dodd GF, Spencer JPE. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients. 2018;10(12).
- Grassi D, Ferri C, Desideri G. Brain Protection and Cognitive Function: Cocoa Flavonoids as Nutraceuticals. Curr Pharm Des. 2016;22(2):145-151.
- Wang J, Varghese M, Ono K, et al. Cocoa extracts reduce oligomerization of amyloid-beta: implications for cognitive improvement in Alzheimer's disease. J Alzheimers Dis. 2014;41(2):643-650.
- Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol. 2013;75(3):716-727.
- Orozco Arbelaez E, Banegas JR, Rodriguez Artalejo F, Lopez Garcia E. [Influence of habitual chocolate consumption over the Mini-Mental State Examination in Spanish older adults]. Nutr Hosp. 2017;34(4):841-846.
- Moreira A, Diogenes MJ, de Mendonca A, Lunet N, Barros H. Chocolate Consumption is Associated with a Lower Risk of Cognitive Decline. J Alzheimers Dis. 2016;53(1):85-93.
- Neshatdoust S, Saunders C, Castle SM, et al. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: Two randomised, controlled trials. Nutr Healthy Aging. 2016;4(1):81-93.
- Mastroiacovo D, Kwik-Uribe C, Grassi D, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study--a randomized controlled trial. Am J Clin Nutr. 2015;101(3):538-548.
- Brickman AM, Khan UA, Provenzano FA, et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17(12):1798-1803.
- Goncalves S, Moreira E, Grosso C, Andrade PB, Valentao P, Romano A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J Food Sci Technol. 2017;54(1):219-227.
- Nabavi SF, Tenore GC, Daglia M, Tundis R, Loizzo MR, Nabavi SM. The cellular protective effects of rosmarinic acid: from bench to bedside. Curr Neurovasc Res. 2015;12(1):98-105.
- Lee AY, Hwang BR, Lee MH, Lee S, Cho EJ. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-beta25-35 induced impairment of cognition and memory function. Nutrition research and practice. 2016;10(3):274-281.
- Paudel P, Seong SH, Zhou Y, et al. Rosmarinic Acid Derivatives' Inhibition of Glycogen Synthase Kinase-3beta Is the Pharmacological Basis of Kangen-Karyu in Alzheimer's Disease. Molecules (Basel, Switzerland). 2018;23(11).
- Herrlinger KA, Nieman KM, Sanoshy KD, et al. Spearmint Extract Improves Working Memory in Men and Women with Age-Associated Memory Impairment. Journal of alternative and complementary medicine (New York, NY). 2018;24(1):37-47.
- Nieman KM. Tolerance, bioavailability, and potential cognitive health implications of a distinct aqueous spearmint extract. Functional Foods in Health and Disease. 2015;5(5):165–187.
- Wong RH, Howe PR, Bryan J, Coates AM, Buckley JD, Berry NM. Chronic effects of a wild green oat extract supplementation on cognitive performance in older adults: a randomised, double-blind, placebo-controlled, crossover trial. Nutrients. 2012;4(5):331-342.
- Dimpfel W, Storni C, Verbruggen M. Ingested oat herb extract (Avena sativa) changes EEG spectral frequencies in healthy subjects. Journal of alternative and complementary medicine (New York, NY). 2011;17(5):427-434.
- Maggiorani D, Manzella N, Edmondson DE, et al. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxid Med Cell Longev. 2017;2017:3017947.
- Weinreb O, Amit T, Bar-Am O, Youdim MB. Neuroprotective effects of multifaceted hybrid agents targeting MAO, cholinesterase, iron and beta-amyloid in ageing and Alzheimer's disease. Br J Pharmacol. 2016;173(13):2080-2094.
- Riederer P, Muller T. Use of monoamine oxidase inhibitors in chronic neurodegeneration. Expert opinion on drug metabolism & toxicology. 2017;13(2):233-240.
- Perrinjaquet-Moccetti T, Wullschleger C, Schmidt A, Aydogan C, Kreuter M. Bioactivity-based development of a wild green oat (Avena sativa L.) extract in support of mental health disorders. Vol 272006.
- Garcia AM, Martinez A, Gil C. Enhancing cAMP Levels as Strategy for the Treatment of Neuropsychiatric Disorders. Current topics in medicinal chemistry. 2016;16(29):3527-3535.
- Heckman PRA, Blokland A, Prickaerts J. From Age-Related Cognitive Decline to Alzheimer's Disease: A Translational Overview of the Potential Role for Phosphodiesterases. Advances in neurobiology. 2017;17:135-168.
- Wong RH, Howe PR, Coates AM, Buckley JD, Berry NM. Chronic consumption of a wild green oat extract (Neuravena) improves brachial flow-mediated dilatation and cerebrovascular responsiveness in older adults. Journal of hypertension. 2013;31(1):192-200.
- Kennedy DO, Jackson PA, Forster J, et al. Acute effects of a wild green-oat (Avena sativa) extract on cognitive function in middle-aged adults: A double-blind, placebo-controlled, within-subjects trial. Nutritional neuroscience. 2017;20(2):135-151.
- Berry NM, Robinson MJ, Bryan J, Buckley JD, Murphy KJ, Howe PR. Acute effects of an Avena sativa herb extract on responses to the Stroop Color-Word test. Journal of alternative and complementary medicine (New York, NY). 2011;17(7):635-637.
- Friedman M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion's Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J Agric Food Chem. 2015;63(32):7108-7123.
- Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother Res. 2009;23(3):367-372.
- Brandalise F, Cesaroni V, Gregori A, et al. Dietary Supplementation of Hericium erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evidence-based complementary and alternative medicine : eCAM. 2017;2017:3864340.
- Tsai-Teng T, Chin-Chu C, Li-Ya L, et al. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer's disease-related pathologies in APPswe/PS1dE9 transgenic mice. J Biomed Sci. 2016;23(1):49.
- Mori K, Obara Y, Moriya T, Inatomi S, Nakahata N. Effects of Hericium erinaceus on amyloid beta(25-35) peptide-induced learning and memory deficits in mice. Biomedical research (Tokyo, Japan). 2011;32(1):67-72.
- Misra HS, Rajpurohit YS, Khairnar NP. Pyrroloquinoline-quinone and its versatile roles in biological processes. Journal of biosciences. 2012;37(2):313-325.
- Rucker R, Chowanadisai W, Nakano M. Potential physiological importance of pyrroloquinoline quinone. Alternative medicine review : a journal of clinical therapeutic. 2009;14(3):268-277.
- Harris CB, Chowanadisai W, Mishchuk DO, Satre MA, Slupsky CM, Rucker RB. Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J Nutr Biochem. 2013;24(12):2076-2084.
- Zhou XQ, Yao ZW, Peng Y, et al. PQQ ameliorates D-galactose induced cognitive impairments by reducing glutamate neurotoxicity via the GSK-3beta/Akt signaling pathway in mouse. Sci Rep. 2018;8(1):8894.
- Qin J, Wu M, Yu S, et al. Pyrroloquinoline quinone-conferred neuroprotection in rotenone models of Parkinson's disease. Toxicol Lett. 2015;238(3):70-82.
- Yang C, Yu L, Kong L, et al. Pyrroloquinoline quinone (PQQ) inhibits lipopolysaccharide induced inflammation in part via downregulated NF-kappaB and p38/JNK activation in microglial and attenuates microglia activation in lipopolysaccharide treatment mice. PLoS One. 2014;9(10):e109502.
- Guan S, Xu J, Guo Y, et al. Pyrroloquinoline quinone against glutamate-induced neurotoxicity in cultured neural stem and progenitor cells. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2015;42:37-45.
- Zhang Q, Chen S, Yu S, et al. Neuroprotective effects of pyrroloquinoline quinone against rotenone injury in primary cultured midbrain neurons and in a rat model of Parkinson's disease. Neuropharmacology. 2016;108:238-251.
- Kim J, Harada R, Kobayashi M, Kobayashi N, Sode K. The inhibitory effect of pyrroloquinoline quinone on the amyloid formation and cytotoxicity of truncated alpha-synuclein. Molecular neurodegeneration. 2010;5:20.
