
Female Infertility
Female Infertility
Last Section Update: 12/2024
Contributor(s): Maureen Williams, ND; Stephen Tapanes, PhD; Shayna Sandhaus, PhD; Carrie Decker, ND, MS
Table of Contents
- Overview
- Structure & Function of the Female Reproductive System
- Causes of Female Infertility
- Risk Factors for Female Infertility
- Nutrients
- Dietary & Lifestyle Changes to Support Fertility
- Diagnosing Female Infertility
- Female Infertility Treatment
- Novel & Emerging Treatments for Female Infertility
- Living With Female Infertility
- Frequently Asked Questions About Female Infertility
- Update History
- References
1 Overview
Summary and Quick Facts for Female Infertility
- Female infertility is diagnosed when a couple is unable to conceive after a year of trying, or six months of trying if the woman is over 35, and diagnostic workup of both partners reveals the cause to be the female partner’s reproductive system. Female infertility can also refer to repeated miscarriages.1
- Infertility affects 12–15% of couples in the United States.264
- In about one-third of infertile couples, the cause is in the female partner. In another third, the cause is in the male partner. And in the remaining third, the problem is either with both partners or cannot be identified.264
- Infrequent or absent ovulation; endometriosis; problems related to the fallopian tubes, uterus, or cervix; and premature ovarian aging are common causes of female infertility, but some cases are unexplained (idiopathic).4
- Clinical trials have shown nutrients such as acetyl-L-carnitine,80 vitamin E,6 melatonin,7 and coenzyme Q108 can enhance female fertility.
- Depending on the cause, female infertility may be treatable with surgery or ovulation-inducing medications (eg, clomiphene citrate, letrozole, and gonadotropins).9
- Assisted reproductive technologies, including intrauterine insemination (IUI), in vitro fertilization, and intracytoplasmic sperm injection, may be options for women whose fertility problems are not otherwise treatable.9
2 Structure & Function of the Female Reproductive System
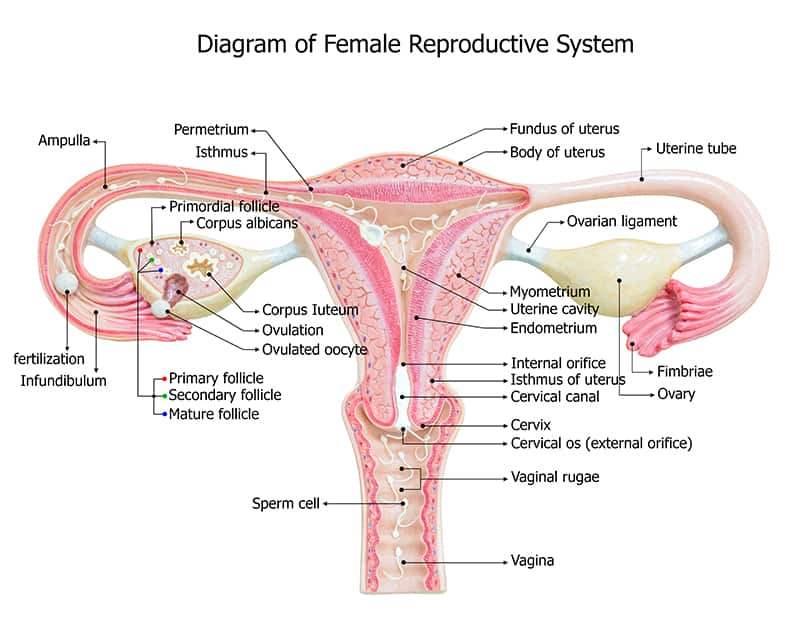
Healthy structure and function of the female reproductive system is essential to fertility. The structures of the female reproductive system (Figure 1) are:
- Ovaries. There are two ovaries present in the female pelvis. Each ovary has an outer cortex, where follicles develop, and an inner medulla comprising connective tissue and blood vessels. Before a woman’s birth, germ cells with the potential to become oocytes (cells that can mature into eggs) reproduce rapidly, reaching a peak of roughly 6–7 million around gestational week 20; however, most of these germ cells degrade before birth. The remaining one million or so germ cells undergo the first stage of transformation into primary oocytes and constitute ovarian reserve. These oocytes exist in dormant follicles until activated by cyclical hormone signals after puberty.10,11 The ovaries are also made up of theca and granulosa cells, responsible for secreting estrogen, progesterone, androgens (male hormones), and other factors that regulate follicular activation and maturation.10 Ovarian reserve declines gradually as follicles degrade with age. Environmental and genetic factors can also contribute to diminished ovarian reserve.10
