Life Extension Magazine®
Significant dollars are being invested to develop methods to turn youthful immune function back “on.”
Yet many of us can’t wait for delays while our immune systems “fall off a cliff.”
This article explains some of the changes that occur with aging that cause our immune functions to become dysfunctional (senescent).
It also describes some of what is available today to help restore immune competence in aging humans, including suppressing the interleukin-6 cytokine.
Much of this article was published by Life Extension® in January 2016.1
Human research has since moved forward on several fronts to induce systemic improvements in immune functions.
Around age 60 a startling change occurs that decimates our ability to combat infections and malignancies.
Some people encounter these immune impairments earlier in life.
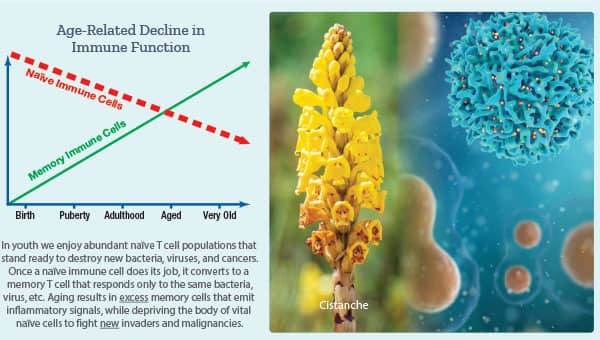
This catastrophic event decreases naïve T cells needed to ward off new bacteria, viruses, and cancers.
At the same time naïve T cells are lost, we accumulate senile memory T cells that emit pro-inflammatory signals that wreak havoc in every organ system.2
One of the deadliest of these inflammatory “signals” is a cytokine called interleukin-6 (IL-6).3
Higher IL-6 levels are associated with a 2-fold greater risk of death.4 Higher levels are also involved with multiple degenerative processes, including frailty, from which so many elderly suffer.5-7
A common trait of healthy centenarians is that they have unusually low levels of IL-6.8
People associate immune senescence with weakened immune function. It turns out that impaired immunity is only half the problem. Spiraling levels of IL-6 that attack our healthy tissues are another component of immune senescence that must be addressed.9
The encouraging news is that significant dollars are being invested to develop technologies to turn youthful immune function back “on.”10 These immune-restoration therapies may add decades to our healthy lifespans.
The problem is that many of us can’t wait for bureaucratic delays while our immune systems fall off a cliff.
There is an urgent need today to accelerate restoration of immune competence in aging humans, including suppressing deadly interleukin-6 levels.
Leading Cause of Disability and Death in Older Persons
Immune senescence is a leading cause of disability and infectious death in aging humans.11
By way of example, deaths from pneumonia are rare in youth, but spiral upward as humans mature.12 If you read obituaries (as I do), the number of once-vigorous individuals who perish from opportunistic illnesses caused by immune senescence is startling.
Life Extension® magazine has published in-depth reports about underlying causes of immune senescence and what stop-gap measures people should initiate to reverse this deadly trend.13-15
In people over age 65, the top 10 causes of death include influenza, pneumonia, and sepsis.16 Immune senescence is a major cause of all these maladies.12,17-22
Cancer, stroke, Alzheimer’s, and heart attack are common diseases of aging.16 These illnesses are all also related to immune senescence.
We often hear the term “immune health” as people seek to protect against winter viral infections. The public does not yet understand what causes our immune system to fail as we age.
“We conclude that CHF (chronic heart failure) patients show a higher degree of immunosenescence than age-matched healthy controls. T-lymphocyte differentiation and IL-6 levels are increased in patients with an advanced clinical status and may contribute to disease impairment through a compromised adaptive immune response due to accelerated aging of their immune system.”
Publication: International Journal of Cardiology – July 1, 2014
Article title: Immunosenescence and inflammation characterize chronic heart failure patients with more advanced disease.
More Naïve T Cells Urgently Needed
Immune imbalance occurs when our aging immune system fails to protect against new cancers/infections and instead generates inflammatory reactions (including increased IL-6) that attack every cell in our body.