- Kim J, Kobayashi M, Fukuda M, et al. Pyrroloquinoline quinone inhibits the fibrillation of amyloid proteins. Prion. 2010;4(1):26-31.
- Kobayashi M, Kim J, Kobayashi N, et al. Pyrroloquinoline quinone (PQQ) prevents fibril formation of alpha-synuclein. Biochemical and biophysical research communications. 2006;349(3):1139-1144.
- Zhang JJ, Zhang RF, Meng XK. Protective effect of pyrroloquinoline quinone against Abeta-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci Lett. 2009;464(3):165-169.
- Yamaguchi K, Sasano A, Urakami T, Tsuji T, Kondo K. Stimulation of nerve growth factor production by pyrroloquinoline quinone and its derivatives in vitro and in vivo. Bioscience, biotechnology, and biochemistry. 1993;57(7):1231-1233.
- Urakami T, Tanaka A, Yamaguchi K, Tsuji T, Niki E. Synthesis of esters of coenzyme PQQ and IPQ, and stimulation of nerve growth factor production. BioFactors (Oxford, England). 1995;5(3):139-146.
- Murase K, Hattori A, Kohno M, Hayashi K. Stimulation of nerve growth factor synthesis/secretion in mouse astroglial cells by coenzymes. Biochemistry and molecular biology international. 1993;30(4):615-621.
- Liu S, Li H, Ou Yang J, et al. Enhanced rat sciatic nerve regeneration through silicon tubes filled with pyrroloquinoline quinone. Microsurgery. 2005;25(4):329-337.
- Li HH, Liu SQ, Peng H, Zhang N. Pyrroloquinoline quinone enhances regeneration of transected sciatic nerve in rats. Chinese journal of traumatology = Zhonghua chuang shang za zhi / Chinese Medical Association. 2005;8(4):225-229.
- Ohwada K, Takeda H, Yamazaki M, et al. Pyrroloquinoline Quinone (PQQ) Prevents Cognitive Deficit Caused by Oxidative Stress in Rats. Journal of clinical biochemistry and nutrition. 2008;42:29-34.
- Itoh Y, Hine K, Miura H, et al. Effect of the Antioxidant Supplement Pyrroloquinoline Quinone Disodium Salt (BioPQQ) on Cognitive Functions. Adv Exp Med Biol. 2016;876:319-325.
- Nakano M, Murayama Y, Hu L, Ikemoto K, Uetake T, Sakatani K. Effects of Antioxidant Supplements (BioPQQ) on Cerebral Blood Flow and Oxygen Metabolism in the Prefrontal Cortex. Adv Exp Med Biol. 2016;923:215-222.
- Srivastava S. Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. Clinical and translational medicine. 2016;5(1):25.
- Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends in endocrinology and metabolism: TEM. 2012;23(9):420-428.
- Mendelsohn AR, Larrick JW. The NAD+/PARP1/SIRT1 Axis in Aging. Rejuvenation research. 2017;20(3):244-247.
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464-471.
- Kulikova V, Shabalin K, Nerinovski K, et al. Generation, Release, and Uptake of the NAD Precursor Nicotinic Acid Riboside by Human Cells. The Journal of biological chemistry. 2015;290(45):27124-27137.
- Yang Y, Sauve AA. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016;1864(12):1787-1800.
- Elhassan YS, Philp AA, Lavery GG. Targeting NAD+ in Metabolic Disease: New Insights Into an Old Molecule. Journal of the Endocrine Society. 2017;1(7):816-835.
- Matasic DS, Brenner C, London B. Emerging potential benefits of modulating NAD(+) metabolism in cardiovascular disease. American journal of physiology Heart and circulatory physiology. 2018;314(4):H839-h852.
- Yoshino J, Baur JA, Imai SI. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell metabolism. 2018;27(3):513-528.
- Dellinger RW, Santos SR, Morris M, et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD(+) levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ aging and mechanisms of disease. 2017;3:17.
- Gong B, Pan Y, Vempati P, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer's mouse models. Neurobiol Aging. 2013;34(6):1581-1588.
- Hou Y, Lautrup S, Cordonnier S, et al. NAD(+) supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(8):E1876-e1885.
- Zhang H, Ryu D, Wu Y, et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, NY). 2016;352(6292):1436-1443.
- Khan NA, Auranen M, Paetau I, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO molecular medicine. 2014;6(6):721-731.
- Singh A, Kumar A. Microglial Inhibitory Mechanism of Coenzyme Q10 Against Abeta (1-42) Induced Cognitive Dysfunctions: Possible Behavioral, Biochemical, Cellular, and Histopathological Alterations. Frontiers in pharmacology. 2015;6:268.
- Bhardwaj M, Kumar A. Neuroprotective mechanism of Coenzyme Q10 (CoQ10) against PTZ induced kindling and associated cognitive dysfunction: Possible role of microglia inhibition. Pharmacological reports : PR. 2016;68(6):1301-1311.
- Yamagishi K, Ikeda A, Moriyama Y, et al. Serum coenzyme Q10 and risk of disabling dementia: the Circulatory Risk in Communities Study (CIRCS). Atherosclerosis. 2014;237(2):400-403.
- Kure CE, Rosenfeldt FL, Scholey AB, et al. Relationships Among Cognitive Function and Cerebral Blood Flow, Oxidative Stress, and Inflammation in Older Heart Failure Patients. Journal of cardiac failure. 2016;22(7):548-559.
- Li Z, Wang P, Yu Z, et al. The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson's disease. European neurology. 2015;73(3-4):205-211.
- Dumont M, Kipiani K, Yu F, et al. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2011;27(1):211-223.
- Hickey MA, Zhu C, Medvedeva V, Franich NR, Levine MS, Chesselet MF. Evidence for behavioral benefits of early dietary supplementation with CoEnzymeQ10 in a slowly progressing mouse model of Huntington's disease. Molecular and cellular neurosciences. 2012;49(2):149-157.
- Bharti VK, Malik JK, Gupta RC. Chapter 52 - Ashwagandha: Multiple Health Benefits. In: Gupta RC, ed. Nutraceuticals. Boston: Academic Press; 2016:717-733.
- Dar NJ, MuzamilAhmad. Neurodegenerative diseases and Withania somnifera (L.): An update. Journal of ethnopharmacology. 2020;256:112769.
- Choudhary D, Bhattacharyya S, Bose S. Efficacy and Safety of Ashwagandha (Withania somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. Journal of dietary supplements. 2017;14(6):599-612.
- Chengappa KN, Bowie CR, Schlicht PJ, Fleet D, Brar JS, Jindal R. Randomized placebo-controlled adjunctive study of an extract of withania somnifera for cognitive dysfunction in bipolar disorder. The Journal of clinical psychiatry. 2013;74(11):1076-1083.
- Birla H, Keswani C, Rai SN, et al. Neuroprotective effects of Withania somnifera in BPA induced-cognitive dysfunction and oxidative stress in mice. Behavioral and brain functions : BBF. 2019;15(1):9.
- Ahmed ME, Javed H, Khan MM, et al. Attenuation of oxidative damage-associated cognitive decline by Withania somnifera in rat model of streptozotocin-induced cognitive impairment. Protoplasma. 2013;250(5):1067-1078.
- Pandey A, Bani S, Dutt P, Kumar Satti N, Avtar Suri K, Nabi Qazi G. Multifunctional neuroprotective effect of Withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine. 2018;102:211-221.
- Vallée M. Neurosteroids and potential therapeutics: Focus on pregnenolone. J Steroid Biochem Mol Biol. 2016;160:78-87.
- Smith CC, Gibbs TT, Farb DH. Pregnenolone sulfate as a modulator of synaptic plasticity. Psychopharmacology. 2014;231(17):3537-3556.
- Murugan S, Jakka P, Namani S, Mujumdar V, Radhakrishnan G. The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. J Biol Chem. 2019;294(12):4596-4607.
- Abdel-Hafiz L, Chao OY, Huston JP, et al. Promnestic effects of intranasally applied pregnenolone in rats. Neurobiology of learning and memory. 2016;133:185-195.
- Vallée M, Mayo W, Darnaudéry M, et al. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proc Natl Acad Sci U S A. 1997;94(26):14865-14870.