- Fallopian tubes. There are two fallopian (or uterine) tubes, which collect oocytes released by the ovaries and carry them to the uterus. The end of each fallopian tube closest to an ovary has finger-like projections called fimbria that help sweep oocytes into the inner tube. The other end of each fallopian tube is in the inner uterus.11 The fallopian tubes are the site of fertilization, which generally occurs within three days of ovulation. During fertilization, a fully mature sperm binds to the outer protective shell (zona pellucida) of the oocyte. This triggers the final maturation of the oocyte to an ovum (egg) and the generation of an embryo. In addition to their role in transport, the fallopian tubes are responsible for producing substances that sustain sperm and provide nutrition to the developing embryo.10
- Uterus. The uterus is the site of implantation, embryonic maturation, and growth of the developing fetus. The uterine cavity is surrounded by walls consisting of three layers: the inner blood vessel-rich endometrium, which expands or thins in response to hormonal signals during the menstrual cycle; middle muscular myometrium, which provides structural support and can contract to induce menses or childbirth; and serosa, which is the outer fibrous layer. A tubular structure known as the uterine cervix acts as a channel between the uterus and the vagina.11 Implantation can occur between seven and 10 days after ovulation when the uterine lining, under the influence of high levels of estrogen and progesterone, is receptive. Even in normal circumstances, only about half of fertilized ova will implant and result in successful pregnancy.10
- Vagina. The vagina is the passageway from outside of the body to the uterus.11
During regular hormone cycles, several oocytes undergo further development, and a dominant follicle emerges. This dominant follicle contains a fluid-filled cavity called the antrum. The oocyte from this dominant follicle is released from the ovary in a process known as ovulation. The remaining follicle cells transform into a progesterone-secreting structure called the corpus luteum.11
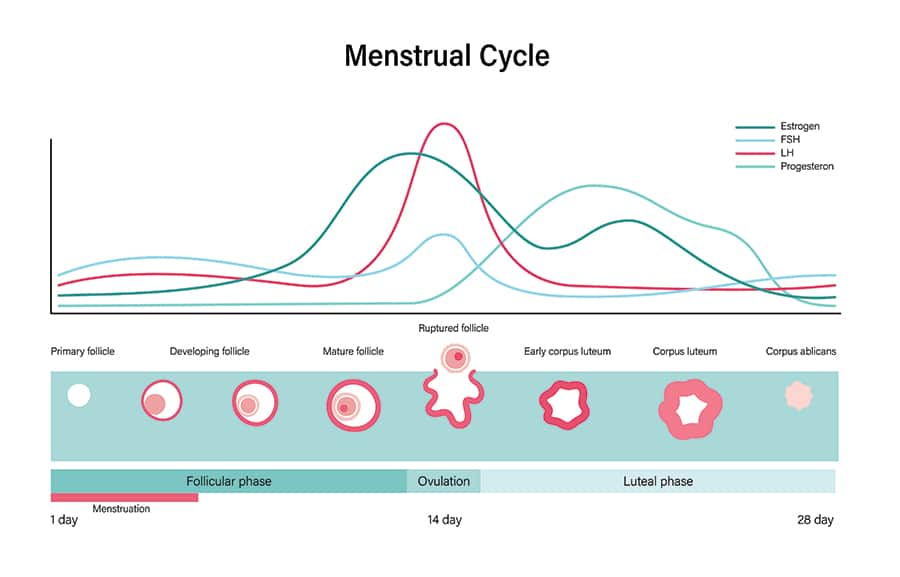
Female Hormonal (Menstrual) Cycle
Female fertility is controlled by cyclic hormone signals from the hypothalamus and pituitary gland via the hypothalamic-pituitary-ovarian (HPO) axis.10
- The female hormone (menstrual) cycle (Figure 2) begins with the follicular phase, which normally lasts approximately 14
days.