A “naïve” immune cell is one that has not yet been activated.23 Since it is “naïve” (not yet exposed to an antigen), naïve immune cells are primed to effectively respond to new infectious agents and malignancies.
Once exposed, naïve immune cells become memory cells or plasma cells specific to the original antigen. As our internal reservoir of naïve immune cells is decreased, we have less ability to respond to new infections/malignancies.23
A deficit of naïve immune cells combined with overaccumulation of exhausted memory cells decreases the efficacy (antibody response) of vaccinations.24
Exhausted memory T cells are associated with increased risks of coronary heart disease and endothelial dysfunction, along with systemic inflammation.25-30
If we are to guard against the ravages of immune senescence, we need to increase our numbers of naïve cells (“virgin” immune cells), while reducing numbers of senile memory cells.
What you need to know
Immune Senescence
- Around age 60, physiological changes decimate our ability to combat infections and malignancies.
- Immune senescence is a leading cause of disability and death in aging humans.
- Most Life Extension® customers take nutrients that boost immune activity, including zinc, DHEA and fish oil.
- Immune imbalance occurs when our immune system fails to protect against new infections and instead generates inflammatory reactions (including increased IL-6) that attack every cell in our body.
- The herb Cistanche helps combat immune senescence, which appears to have rejuvenating effects on bone marrow.
- Supplementation with Cistanche has been shown to increase naïve T cells and natural killer (NK) cells while decreasing memory T cells and pro-inflammatory IL-6.
Protecting against Immune Senescence

Most Life Extension® customers take nutrients that exert beneficial effects on immune activity.
Zinc and DHEA partially restore thymus function, which is vital to transforming bone-marrow-derived immune cells into activated T cells. 31-35
DHEA and fish oil help suppress deadly interleukin-6.36-41
An advance in combatting immune senescence is an herb called Cistanche. This medicinal plant has been used extensively in China to treat the “ailments of aging.”42
Supplementation with Cistanche has been shown to increase naïve T cells and natural killer (NK) cells while decreasing memory T cells and pro-inflammatory interleukin-6.43
A prime cause of the severe immune dysfunction suffered by the elderly is a marked decrease in naïve T cells44-46 and functional natural killer cells,47-49 with a concomitant increase in memory T cells.50,51
Cistanche counteracts these pathological trends that characterize immune senescence.43
How Cistanche Boosts T Cell Production and Healthy Longevity

Cistanche helps restore progenitors of peripheral naïve T cells, which explains the increase seen in these vital immune cells in response to Cistanche.43
Animals supplemented with Cistanche have increased lifespans, which would be expected from a compound that counteracts immune senescence.43
Cistanche is one of the most popular Chinese herbal medicines and is listed in the Chinese herbal pharmacopeias as having “anti-aging” properties.
One reason Chinese physicians see such impressive therapeutic results is that Cistanche restores one of the most prominent bone marrow biomarkers of immune cell formation called stem cell antigen-1.43
Senile bone marrow loses its ability to produce fresh, naïve immune cells, which are launched into the bloodstream to differentiate into mature naïve T and natural killer cells.
Bone marrow stem cell antigen-1 represents the body’s main source of naïve T cells in the blood.43
Cistanche appears to have a rejuvenating effect on the bone marrow, something that is generally available only through expensive recombinant drugs. 52-54
The beneficial impact of Cistanche was demonstrated in an open-label, pilot trial of elderly people. This study combined a low dose of Cistanche (100 mg) with zinc, vitamin E, vitamin B6, fucoidan, and coenzyme Q10. Not only were markers of immune senescence reversed, but the test subjects reported improvements in quality of life, such as not “feeling tired all the time.”
This makes sense in light of the multiple adverse effects immune senescence inflicts on the body, which include increased levels of frailty.55
Cistanche represents an opportunity to restore vital components of our aging immune systems. Its low cost makes it readily affordable.
Suppressing Deadly Impact of IL-6
One way of describing “aging” is that beneficial factors (such as naïve T cell production) decrease while detrimental ones (like interleukin-6) increase.
IL-6 levels are especially high in patients with autoimmune conditions in which an out-of-control immune system attacks one’s own tissues.