- Kreinin A, Bawakny N, Ritsner MS. Adjunctive Pregnenolone Ameliorates the Cognitive Deficits in Recent-Onset Schizophrenia: An 8-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Clin Schizophr Relat Psychoses. 2017;10(4):201-210.
- Ritsner MS, Gibel A, Shleifer T, et al. Pregnenolone and dehydroepiandrosterone as an adjunctive treatment in schizophrenia and schizoaffective disorder: an 8-week, double-blind, randomized, controlled, 2-center, parallel-group trial. The Journal of clinical psychiatry. 2010;71(10):1351-1362.
- Horowitz AM, Fan X, Bieri G, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science (New York, NY). 2020;369(6500):167-173.
- Chandrika UG, Prasad Kumarab PA. Gotu Kola (Centella asiatica): Nutritional Properties and Plausible Health Benefits. Advances in food and nutrition research. 2015;76:125-157.
- Chaisawang P, Sirichoat A, Chaijaroonkhanarak W, et al. Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5-fluorouracil chemotherapy. PLoS One. 2017;12(7):e0180650.
- Umka Welbat J, Sirichoat A, Chaijaroonkhanarak W, et al. Asiatic Acid Prevents the Deleterious Effects of Valproic Acid on Cognition and Hippocampal Cell Proliferation and Survival. Nutrients. 2016;8(5).
- Hamid K, Ng I, Tallapragada VJ, et al. An Investigation of the Differential Effects of Ursane Triterpenoids from Centella asiatica, and Their Semisynthetic Analogues, on GABAA Receptors. Chem Biol Drug Des. 2016;88(3):386-397.
- Xu MF, Xiong YY, Liu JK, Qian JJ, Zhu L, Gao J. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol Sin. 2012;33(5):578-587.
- Nagoor Meeran MF, Goyal SN, Suchal K, Sharma C, Patil CR, Ojha SK. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Frontiers in pharmacology. 2018;9(892).
- Matthews DG, Caruso M, Murchison CF, et al. Centella Asiatica Improves Memory and Promotes Antioxidative Signaling in 5XFAD Mice. Antioxidants (Basel, Switzerland). 2019;8(12).
- Chiroma SM, Baharuldin MTH, Mat Taib CN, et al. Protective Effects of Centella asiatica on Cognitive Deficits Induced by D-gal/AlCl(3) via Inhibition of Oxidative Stress and Attenuation of Acetylcholinesterase Level. Toxics. 2019;7(2).
- Veerendra Kumar MH, Gupta YK. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. Journal of ethnopharmacology. 2002;79(2):253-260.
- Chiroma SM, Hidayat Baharuldin MT, Mat Taib CN, et al. Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: Behavioral and ultrastructural approaches. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;109:853-864.
- Gray NE, Harris CJ, Quinn JF, Soumyanath A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. Journal of ethnopharmacology. 2016;180:78-86.
- Wattanathorn J, Mator L, Muchimapura S, et al. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. Journal of ethnopharmacology. 2008;116(2):325-332.
- Puttarak P, Dilokthornsakul P, Saokaew S, et al. Effects of Centella asiatica (L.) Urb. on cognitive function and mood related outcomes: A Systematic Review and Meta-analysis. Sci Rep. 2017;7(1):10646.
- Tan BL, Norhaizan ME. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules (Basel, Switzerland). 2019;24(9):1801.
- Mewborn CM, Terry DP, Renzi-Hammond LM, Hammond BR, Miller LS. Relation of Retinal and Serum Lutein and Zeaxanthin to White Matter Integrity in Older Adults: A Diffusion Tensor Imaging Study. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2018;33(7):861-874.
- Christensen K, Gleason CE, Mares JA. Dietary carotenoids and cognitive function among US adults, NHANES 2011-2014. Nutritional neuroscience. 2020;23(7):554-562.
- Araki A, Yoshimura Y, Sakurai T, et al. Low intakes of carotene, vitamin B2 , pantothenate and calcium predict cognitive decline among elderly patients with diabetes mellitus: The Japanese Elderly Diabetes Intervention Trial. Geriatrics & gerontology international. 2017;17(8):1168-1175.
- Mewborn CM, Lindbergh CA, Robinson TL, et al. Lutein and Zeaxanthin Are Positively Associated with Visual-Spatial Functioning in Older Adults: An fMRI Study. Nutrients. 2018;10(4).
- Yuan C, Fondell E, Ascherio A, et al. Long-Term Intake of Dietary Carotenoids Is Positively Associated with Late-Life Subjective Cognitive Function in a Prospective Study in US Women. J Nutr. 2020;150(7):1871-1879.
- Ceravolo SA, Hammond BR, Oliver W, Clementz B, Miller LS, Renzi-Hammond LM. Dietary Carotenoids Lutein and Zeaxanthin Change Brain Activation in Older Adult Participants: A Randomized, Double-Masked, Placebo-Controlled Trial. Mol Nutr Food Res. 2019;63(15):1801051.
- Power R, Coen RF, Beatty S, et al. Supplemental Retinal Carotenoids Enhance Memory in Healthy Individuals with Low Levels of Macular Pigment in A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Alzheimers Dis. 2018;61(3):947-961.
- Hammond BR, Jr., Miller LS, Bello MO, Lindbergh CA, Mewborn C, Renzi-Hammond LM. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Frontiers in aging neuroscience. 2017;9:254.
- Ghossein N, Kang M, Lakhkar A. Anticholinergic Medications. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK555893/. Accessed October 19, 2020.
- Weigand AJ, Bondi MW, Thomas KR, et al. Association of anticholinergic medications and AD biomarkers with incidence of MCI among cognitively normal older adults. Neurology. 2020;95(16):e2295.
- Graff-Radford J. Vascular Cognitive Impairment. Continuum (Minneapolis, Minn). Feb 2019;25(1):147-164. doi:10.1212/CON.0000000000000684
- Li X, Lyu P, Ren Y, An J, Dong Y. Arterial stiffness and cognitive impairment. J Neurol Sci. Sep 15 2017;380:1-10. doi:10.1016/j.jns.2017.06.018
- Li Q, Yang Y, Reis C, et al. Cerebral Small Vessel Disease. Cell transplantation. Dec 2018;27(12):1711-1722. doi:10.1177/0963689718795148
- Caplan LR. Clinical diagnosis of stroke subtypes. UpToDate. Updated 8/5/2019. Accessed 2/5/2021, https://www.uptodate.com/contents/clinical-diagnosis-of-stroke-subtypes?search=Clinical%20diagnosis%20of%20stroke%20subtypes&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag. 2016;12:105-16. doi:10.2147/VHRM.S75306
- Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. May 2016;1862(5):915-25. doi:10.1016/j.bbadis.2016.01.015
- Levine DA, Wadley VG, Langa KM, et al. Risk Factors for Poststroke Cognitive Decline: The REGARDS Study (Reasons for Geographic and Racial Differences in Stroke). Stroke. Apr 2018;49(4):987-994. doi:10.1161/STROKEAHA.117.018529
- Ganzer CA, Barnes A, Uphold C, Jacobs AR. Transient Ischemic Attack and Cognitive Impairment: A Review. J Neurosci Nurs. Dec 2016;48(6):322-327. doi:10.1097/JNN.0000000000000236
- Gardener H, Caunca MR. Mediterranean Diet in Preventing Neurodegenerative Diseases. Current nutrition reports. 2018;7(1):10-20. doi:10.1007/s13668-018-0222-5
- Gardener SL, Rainey-Smith SR. The Role of Nutrition in Cognitive Function and Brain Ageing in the Elderly. Current nutrition reports. Sep 2018;7(3):139-149. doi:10.1007/s13668-018-0229-y
- van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer's Disease-A Review. Adv Nutr. Nov 1 2019;10(6):1040-1065. doi:10.1093/advances/nmz054
- Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean Diet; a Literature Review. Nutrients. Nov 5 2015;7(11):9139-53. doi:10.3390/nu7115459
- Sanchez-Villegas A, Galbete C, Martinez-Gonzalez MA, et al. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: the PREDIMED-NAVARRA randomized trial. Nutritional neuroscience. Sep 2011;14(5):195-201. doi:10.1179/1476830511Y.0000000011