- Estrogen and progesterone levels are at their lowest, and the hypothalamus responds by increasing secretion of gonadotropin releasing hormone (GnRH), which triggers pituitary production of gonadotropins—follicle stimulating hormone (FSH) and luteinizing hormone (LH). Rising FSH levels stimulate maturation of several ovarian follicles and the emergence of a dominant follicle. Estrogen production by granulosa cells also increases, causing thickening of the uterine endometrium to support implantation should fertilization occur.
- A surge in LH around mid-cycle is followed by ovulation, and the second
phase of the cycle, the luteal phase, begins. This phase
usually lasts from about days 15 through 28.
- The luteal phase is marked by increasing progesterone production by the corpus luteum, which exerts negative feedback signaling that inhibits GnRH, FSH, and LH release and promotes maturation of the endometrium. The developing endometrium produces substances that support implantation of a fertilized ovum, or embryo. If implantation occurs, cells associated with the embryo produce human chorionic gonadotropin (hCG), which maintains the corpus luteum and keeps progesterone levels high.
- In the absence of implantation, diminishing LH and FSH levels result in corpus luteum breakdown, decreased production of progesterone and estrogen, sloughing away of the endometrium through menstruation, and the start of a new cycle.11
3 Causes of Female Infertility
The causes of fertility problems in women can be categorized as ovulation dysfunction, tubal infertility, uterine and cervical issues, and diminished ovarian reserve. It is important to note that, even when female causes are identified, factors related to the male partner may still be contributing to infertility; in fact, it is estimated male factors play a role in roughly 50% of couples with infertility.2,12
Ovulation Dysfunction
Ovulation disorders are caused by hormonal imbalance and account for approximately 25% of infertility cases,3 and may be caused by one or more of the following:
- Genetics. Certain genetic mutations, particularly those affecting the X chromosome, can be an underlying cause of premature ovarian insufficiency (also known as primary ovarian insufficiency), a condition in which menstrual cycles become irregular or cease and FSH levels are persistently high before the age of 40 years. In vitro fertilization using donor eggs is generally the only treatment option for women with X chromosome-related infertility. Other gene mutations can affect function of the HPO axis and contribute to hormone imbalance.10
- Polycystic ovary syndrome (PCOS), a condition characterized by excess androgen (male hormone) production, ovarian structural and functional abnormalities, and metabolic disturbance, is a common hormonal cause of infertility. In the United States, PCOS is the most common cause of female infertility.3,265,266
- Classical and non-classical congenital adrenal hyperplasia (CAH and NC-CAH) are autosomal recessive diseases that cause adrenal steroid 21-hydroxylase deficiency. The classical forms of the disease are more severe and are likely to be diagnosed early in life, as opposed to the non-classical form, which may be confused with PCOS due to overlapping symptoms, including female infertility.262,263
- Hypogonadotropic hypogonadism is a condition marked by levels of gonadotropins (LH and FSH) that are too low to support normal ovarian function. This results in low production of estrogen and progesterone and anovulation. Hypogonadotropic hypogonadism may be related to genetic mutations, medical conditions, eating disorders, or drugs that affect hypothalamic or pituitary function.10
- Hyperprolactinemia (high blood levels of the hormone prolactin) causes inhibition of gonadotropin release and can lead to anovulation. Pregnancy, breastfeeding, hypothyroidism, and some pituitary tumors raise prolactin levels and thereby reduce fertility. In addition, stress, excessive exercise (ie, more than one hour of high-intensity exercise daily), and some medications are possible causes of hyperprolactinemia.10,13
- Hypothyroidism can be a cause of infertility and pregnancy complications. Thyroid hormone is needed for normal ovarian function and ovulatory menstrual cycles to occur. In addition, high levels of thyroid stimulating hormone (TSH), a pituitary hormone that activates the thyroid gland, have been noted to be correlated with high levels of prolactin. Untreated hypothyroidism, even when subclinical (normal free thyroxine [fT4] levels with high TSH levels), is associated with poor pregnancy outcomes.14,15