High-serum IL-6, as seen in rheumatoid arthritis, for instance, is regarded as a reliable biomarker of high-grade inflammation.56-58
When it comes to “normal” aging, elevated IL-6 contributes to the destruction of bone, heart valves, neurons, and other tissues.
The DNA damage that IL-6 inflicts accelerates aging processes and malignant transformation of healthy cells.59-65
Life Extension® has published a number of articles about the critical need for aging humans to suppress chronic inflammatory inducers like interleukin-6.
As will be described in the article on page 58, a low-cost tea extract has demonstrated the ability to reduce IL-6.
When this tea extract by itself was given to 90 patients (30-65 years old) with metabolic syndrome, the following reductions in inflammatory markers were observed:66
- C-reactive protein (CRP) was reduced by 26%
- Tumor necrosis factor (TNF-a) was reduced by 23%
- Interleukin-6 (IL-6) was reduced by 21%
In addition to suppressing IL-6 and other inflammatory factors, this tea extract was shown to favorably alter genes (such as mutant p53) involved in tumor cell growth.67
Making Major Strides… but Not Fast Enough
For the past five years, significant resources have been expended to initiate studies aimed at counteracting age-related disease.
Scientific studies document how certain nutrients that Life Extension® supporters have taken for decades (like DHEA and zinc) help protect against immune decline, while guarding against chronic inflammatory factors.68-73
Consumers have access to an arsenal of novel compounds to help counteract the underlying factors that characterize immune senescence.
An impressive array of clinical research is being investigated to induce systemic age reversal in elderly people, including restoration of youthful immune function .
The carnage inflicted by dysfunctional immunity in the elderly mandates that research accelerate faster, so that more lives can be saved.
If you have any questions on the scientific content of this article, please call a Life Extension® Wellness Specialist at 1-866-864-3027.
References
- Available at: https://www.lifeextension.com/magazine/2016/1/when-immune-function-falls-off-a-cliff. Accessed April 5, 2020.
- Available at: http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2567.2002.01447.x/epdf. Accessed Aprl 5, 2020.
- Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011 May;1813(5):878-88.
- Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999May;106(5):506-12.
- Hubbard RE, O’Mahony MS, Savva GM, et al. Inflammation and frailty measures in older people. J Cell Mol Med. 2009 Sep;13(9B):3103-9.
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245-70.
- Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006 Jun;61(6):575-84.
- Franceschi C, Olivieri F, Marchegiani F, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev. 2005 Feb;126(2): 351-61.
- Simpson EE, Hodkinson CF, Maylor EA, et al. Intracellular cytokine production and cognition in healthy older adults. Psychoneuroendocrinology. 2013 Oct;38(10):2196-208.
- Available at: https://www.sens.org/wp-content/uploads/2019/05/SENS-Research-Foundation-2019-Annual-Report.pdf. Accessed April 5, 2020.
- High KP, Akbar AN, Nikolich-Zugich J. Translational research in immune senescence: assessing the relevance of current models. Semin Immunol. 2012 Oct;24(5):373-82.
- Shivshankar P, Boyd AR, Le Saux CJ, et al. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell. 2011 Oct;10(5):798-806.
- Available at: https://www.lifeextension.com/magazine/2015/2/major-advance-in-slowing-aging. Accessed April 5, 2020.
- Available at: https://www.lifeextension.com/magazine/2015/1/activate-your-natural-killer-cells. Accessed April 5, 2020.
- Available at: https://www.lifeextension.com/magazine/2015/1/how-immune-decline-hastens-aging. Accessed April 5, 2020.
- Available at: http://www.cdc.gov/nchs/data/ahcdagingtrends/01death.pdf. Accessed April 5, 2020.
- Bartling B. Cellular senescence in normal and premature lung aging. Z Gerontol Geriatr. 2013 Oct;46(7):613-22.
- Glennie SJ, Sepako E, Mzinza D, et al. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS One. 2011;6(9):e25610.
- Hernandez-Vargas EA, Wilk E, Canini L, et al. Effects of aging on influenza virus infection dynamics. J Virol. 2014 Apr;88(8):4123-31.
- Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011 Mar 3;29(11):2169-77.
- Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005 Nov 15;41 Suppl 7:S504-12.
- Vollmar B, Pradarutti S, Nickels RM, et al. Age-associated loss of immunomodulatory protection by granulocyte-colony stimulating factor in endotoxic rats. Shock. 2002 Oct;18(4):348-54.
- Available at: http://www.ncbi.nlm.nih.gov/books/NBK27090/. Accessed April 5, 2020.
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, et al. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008 Apr 1;46(7):1078-84.
- Wehrens EJ, Prakken BJ, van Wijk F. T cells out of control--impaired immune regulation in the inflamed joint. Nat Rev Rheumatol. 2013 Jan;9(1):34-42.
- Nakajima T, Schulte S, Warrington KJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002 Feb 5;105(5):570-5.
- Terrazzini N, Bajwa M, Vita S, et al. A novel cytomegalovirus-induced regulatory-type T-cell subset increases in size during older life and links virus-specific immunity to vascular pathology. J Infect Dis. 2014 May 1;209(9):1382-92.
- Olson NC, Doyle MF, Jenny NS, et al. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One. 2013;8(8):e71498.
- Ammirati E, Cianflone D, Vecchio V, et al. Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. J Am Heart Assoc. 2012 Feb;1(1):27-41.
- Dumitriu IE, Araguas ET, Baboonian C, et al. CD4+ CD28 null T cells in coronary artery disease: when helpers become killers. Cardiovasc Res. 2009 Jan 1;81(1):11-9.
- Filipin Mdel V, Caetano LC, Brazao V, et al. DHEA and testosterone therapies in Trypanosoma cruzi-infected rats are associated with thymic changes. Res Vet Sci. 2010 Aug;89(1):98-103.
- May M, Holmes E, Rogers W, et al. Protection from glucocorticoid induced thymic involution by dehydroepiandrosterone. Life Sci. 1990;46(22):1627-31.
- Mitchell WA, Meng I, Nicholson SA, et al. Thymic output, ageing and zinc. Biogerontology. 2006 Oct-Dec;7(5-6):461-70.
- Wong CP, Song Y, Elias VD, et al. Zinc supplementation increases zinc status and thymopoiesis in aged mice. J Nutr. 2009 Jul;139(7):1393-7.
- Dardenne M, Boukaiba N, Gagnerault MC, et al. Restoration of the thymus in aging mice by in vivo zinc supplementation. Clin Immunol Immunopathol. 1993 Feb;66(2):127-35.
- Maes M, Christophe A, Bosmans E, et al. In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol Psychiatry. 2000 May 15;47(10):910-20.
- Khalfoun B, Thibault F, Watier H, et al. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400B:589-97.
- Moon Y, Pestka JJ. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J Nutr Biochem. 2003 Dec;14(12):717-26.
- Shi Y, Pestka JJ. Mechanisms for suppression of interleukin-6 expression in peritoneal macrophages from docosahexaenoic acid-fed mice. J Nutr Biochem. 2009 May;20(5):358-68.
- Araghi-Niknam M, Zhang Z, Jiang S, et al. Cytokine dysregulation and increased oxidation is prevented by dehydroepiandrosterone in mice infected with murine leukemia retrovirus. Proc Soc Exp Biol Med. 1997 Dec;216(3):386-91.
- Liu S, Ishikawa H, Li FJ, et al. Dehydroepiandrosterone can inhibit the proliferation of myeloma cells and the interleukin-6 production of bone marrow mononuclear cells from patients with myeloma. Cancer Res. 2005 Mar 15;65(6):2269-76.
- Jiang Y, Tu PF. Analysis of chemical constituents in Cistanche species. J Chromatogr A. 2009 Mar 13;1216(11):1970-9.
- Zhang K, Ma X, He W, et al. Extracts of Cistanche deserticola Can Antagonize Immunosenescence and Extend Life Span in Senescence-Accelerated Mouse Prone 8 (SAM-P8) Mice. Evid Based Complement Alternat Med. 2014;2014:601383.