- DHHS. YOUR GUIDE TO LOWERING YOUR BLOOD PRESSURE. DASH Eating Plan: Lower Your Blood Pressure. U.S. Department of Health and Human Services. Updated 4/2006. Accessed 7/14/2021, https://www.nhlbi.nih.gov/files/docs/public/heart/new_dash.pdf
- HARVARD T.H. CHAN. Diet Review: DASH. Harvard. Accessed 7/14/2021, https://www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/dash-diet/
- Asemi Z, Samimi M, Tabassi Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized clinical trial. Nutrition (Burbank, Los Angeles County, Calif). Nov-Dec 2014;30(11-12):1287-93. doi:10.1016/j.nut.2014.03.008
- NLM. U.S. National Library of Medicine. DASH Eating Plan. MedlinePlus. Updated 7/2/2021. Accessed 7/14/2021, https://medlineplus.gov/dasheatingplan.html
- Pirouzeh R, Heidarzadeh-Esfahani N, Morvaridzadeh M, et al. Effect of DASH diet on oxidative stress parameters: A systematic review and meta-analysis of randomized clinical trials. Diabetes Metab Syndr. Nov-Dec 2020;14(6):2131-2138. doi:10.1016/j.dsx.2020.10.031
- Berendsen AAM, Kang JH, van de Rest O, Feskens EJM, de Groot L, Grodstein F. The Dietary Approaches to Stop Hypertension Diet, Cognitive Function, and Cognitive Decline in American Older Women. Journal of the American Medical Directors Association. May 1 2017;18(5):427-432. doi:10.1016/j.jamda.2016.11.026
- Blumenthal JA, Smith PJ, Mabe S, et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology. Jan 15 2019;92(3):e212-e223. doi:10.1212/WNL.0000000000006784
- Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. Sep 2015;11(9):1007-14. doi:10.1016/j.jalz.2014.11.009
- Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimer's & dementia : the journal of the Alzheimer's Association. Apr 2019;15(4):581-589. doi:10.1016/j.jalz.2018.12.011
- Kheirouri S, Alizadeh M. MIND diet and cognitive performance in older adults: a systematic review. Crit Rev Food Sci Nutr. May 14 2021:1-19. doi:10.1080/10408398.2021.1925220
- Cherian L, Wang Y, Fakuda K, Leurgans S, Aggarwal N, Morris M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diet Slows Cognitive Decline After Stroke. The journal of prevention of Alzheimer's disease. 2019;6(4):267-273. doi:10.14283/jpad.2019.28
- Yeh TS, Yuan C, Ascherio A, Rosner B, Willett W, Blacker D. Long-term Dietary Flavonoid Intake and Subjective Cognitive Decline in US Men and Women. Neurology. Jul 28 2021;doi:10.1212/WNL.0000000000012454
- Simpson T, Kure C, Stough C. Assessing the Efficacy and Mechanisms of Pycnogenol((R)) on Cognitive Aging From In Vitro Animal and Human Studies. Front Pharmacol. 2019;10:694. doi:10.3389/fphar.2019.00694
- Hosoi M, Belcaro G, Saggino A, Luzzi R, Dugall M, Feragalli B. Pycnogenol(R) supplementation in minimal cognitive dysfunction. Journal of neurosurgical sciences. Jun 2018;62(3):279-284. doi:10.23736/S0390-5616.18.04382-5
- Ishrat T, Parveen K, Hoda MN, et al. Effects of Pycnogenol and vitamin E on cognitive deficits and oxidative damage induced by intracerebroventricular streptozotocin in rats. Behavioural pharmacology. Oct 2009;20(7):567-75. doi:10.1097/FBP.0b013e32832c7125
- Luzzi R, Belcaro G, Zulli C, et al. Pycnogenol(R) supplementation improves cognitive function, attention and mental performance in students. Panminerva Med. Sep 2011;53(3 Suppl 1):75-82.
- Ryan J, Croft K, Mori T, et al. An examination of the effects of the antioxidant Pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. Jul 2008;22(5):553-62. doi:10.1177/0269881108091584
- Cesarone MR, Belcaro G, Hosoi M, et al. Supplementary management with Pycnogenol(R) in Parkinson's disease to prevent cognitive impairment. Journal of neurosurgical sciences. Jun 2020;64(3):258-262. doi:10.23736/S0390-5616.19.04839-2
- Paarmann K, Prakash SR, Krohn M, et al. French maritime pine bark treatment decelerates plaque development and improves spatial memory in Alzheimer's disease mice. Phytomedicine. Apr 2019;57:39-48. doi:10.1016/j.phymed.2018.11.033
- Belcaro G, Dugall M, Ippolito E, Hu S, Saggino A, Feragalli B. The COFU3 Study. Improvement in cognitive function, attention, mental performance with Pycnogenol(R) in healthy subjects (55-70) with high oxidative stress. Journal of neurosurgical sciences. Dec 2015;59(4):437-46.
- Belcaro G, Luzzi R, Dugall M, Ippolito E, Saggino A. Pycnogenol(R) improves cognitive function, attention, mental performance and specific professional skills in healthy professionals aged 35-55. Journal of neurosurgical sciences. Dec 2014;58(4):239-48.
- Stough CK, Pase MP, Cropley V, et al. A randomized controlled trial investigating the effect of Pycnogenol and Bacopa CDRI08 herbal medicines on cognitive, cardiovascular, and biochemical functioning in cognitively healthy elderly people: the Australian Research Council Longevity Intervention (ARCLI) study protocol (ANZCTR12611000487910). Nutr J. Mar 6 2012;11:11. doi:10.1186/1475-2891-11-11
- Petersen RC, Yaffe K, Wilterdink JL. Mild cognitive impairment: Epidemiology, pathology, and clinical assessment. UpToDate. Updated Dec. 10, 2020. Accessed Dec. 14, 2021, https://www.uptodate.com/contents/mild-cognitive-impairment-epidemiology-pathology-and-clinical-assessment?source=history_widget#H2
- Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern Med. Sep 2015;175(9):1450-8. doi:10.1001/jamainternmed.2015.2152
- Braekhus A, Laake K, Engedal K. A low, 'normal' score on the Mini-Mental State Examination predicts development of dementia after three years. J Am Geriatr Soc. Jun 1995;43(6):656-61. doi:10.1111/j.1532-5415.1995.tb07201.x
- Zaudig M. A new systematic method of measurement and diagnosis of "mild cognitive impairment" and dementia according to ICD-10 and DSM-III-R criteria. International psychogeriatrics. 1992;4 Suppl 2:203-19. doi:10.1017/s1041610292001273
- White HL, Scates PW. Acetyl-L-carnitine as a precursor of acetylcholine. Neurochem Res. Jun 1990;15(6):597-601. doi:10.1007/BF00973749
- Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. Jul 25 2006;67(2):229-34. doi:10.1212/01.wnl.0000224748.48011.84
- Lopez-Rios L, Wiebe JC, Vega-Morales T, Gericke N. Central nervous system activities of extract Mangifera indica L. J Ethnopharmacol. Oct 5 2020;260:112996. doi:10.1016/j.jep.2020.112996
- Wightman EL, Jackson PA, Forster J, et al. Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study. Nutrients. Jul 23 2020;12(8)doi:10.3390/nu12082194
- Feng ST, Wang ZZ, Yuan YH, Sun HM, Chen NH, Zhang Y. Mangiferin: A multipotent natural product preventing neurodegeneration in Alzheimer's and Parkinson's disease models. Pharmacol Res. Aug 2019;146:104336. doi:10.1016/j.phrs.2019.104336
- Marquez L, Garcia-Bueno B, Madrigal JL, Leza JC. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur J Nutr. Sep 2012;51(6):729-39. doi:10.1007/s00394-011-0252-x
- UniProt. UniProtKB - P21964 (COMT_HUMAN). Updated 9/29/2021. Accessed 2/15/2022, https://www.uniprot.org/uniprot/P21964
- Lum PT, Sekar M, Gan SH, Pandy V, Bonam SR. Protective effect of mangiferin on memory impairment: A systematic review. Saudi J Biol Sci. Jan 2021;28(1):917-927. doi:10.1016/j.sjbs.2020.11.037
- Ayaz M, Sadiq A, Junaid M, Ullah F, Subhan F, Ahmed J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front Aging Neurosci. 2017;9:168. doi:10.3389/fnagi.2017.00168