- Extreme stress and eating disorders can disrupt hypothalamic signaling, leading to ovarian dysfunction and anovulation.3,10,16
- Idiopathic chronic anovulation (anovulation without a known cause) accounts for about 7–8% of cases of anovulatory infertility.16
Endometriosis
Endometriosis is the presence of endometrial tissue outside of the uterine cavity in any region of the pelvis or abdomen.16 Approximately 10–15% of reproductive-aged women have endometriosis and an estimated 30–50% of these women will experience infertility. In its early stages, endometriosis can limit fertility through inflammatory signaling, which impairs both ovarian and fallopian tube function and can reduce normal follicle formation, fertilization, and implantation; advanced endometriosis is associated with adhesions and altered pelvic anatomy that can impair the movement of oocytes and sperm, induce uterine dysfunction, and prevent normal fertilization and embryonic development.267
Tubal Infertility
Any type of fallopian tube abnormality can contribute to female infertility.20 Scarring and adhesions affecting one or both fallopian tubes can block movement of oocytes and sperm, preventing fertilization. Adhesions surrounding the tubes can also impair their ability to retrieve oocytes after ovulation.16 Endometriosis, infections, and other acute and chronic inflammatory conditions can damage the integrity of the fallopian tubes, resulting in obstruction which can prevent normal transport of sperm and oocytes. Tubal obstruction can also block the normal movement of fluid, causing fluid buildup in the tubes, known as hydrosalpinges. Hydrosalpinges not only prevents natural fertilization, but also appears to inhibit implantation after in vitro fertilization by creating a hostile environment in the uterine cavity.4
The most common cause of tubal dysfunction is sexually transmitted infections. Other possible causes include endometriosis, abdominal or pelvic surgery, pelvic inflammatory disease, other abdominal or pelvic infections, and previous tubal pregnancy (a dangerous condition in which the fertilized oocyte is implanted into the wall of a fallopian tube).16,21
Uterine and Cervical Issues
Problems affecting the uterine cavity can interfere with implantation, leading to infertility. They also increase the risk of poor pregnancy outcomes, such as miscarriage and pre-term birth. Examples include16:
- Endometrial uterine polyps
- Uterine fibroids (leiomyomata)
- Endometriosis
- Congenital uterine malformations
- Intrauterine scarring and adhesions (synechiae), also known as Asherman syndrome22
Abnormalities of the cervix, whether congenital or acquired through surgery or dysplasia (a precancerous condition), as well as decreased cervical mucous, can disrupt sperm progression and prevent fertilization.16
Diminished Ovarian Reserve
The number and quality of oocytes and follicles present in the ovaries diminish throughout a woman’s life, resulting in declining fertility. In addition to aging, ovarian reserve may be decreased due to ovarian surgery, chemotherapy, radiation therapy, and certain genetic factors. Decreasing levels of anti-Müllerian hormone (AMH), which is released by small growing ovarian follicles, is a marker of diminishing ovarian reserve. An AMH level below 1.66 ng/mL suggests the ovarian reserve may be diminished.16
4 Risk Factors for Female Infertility
Many factors can increase a woman’s risk of infertility. Chronic health problems, genetic (inherited) traits, lifestyle choices, and age can all contribute to female infertility. Some specific factors include:
Age. Women’s fertility naturally declines with age, as both the quantity and quality of oocytes diminish. This is due partly to cumulative effects of oxidative stress, which can damage DNA and impair oocyte viability.24,28 Age-related inhibition of cellular growth and function, a process known as senescence, is also believed to play a role.32 Women over 35 years old in particular have a higher risk of infertility, lower chance of benefiting from treatment, and greater likelihood of poor pregnancy outcomes.26,33
Abnormal menstrual cycles. Menstrual cycles that are shorter than 21 days or longer than 35 days may indicate hormone imbalance and anovulation.16 In addition, heavy or painful menses, bleeding between periods, pelvic pain, and pain during intercourse may be signs of problems such as endometriosis or fibroids.34,35