- Pfister G, Weiskopf D, Lazuardi L, et al. Naive T cells in the elderly: are they still there? Ann N Y Acad Sci. 2006 May;1067:152-7.
- Ferrando-Martinez S, Ruiz-Mateos E, Hernandez A, et al. Age-related deregulation of naive T cell homeostasis in elderly humans. Age (Dordr). 2011 Jun;33(2):197-207.
- Fagnoni FF, Vescovini R, Passeri G, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000 May 1;95(9):2860-8.
- Camous X, Pera A, Solana R, et al. NK cells in healthy aging and age-associated diseases. J Biomed Biotechnol. 2012;2012:195956.
- Beli E, Duriancik DM, Clinthorne JF, et al. Natural killer cell development and maturation in aged mice. Mech Ageing Dev. 2014 Jan;135:33-40.
- Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 2013 Sep;12(4):1069-78.
- Kovaiou RD, Weiskirchner I, Keller M, et al. Age-related differences in phenotype and function of CD4+ T cells are due to a phenotypic shift from naive to memory effector CD4+ T cells. Int Immunol. 2005 Oct;17(10):1359-66.
- Wikby A, Maxson P, Olsson J, et al. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998 May 15;102(2-3):187-98.
- Liang HD, Yu F, Tong ZH, et al. Cistanches Herba aqueous extract affecting serum BGP and TRAP and bone marrow Smad1 mRNA, Smad5 mRNA, TGF-beta1 mRNA and TIEG1 mRNA expression levels in osteoporosis disease. Mol Biol Rep. 2013 Feb;40(2):757-63.
- Zeng JC, Fan YG, Liu JR, et al. [Experimental study of directional differentiation of bone mesenchymal stem cells (BMSCs) to osteoblasts guided by serum containing cistanche deserticola]. Zhongguo Gu Shang. 2010 Aug;23(8):606-8.
- Liang H, Yu F, Tong Z, et al. Effect of Cistanches Herba aqueous extract on bone loss in ovariectomized rat. Int J Mol Sci. 2011;12(8):5060-9.
- van den Biggelaar AH, Huizinga TW, de Craen AJ, et al. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004 Sep;39(9):1407-14.
- Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002 Aug-Oct;13(4-5):357-68.
- Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014 Feb;141(2):125-39.
- Nowell MA, Williams AS, Carty SA, et al. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J Immunol. 2009 Jan 1;182(1):613-22.
- Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009 Aug;11(8):973-9.
- Freund A, Orjalo AV, Desprez PY, et al. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010 May;16(5):238-46.
- Wei H, Chadman KK, McCloskey DP, et al. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2012 Jun;1822(6):831-42.
- Quintanilla RA, Orellana DI, Gonzalez-Billault C, et al. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004 Apr 15;295(1):245-57.
- Mahler GJ, Farrar EJ, Butcher JT. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol. 2013 Jan;33(1):121-30.
- Mathieu P, Bouchareb R, Boulanger MC. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. J Immunol Res. 2015;2015:851945.
- Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990 Nov 15;145(10):3297-303.
- Chu SL, Fu H, Yang JX, et al. A randomized double-blind placebo-controlled study of Pu’er tea extract on the regulation of metabolic syndrome. Chin J Integr Med. 2011 Jul;17(7):492-8.
- Zhao L, Jia S, Tang W, et al. Pu-erh tea inhibits tumor cell growth by down-regulating mutant p53. Int J Mol Sci. 2011;12(11):7581-93.
- Prasad AS. Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol. 2014 Oct;28(4):364-71.
- Wong CP, Ho E. Zinc and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res. 2012 Jan;56(1):77-87.
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009 Jun 12;6:9.
- Lichte P, Pfeifer R, Werner BE, et al. Dehydroepiandrosterone modulates the inflammatory response in a bilateral femoral shaft fracture model. Eur J Med Res. 2014 May 19;19:27.
- Available at: https://www.lifeextension.com/magazine/2002/1/awsi. Accessed April 5, 2020.
- Available at: https://www.lifeextension.com/magazine/2001/8/report_dhea. Accessed April 5, 2020.