- Kennedy D, Okello E, Chazot P, et al. Volatile Terpenes and Brain Function: Investigation of the Cognitive and Mood Effects of Mentha x Piperita L. Essential Oil with In Vitro Properties Relevant to Central Nervous System Function. Nutrients. Aug 7 2018;10(8)doi:10.3390/nu10081029
- Murbach TS, Glávits R, Endres JR, et al. A toxicological evaluation of lithium orotate. Regulatory toxicology and pharmacology : RTP. Aug 2021;124:104973. doi:10.1016/j.yrtph.2021.104973
- Zhao Y, Zhang J, Zheng Y, et al. NAD(+) improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1alpha pathway. J Neuroinflammation. Sep 16 2021;18(1):207. doi:10.1186/s12974-021-02250-8
- Kinoshita T, Maruyama K, Tanigawa T. The effects of long-term Ubiquinol intake on improving the Quality of Life of community residents. Functional Foods in Health and Disease. 2016;6(1):16-32. doi:10.31989/ffhd.v6i1.225
- Hrcka Krausova B, Kysilov B, Cerny J, et al. Site of Action of Brain Neurosteroid Pregnenolone Sulfate at the N-Methyl-D-Aspartate Receptor. J Neurosci. Jul 29 2020;40(31):5922-5936. doi:10.1523/JNEUROSCI.3010-19.2020
- Rajagopal L, Soni D, Meltzer HY. Neurosteroid pregnenolone sulfate, alone, and as augmentation of lurasidone or tandospirone, rescues phencyclidine-induced deficits in cognitive function and social interaction. Behav Brain Res. Sep 17 2018;350:31-43. doi:10.1016/j.bbr.2018.05.005
- Lopresti AL. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs in R&D. Mar 2017;17(1):53-64. doi:10.1007/s40268-016-0157-5
- Foolad F, Khodagholi F. Dietary supplementation with Salvia sahendica attenuates acetylcholinesterase activity and increases mitochondrial transcription factor A and antioxidant proteins in the hippocampus of amyloid beta-injected rats. J Pharm Pharmacol. Oct 2013;65(10):1555-62. doi:10.1111/jphp.12116
- Smach MA, Hafsa J, Charfeddine B, Dridi H, Limem K. Effects of sage extract on memory performance in mice and acetylcholinesterase activity. Ann Pharm Fr. Jul 2015;73(4):281-8. doi:10.1016/j.pharma.2015.03.005
- Qin XY, Cao C, Cawley NX, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer's disease: a meta-analysis study (N=7277). Molecular psychiatry. Feb 2017;22(2):312-320. doi:10.1038/mp.2016.62
- Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther. Feb 2003;28(1):53-9. doi:10.1046/j.1365-2710.2003.00463.x
- Scholey AB, Tildesley NT, Ballard CG, et al. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology (Berl). May 2008;198(1):127-39. doi:10.1007/s00213-008-1101-3
- Edwards KD, Dubberke A, Meyer N, Kugel S, Hellhammer J. Assessment of the Effects of a Sage (Salvia officinalis) Extract on Cognitive Performance in Adolescents and Young Adults. medRxiv. 2021;(January 27th, 2020):2021.05.28.21257776. doi:10.1101/2021.05.28.21257776
- Kennedy DO, Pace S, Haskell C, Okello EJ, Milne A, Scholey AB. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology. Apr 2006;31(4):845-52. doi:10.1038/sj.npp.1300907
- Miroddi M, Navarra M, Quattropani MC, Calapai F, Gangemi S, Calapai G. Systematic review of clinical trials assessing pharmacological properties of Salvia species on memory, cognitive impairment and Alzheimer's disease. CNS neuroscience & therapeutics. Jun 2014;20(6):485-95. doi:10.1111/cns.12270
- Grima NA, Pase MP, Macpherson H, Pipingas A. The Effects of Multivitamins on Cognitive Performance: A Systematic Review and Meta-Analysis. Journal of Alzheimer's Disease. 2012;29:561-569. doi:10.3233/JAD-2011-111751
- Grodstein F, O’Brien J, Kang JH, et al. Long-Term Multivitamin Supplementation and Cognitive Function in Men. Annals of Internal Medicine. 2013;159(12):806-814. doi:10.7326/0003-4819-159-12-201312170-00006 %m 24490265
- Baker LD, Manson JE, Rapp SR et al. Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial. Alzheimers Dement. 2022 Sep 14. doi: 10.1002/alz.12767.
- Charisis S, Ntanasi E, Yannakoulia M, et al. Diet Inflammatory Index and Dementia Incidence: A Population-Based Study. Neurology. Dec 14 2021;97(24):e2381-e2391. doi:10.1212/WNL.0000000000012973
- Dhana K, James BD, Agarwal P, et al. MIND Diet, Common Brain Pathologies, and Cognition in Community-Dwelling Older Adults. J Alzheimers Dis. 2021;83(2):683-692. doi:10.3233/JAD-210107
- Watson NF, Badr MS, Belenky G, et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843-844. doi:10.5665/sleep.4716
- Sabia S, Fayosse A, Dumurgier J, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. Apr 20 2021;12(1):2289. doi:10.1038/s41467-021-22354-2
- Dove A, Shang Y, Xu W, et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimer's & Dementia. 2021;17(11):1769-1778. doi:https://doi.org/10.1002/alz.12482
- Lin FR, Yaffe K, Xia J, et al. Hearing Loss and Cognitive Decline in Older Adults. JAMA Internal Medicine. 2013;173(4):293-299. doi:10.1001/jamainternmed.2013.1868
- Karasek M, Reiter RJ. Melatonin and aging. Neuro Endocrinol Lett. Apr 2002;23 Suppl 1:14-6.
- Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Annals of neurology. 2011;70(6):871-880.
- Xia X, Wang R, Vetrano DL, et al. From Normal Cognition to Cognitive Impairment and Dementia: Impact of Orthostatic Hypotension. Hypertension. Sep 2021;78(3):769-778. doi:10.1161/HYPERTENSIONAHA.121.17454
- Ernst ME, Ryan J, Chowdhury EK, et al. Long-Term Blood Pressure Variability and Risk of Cognitive Decline and Dementia Among Older Adults. J Am Heart Assoc. Jul 6 2021;10(13):e019613. doi:10.1161/JAHA.120.019613
- Guilliams TG. The role of stress and the HPA axis in chronic disease management. Stevens Point: Point Institute. 2015;
- Payne AH, Jaffe RB. Androgen formation from pregnenolone sulfate by fetal, neonatal, prepubertal and adult human testes. J Clin Endocrinol Metab. Jan 1975;40(1):102-7. doi:10.1210/jcem-40-1-102
- Ryan KJ, Petro Z. Steroid biosynthesis by human ovarian granulosa and thecal cells. J Clin Endocrinol Metab. Jan 1966;26(1):46-52. doi:10.1210/jcem-26-1-46
- Vallee M. Neurosteroids and potential therapeutics: Focus on pregnenolone. J Steroid Biochem Mol Biol. Jun 2016;160:78-87. doi:10.1016/j.jsbmb.2015.09.030
- Hill M, Lukáč D, Lapčík O, et al. Age relationships and sex differences in serum levels of pregnenolone and 17-hydroxypregnenolone in normal subjects. 1999;
- Weill-Engerer S, David JP, Sazdovitch V, et al. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J Clin Endocrinol Metab. Nov 2002;87(11):5138-43. doi:10.1210/jc.2002-020878
- Marx CE, Trost WT, Shampine LJ, et al. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry. Dec 15 2006;60(12):1287-94. doi:10.1016/j.biopsych.2006.06.017
- Weng JH, Chung BC. Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids. Jul 2016;111:54-59. doi:10.1016/j.steroids.2016.01.017
- Mayo W, Le Moal M, Abrous DN. Pregnenolone sulfate and aging of cognitive functions: behavioral, neurochemical, and morphological investigations. Hormones and behavior. Sep 2001;40(2):215-7. doi:10.1006/hbeh.2001.1677
- Akwa Y, Sananès N, Gouézou M, Robel P, Baulieu EE, Le Goascogne C. Astrocytes and neurosteroids: metabolism of pregnenolone and dehydroepiandrosterone. Regulation by cell density. J Cell Biol. Apr 1993;121(1):135-43. doi:10.1083/jcb.121.1.135
- Wojtal K, Trojnar MK, Czuczwar SJ. Endogenous neuroprotective factors: neurosteroids. Pharmacological reports : PR. May-Jun 2006;58(3):335-40.
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. Journal of neuroendocrinology. 1995;7(3):171-177.
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABAA receptors. European journal of pharmacology. 1997;325(1):1-7.
- Marx CE, Bradford DW, Hamer RM, et al. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011;191:78-90.
- Brown ES, Park J, Marx CE, et al. A randomized, double-blind, placebo-controlled trial of pregnenolone for bipolar depression. Neuropsychopharmacology. Nov 2014;39(12):2867-73. doi:10.1038/npp.2014.138
- Harteneck C. Pregnenolone sulfate: from steroid metabolite to TRP channel ligand. Molecules. Sep 27 2013;18(10):12012-28. doi:10.3390/molecules181012012
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. The Journal of Physiology. 2001;532(3):673-684.
- Smith CC, Gibbs TT, Farb DH. Pregnenolone sulfate as a modulator of synaptic plasticity. Psychopharmacology. 2014;231(17):3537-3556.
- Hajjar I, Okafor M, McDaniel D, et al. Effects of Candesartan vs Lisinopril on Neurocognitive Function in Older Adults With Executive Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA Netw Open. Aug 3 2020;3(8):e2012252. doi:10.1001/jamanetworkopen.2020.12252
- Teng Z, Feng J, Qi Q, et al. Long-Term Use of Metformin Is Associated With Reduced Risk of Cognitive Impairment With Alleviation of Cerebral Small Vessel Disease Burden in Patients With Type 2 Diabetes. Front Aging Neurosci. 2021;13:773797. doi:10.3389/fnagi.2021.773797
- Madhu LN, Kodali M, Shetty AK. Promise of metformin for preventing age-related cognitive dysfunction. Neural regeneration research. Mar 2022;17(3):503-507. doi:10.4103/1673-5374.320971
- Beydoun MA, Beydoun HA, Fanelli-Kuczmarski MT, et al. Association of Serum Antioxidant Vitamins and Carotenoids With Incident Alzheimer Disease and All-Cause Dementia Among US Adults. Neurology. 2022:10.1212/WNL.0000000000200289. doi:10.1212/WNL.0000000000200289
- Okano T, Shimomura Y, Yamane M, et al. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. Apr 25 2008;283(17):11270-9. doi:10.1074/jbc.M702971200. https://www.ncbi.nlm.nih.gov/pubmed/18083713
- Popescu A, German M. Vitamin K2 Holds Promise for Alzheimer's Prevention and Treatment. Nutrients. Jun 27 2021;13(7)doi:10.3390/nu13072206. https://www.ncbi.nlm.nih.gov/pubmed/34199021
- Maresz K. Growing Evidence of a Proven Mechanism Shows Vitamin K2 Can Impact Health Conditions Beyond Bone and Cardiovascular. Integr Med (Encinitas). Aug 2021;20(4):34-38. https://www.ncbi.nlm.nih.gov/pubmed/34602875
- Booth SL, Shea MK, Barger K, et al. Association of vitamin K with cognitive decline and neuropathology in community-dwelling older persons. Alzheimers Dement (N Y). 2022;8(1):e12255. doi:10.1002/trc2.12255. https://www.ncbi.nlm.nih.gov/pubmed/35475263
- Alessenko AV, Albi E. Exploring Sphingolipid Implications in Neurodegeneration. Front Neurol. 2020;11:437. doi:10.3389/fneur.2020.00437. https://www.ncbi.nlm.nih.gov/pubmed/32528400
- Yang RY, Pan JY, Chen Y, Li Y, Wu J, Wang XD. Menaquinone-7 protects astrocytes by regulating mitochondrial function and inflammatory response under hypoxic conditions. Eur Rev Med Pharmacol Sci. Oct 2020;24(19):10181-10193. doi:10.26355/eurrev_202010_23239. https://www.ncbi.nlm.nih.gov/pubmed/33090426
- Hadipour E, Tayarani-Najaran Z, Fereidoni M. Vitamin K2 protects PC12 cells against Abeta (1-42) and H2O2-induced apoptosis via p38 MAP kinase pathway. Nutritional neuroscience. May 2020;23(5):343-352. doi:10.1080/1028415X.2018.1504428. https://www.ncbi.nlm.nih.gov/pubmed/30058479
- Huang SH, Fang ST, Chen YC. Molecular Mechanism of Vitamin K2 Protection against Amyloid-beta-Induced Cytotoxicity. Biomolecules. Mar 13 2021;11(3)doi:10.3390/biom11030423. https://www.ncbi.nlm.nih.gov/pubmed/33805625
- Saputra WD, Aoyama N, Komai M, Shirakawa H. Menaquinone-4 Suppresses Lipopolysaccharide-Induced Inflammation in MG6 Mouse Microglia-Derived Cells by Inhibiting the NF-kappaB Signaling Pathway. Int J Mol Sci. May 10 2019;20(9)doi:10.3390/ijms20092317. https://www.ncbi.nlm.nih.gov/pubmed/31083359
- Elkattawy HA, Ghoneim FM, Eladl MA, et al. Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats. Antioxidants (Basel). Mar 8 2022;11(3)doi:10.3390/antiox11030514. https://www.ncbi.nlm.nih.gov/pubmed/35326164
- Yu YX, Yu XD, Cheng QZ, Tang L, Shen MQ. The association of serum vitamin K2 levels with Parkinson's disease: from basic case-control study to big data mining analysis. Aging (Albany NY). Aug 29 2020;12(16):16410-16419. doi:10.18632/aging.103691. https://www.ncbi.nlm.nih.gov/pubmed/32862152
- Gáll Z, Székely O. Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients. Oct 20 2021;13(11)doi:10.3390/nu13113672. https://pubmed.ncbi.nlm.nih.gov/34835929/
- Terock J, Bonk S, Frenzel S, et al. Vitamin D deficit is associated with accelerated brain aging in the general population. Psychiatry Res Neuroimaging. Dec 2022;327:111558. doi:10.1016/j.pscychresns.2022.111558. https://pubmed.ncbi.nlm.nih.gov/36302278/
- Ghahremani M, Smith EE, Chen HY, Creese B, Goodarzi Z, Ismail Z. Vitamin D supplementation and incident dementia: Effects of sex, APOE, and baseline cognitive status. Alzheimer's & dementia (Amsterdam, Netherlands). Jan-Mar 2023;15(1):e12404. doi:10.1002/dad2.12404. https://pubmed.ncbi.nlm.nih.gov/36874594/
- Yang T, Wang H, Xiong Y, et al. Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J Alzheimers Dis. 2020;78(4):1509-1518. doi:10.3233/jad-200926. https://pubmed.ncbi.nlm.nih.gov/33164936/
- Kang JH, Vyas CM, Okereke OI, et al. Effect of vitamin D on cognitive decline: results from two ancillary studies of the VITAL randomized trial. Sci Rep. Dec 1 2021;11(1):23253. doi:10.1038/s41598-021-02485-8. https://pubmed.ncbi.nlm.nih.gov/34853363/
- Cui X, Eyles DW. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients. Oct 17 2022;14(20)doi:10.3390/nu14204353. https://pubmed.ncbi.nlm.nih.gov/36297037/
- Barnes LL, Dhana K, Liu X, et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. New England Journal of Medicine. 2023;doi:10.1056/NEJMoa2302368. https://www.nejm.org/doi/full/10.1056/NEJMoa2302368
- Sattayakhom A, Wichit S, Koomhin P. The Effects of Essential Oils on the Nervous System: A Scoping Review. Molecules. Apr 27 2023;28(9)doi:10.3390/molecules28093771
- Liu B, Kou J, Li F, et al. Lemon essential oil ameliorates age-associated cognitive dysfunction via modulating hippocampal synaptic density and inhibiting acetylcholinesterase. Aging (Albany NY). May 11 2020;12(9):8622-8639. doi:10.18632/aging.103179
- Okuda M, Fujita Y, Takada-Takatori Y, Sugimoto H, Urakami K. Aromatherapy improves cognitive dysfunction in senescence-accelerated mouse prone 8 by reducing the level of amyloid beta and tau phosphorylation. PLoS One. 2020;15(10):e0240378. doi:10.1371/journal.pone.0240378
- Vance DE, Del Bene VA, Kamath V, et al. Does Olfactory Training Improve Brain Function and Cognition? A Systematic Review. Neuropsychol Rev. Feb 2 2023:1-37. doi:10.1007/s11065-022-09573-0
- Al Ain S, Poupon D, Hetu S, Mercier N, Steffener J, Frasnelli J. Smell training improves olfactory function and alters brain structure. Neuroimage. Apr 1 2019;189:45-54. doi:10.1016/j.neuroimage.2019.01.008
- Woo CC, Miranda B, Sathishkumar M, Dehkordi-Vakil F, Yassa MA, Leon M. Overnight olfactory enrichment using an odorant diffuser improves memory and modifies the uncinate fasciculus in older adults. Front Neurosci. 2023;17:1200448. doi:10.3389/fnins.2023.1200448
- Birte-Antina W, Ilona C, Antje H, Thomas H. Olfactory training with older people. International journal of geriatric psychiatry. Jan 2018;33(1):212-220. doi:10.1002/gps.4725
- Haehner A, Chen B, Espin M, et al. Training with Odors Impacts Hippocampal Thickness in Patients with Mild Cognitive Impairment. J Alzheimers Dis. 2022;88(2):743-755. doi:10.3233/JAD-220248
- Jimbo D, Kimura Y, Taniguchi M, Inoue M, Urakami K. Effect of aromatherapy on patients with Alzheimer's disease. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society. Dec 2009;9(4):173-9. doi:10.1111/j.1479-8301.2009.00299.x
- Cha H, Kim S, Seo MS, Kim HS. Effects of olfactory stimulation on cognitive function and behavior problems in older adults with dementia: A systematic literature review. Geriatr Nurs. Sep-Oct 2021;42(5):1210-1217. doi:10.1016/j.gerinurse.2021.07.003
- Won J, Nielson KA, Smith JC. Large-Scale Network Connectivity and Cognitive Function Changes After Exercise Training in Older Adults with Intact Cognition and Mild Cognitive Impairment. J Alzheimers Dis Rep. 2023;7(1):399-413. doi:10.3233/ADR-220062. https://pubmed.ncbi.nlm.nih.gov/37220620/
- Watanabe N. Effect of ergothioneine on the cognitive function improvement in healthy volunteers and mild cognitive impairment subjects–A randomized, double‐blind, parallel‐group comparison study. Jpn Pharmacol Ther . 2020;48:685. https://www.ls-corporation.co.jp/wp-content/uploads/2022/10/ergothioneine_congitive.pdf
- Cheah IK, Feng L, Tang RMY, Lim KHC, Halliwell B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochemical and biophysical research communications . Sep 9 2016;478(1):162-167. doi:10.1016/j.bbrc.2016.07.074. https://www.sciencedirect.com/science/article/abs/pii/S0006291X16311962?via%3Dihub
- Cha S, Bell L, Williams CM. The Relationship between Mushroom Intake and Cognitive Performance: An Epidemiological Study in the European Investigation of Cancer-Norfolk Cohort (EPIC-Norfolk). Nutrients. Jan 25 2024;16(3)doi:10.3390/nu16030353. https://www.ncbi.nlm.nih.gov/pubmed/38337638
- Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg . Feb 1 2018;144(2):115-126. doi:10.1001/jamaoto.2017.2513. https://www.ncbi.nlm.nih.gov/pubmed/29222544
- Ying G, Zhao G, Xu X, Su S, Xie X. Association of age-related hearing loss with cognitive impairment and dementia: an umbrella review. Frontiers in aging neuroscience. 2023;15:1241224. doi:10.3389/fnagi.2023.1241224. https://www.ncbi.nlm.nih.gov/pubmed/37790283
- Conceicao Santos de Oliveira D, Gomes-Filho IS, Araujo EM, et al. Association between hearing loss and cognitive decline in the elderly: A systematic review with meta-analysis study. PLoS One. 2023;18(11):e0288099. doi:10.1371/journal.pone.0288099. https://www.ncbi.nlm.nih.gov/pubmed/37943811
- Sarant J, Harris D, Busby P, et al. The Effect of Hearing Aid Use on Cognition in Older Adults: Can We Delay Decline or Even Improve Cognitive Function? J Clin Med. Jan 17 2020;9(1)doi:10.3390/jcm9010254. https://www.ncbi.nlm.nih.gov/pubmed/31963547
- Yeo BSY, Song H, Toh EMS, et al. Association of Hearing Aids and Cochlear Implants With Cognitive Decline and Dementia: A Systematic Review and Meta-analysis. JAMA Neurol. Feb 1 2023;80(2):134-141. doi:10.1001/jamaneurol.2022.4427. https://www.ncbi.nlm.nih.gov/pubmed/36469314
- Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. Aug 8 2020;396(10248):413-446. doi:10.1016/S0140-6736(20)30367-6. https://www.ncbi.nlm.nih.gov/pubmed/32738937
- Wan Z, Zhang X, He H, et al. Secondhand smoke exposure and risk of dementia in non-smokers: A population-based cohort study. Neuroepidemiology . Feb 28 2024;doi:10.1159/000535828. https://www.ncbi.nlm.nih.gov/pubmed/38417408
- Jeong SM, Park J, Han K, et al. Association of Changes in Smoking Intensity With Risk of Dementia in Korea. JAMA Netw Open. Jan 3 2023;6(1):e2251506. doi:10.1001/jamanetworkopen.2022.51506. https://www.ncbi.nlm.nih.gov/pubmed/36656579
- Benito-Leon J, Ghosh R, Lapena-Motilva J, Martin-Arriscado C, Bermejo-Pareja F. Association between cumulative smoking exposure and cognitive decline in non-demented older adults: NEDICES study. Sci Rep . Apr 8 2023;13(1):5754. doi:10.1038/s41598-023-32663-9. https://www.ncbi.nlm.nih.gov/pubmed/37031269
- Dai X, Gil GF, Reitsma MB, et al. Health effects associated with smoking: a Burden of Proof study. Nat Med. Oct 2022;28(10):2045-2055. doi:10.1038/s41591-022-01978-x. https://www.ncbi.nlm.nih.gov/pubmed/36216941
- Kurihara K, Shiroma A, Koda M, Shinzato H, Takaesu Y, Kondo T. Age-related cognitive decline is accelerated in alcohol use disorder. Neuropsychopharmacol Rep . Dec 2023;43(4):587-595. doi:10.1002/npr2.12395. https://www.ncbi.nlm.nih.gov/pubmed/37926928
- 656. Wang G, Li DY, Vance DE, Li W. Alcohol Use Disorder as a Risk Factor for Cognitive Impairment. J Alzheimers Dis. 2023;94(3):899-907. doi:10.3233/JAD-230181. https://www.ncbi.nlm.nih.gov/pubmed/37355899
- Kilian C, Klinger S, Rehm J, Manthey J. Alcohol use, dementia risk, and sex: a systematic review and assessment of alcohol-attributable dementia cases in Europe. BMC geriatrics. Apr 25 2023;23(1):246. doi:10.1186/s12877-023-03972-5. https://www.ncbi.nlm.nih.gov/pubmed/37098501
- Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J, QalyDays Study G. Contribution of alcohol use disorders to the burden of dementia in France 2008-13: a nationwide retrospective cohort study. Lancet Public Health . Mar 2018;3(3):e124-e132. doi:10.1016/S2468-2667(18)30022-7. https://www.ncbi.nlm.nih.gov/pubmed/29475810
- Lopez-Granero C, Polyanskaya L, Ruiz-Sobremazas D, Barrasa A, Aschner M, Alique M. Particulate Matter in Human Elderly: Higher Susceptibility to Cognitive Decline and Age-Related Diseases. Biomolecules . Dec 26 2023;14(1)doi:10.3390/biom14010035. https://www.ncbi.nlm.nih.gov/pubmed/38254635
- Ge R, Wang Y, Zhang Z, Sun H, Chang J. Association of long-term exposure to various ambient air pollutants, lifestyle, and genetic predisposition with incident cognitive impairment and dementia. BMC Public Health . Jan 15 2024;24(1):179. doi:10.1186/s12889-024-17702-y. https://www.ncbi.nlm.nih.gov/pubmed/38225615
- Wood D, Evangelopoulos D, Beevers S, Kitwiroon N, Demakakos P, Katsouyanni K. Exposure to ambient air pollution and cognitive function: an analysis of the English Longitudinal Study of Ageing cohort. Environmental health : a global access science source . Apr 5 2024;23(1):35. doi:10.1186/s12940-024-01075-1. https://www.ncbi.nlm.nih.gov/pubmed/38575976
- Wang X, Younan D, Millstein J, et al. Association of improved air quality with lower dementia risk in older women. Proc Natl Acad Sci U S A . Jan 11 2022;119(2)doi:10.1073/pnas.2107833119. https://www.ncbi.nlm.nih.gov/pubmed/34983871
- Flaxman AD, Wittenborn JS, Robalik T, et al. Prevalence of Visual Acuity Loss or Blindness in the US: A Bayesian Meta-analysis. JAMA Ophthalmol. Jul 1 2021;139(7):717-723. doi:10.1001/jamaophthalmol.2021.0527. https://pubmed.ncbi.nlm.nih.gov/33983373/
- Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. Aug 10 2024;404(10452):572-628. doi:10.1016/S0140-6736(24)01296-0. https://pubmed.ncbi.nlm.nih.gov/39096926/
- Kuzma E, Littlejohns TJ, Khawaja AP, Llewellyn DJ, Ukoumunne OC, Thiem U. Visual Impairment, Eye Diseases, and Dementia Risk: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2021;83(3):1073-1087. doi:10.3233/JAD-210250. https://pubmed.ncbi.nlm.nih.gov/34397414/
- Shang X, Zhu Z, Wang W, Ha J, He M. The Association between Vision Impairment and Incidence of Dementia and Cognitive Impairment: A Systematic Review and Meta-analysis. Ophthalmology. Aug 2021;128(8):1135-1149. doi:10.1016/j.ophtha.2020.12.029. https://pubmed.ncbi.nlm.nih.gov/33422559/
- Lee CS, Gibbons LE, Lee AY, et al. Association Between Cataract Extraction and Development of Dementia. JAMA Intern Med. Feb 1 2022;182(2):134-141. doi:10.1001/jamainternmed.2021.6990. https://pubmed.ncbi.nlm.nih.gov/34870676/
- Ma LZ, Zhang YR, Li YZ, et al. Cataract, Cataract Surgery, and Risk of Incident Dementia: A Prospective Cohort Study of 300,823 Participants. Biol Psychiatry. May 1 2023;93(9):810-819. doi:10.1016/j.biopsych.2022.06.005. https://pubmed.ncbi.nlm.nih.gov/35940935/
- Reuben DB, Kremen S, Maust DT. Dementia Prevention and Treatment: A Narrative Review. JAMA Intern Med. May 1 2024;184(5):563-572. doi:10.1001/jamainternmed.2023.8522. https://www.ncbi.nlm.nih.gov/pubmed/38436963
- Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. Nov 2018;14(11):653-666. doi:10.1038/s41582-018-0070-3. https://www.ncbi.nlm.nih.gov/pubmed/30291317
- Hafdi M, Hoevenaar-Blom MP, Richard E. Multi-domain interventions for the prevention of dementia and cognitive decline. The Cochrane database of systematic reviews. Nov 8 2021;11(11):CD013572. doi:10.1002/14651858.CD013572.pub2. https://www.ncbi.nlm.nih.gov/pubmed/34748207
- Vaskivuo L, Hokkanen L, Levalahti E, et al. Subjective Memory Complaints and the Effect of a Multidomain Lifestyle Intervention on Cognition: The FINGER Trial. The journals of gerontology Series B, Psychological sciences and social sciences. Dec 11 2024;80(1)doi:10.1093/geronb/gbae179. https://www.ncbi.nlm.nih.gov/pubmed/39450437
- Baker LD, Espeland MA, Whitmer RA, et al. Structured vs Self-Guided Multidomain Lifestyle Interventions for Global Cognitive Function: The US POINTER Randomized Clinical Trial. JAMA. Jul 28 2025;doi:10.1001/jama.2025.12923. https://www.ncbi.nlm.nih.gov/pubmed/40720610
- Harasym J, Tiupova A, Pejcz E. Ergothioneine: An Antioxidative, Neuroprotective and Anti-Inflammatory Compound from Mushroom Residuals. Molecules. Dec 1 2025;30(23)doi:10.3390/molecules30234621 https://mdpi-res.com/d_attachment/molecules/molecules-30-04621/article_deploy/molecules-30-04621.pdf?version=1764605074
- May-Zhang LS, Cheah IK, Zajac IT, Brindal E, Kakoschke N. Ergothioneine for cognitive health, longevity and healthy ageing: where are we now? The Proceedings of the Nutrition Society. Sep 19 2025:1-15. doi:10.1017/s0029665125101754 https://www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/abs/ergothioneine-for-cognitive-health-longevity-and-healthy-ageing-where-are-we-now/06FD6132F86C840EC79C81D95DE3EF1B
- Takhor NH, Phan CW. The role of Ergothioneine in cognition and age-related neurodegenerative disease: a systematic review. Inflammopharmacol. May 2025;33(5):2351-2375. doi:10.1007/s10787-025-01746-6 https://link.springer.com/article/10.1007/s10787-025-01746-6
- Gede A, Gu Q, Phukhatmuen P, et al. Advances and prospects of ergothioneine in the treatment of cognitive frailty. Ann Med. Dec 2025;57(1):2555742. doi:10.1080/07853890.2025.2555742 https://www.tandfonline.com/doi/pdf/10.1080/07853890.2025.2555742
- Yau YF, Cheah IK, Mahendran R, et al. Investigating the efficacy of ergothioneine to delay cognitive decline in mild cognitively impaired subjects: A pilot study. J Alzheimers Dis. Dec 2024;102(3):841-854. doi:10.1177/13872877241291253 https://journals.sagepub.com/doi/10.1177/13872877241291253
- Zajac IT, Kakoschke N, Kuhn-Sherlock B, May-Zhang LS. The Effect of Ergothioneine Supplementation on Cognitive Function, Memory, and Sleep in Older Adults with Subjective Memory Complaints: A Randomized Placebo-Controlled Trial. Nutraceuticals. 2025;5(3):15. https://www.mdpi.com/1661-3821/5/3/15
- Meng X, Ohara T, Nishioka K, et al. Serum ergothioneine and risk of dementia in a general older Japanese population: the Hisayama Study. Psychiatry and clinical neurosciences. Dec 2025;79(12):808-816. doi:10.1111/pcn.13893 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/pcn.13893?download=true
- Wu LY, Cheah IK, Chong JR, et al. Low plasma ergothioneine levels are associated with neurodegeneration and cerebrovascular disease in dementia. Free Radic Biol Med. Dec 2021;177:201-211. doi:10.1016/j.freeradbiomed.2021.10.019 https://www.sciencedirect.com/science/article/abs/pii/S089158492100770X?via%3Dihub
- Ba DM, Gao X, Al-Shaar L, et al. Mushroom intake and cognitive performance among US older adults: the National Health and Nutrition Examination Survey, 2011-2014. Br J Nutr. Dec 14 2022;128(11):2241-2248. doi:10.1017/s0007114521005195 https://www.cambridge.org/core/services/aop-cambridge-core/content/view/C28993998C223626CD7D42C748ACD47B/S0007114521005195a.pdf/div-class-title-mushroom-intake-and-cognitive-performance-among-us-older-adults-the-national-health-and-nutrition-examination-survey-2011-2014-div.pdf