Obesity. Obesity, even in women without PCOS, contributes to female infertility by disrupting the HPO axis and decreasing oocyte quantity and quality. In addition, multiple studies report a link between obesity and diminished ovarian reserve.36,37 It is thought to negatively impact reproductive function by increasing inflammation, insulin resistance, and oxidative stress.36
Type 2 diabetes. Women with type 2 diabetes have been found to have higher risks of infertility and miscarriage, as well as lower chances of viable pregnancy and childbirth, compared with non-diabetic women, and not all these differences are explained by obesity or PCOS.38
Underweight and low body fat percentage. Being underweight and having a low body fat percentage (<26%) limits the energy available to the body for normal functioning and can suppress hypothalamic GnRH release, resulting in loss of menstrual cycles and ovulation.39,40 Extremely intense exercise, stress, and eating disorders are known to increase the risk of infertility due to this type of hypothalamic suppression.39
Autoimmune disorders. Systemic autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, Sjögren syndrome, and mixed connective tissue disease increase the risk of female infertility.41 In addition, celiac disease has been correlated with increased risk of infertility, and Hashimoto’s disease can cause hypothyroidism-related ovulation dysfunction.15,42
Other chronic health problems. Chronic health problems can predispose women to infertility, and female infertility may be an indicator of underlying health issues.16,43
Sexually transmitted infections and pelvic inflammatory disease. Pelvic inflammatory disease, an infection of the upper female reproductive tract, is frequently caused by sexually transmitted infections such as gonorrhea and chlamydia. In some cases, it can be caused by microorganisms residing in the vagina. These infections can result in long-term scarring of the fallopian tubes and inability to conceive, as well as ectopic (tubal) pregnancy. Adolescents and young adults, especially those with multiple sexual partners and those who do not routinely use condoms, have a higher risk of sexually transmitted infections and pelvic inflammatory disease, which increase their risk of future infertility.44
Intrauterine procedures. Dilation and curettage (D&C) is a procedure in which the uterine cervix is dilated and the uterine lining is partially or completely removed. It can be performed diagnostically, such as in women with abnormal uterine bleeding, or to clear the uterine lining after a miscarriage or termination (abortion). Curettage is a potential cause of Asherman syndrome, a condition marked by endometrial or cervical scarring and adhesions that can contribute to recurrent miscarriages and infertility.45
Bacterial vaginosis. A healthy vaginal microbiome is essential for reproductive health. Bacterial vaginosis, a dysbiotic condition in which relative amounts of Lactobacillus species and other beneficial bacteria are reduced, has been linked to increased risk of infertility, as well as pregnancy complications.46
Excessive alcohol consumption. Drinking more than two alcoholic drinks per day has been associated with a 60% increase in infertility risk.47 Even moderate alcohol use (3–6 drinks per week) during the luteal (post-ovulatory) phase of the menstrual cycle has been shown to reduce fertility, while heavy drinking (defined in the study as more than six drinks per week) around ovulation and throughout the luteal phase appeared to disrupt reproductive function.48 Alcohol also appears to have a detrimental effect on assisted reproductive technique outcomes.49
Smoking and substance abuse. Smoking has multiple negative impacts on female reproductive function and has been associated with decreased fertility. Marijuana use appears to increase ovulation dysfunction-related infertility. Preclinical research suggests cocaine, methamphetamine, and heroin can each impair fertility by disrupting reproductive ovarian function, hormone balance, and menstrual cycles.
Medications. A number of medications can raise prolactin levels and negatively affect ovarian function and fertility by this or other mechanisms.50,51 Many commonly used medications can adversely impact female fertility by affecting the HPO axis or through direct actions on the ovaries or uterus. The following are some important examples of drugs that can impact fertility, but women planning to conceive should discuss any medication use with their doctor.
| Table 1: Drugs that can Adversely Affect Female Fertility | ||
|---|---|---|
| Drug Category | Examples | Notes |
| Antipsychotics | haloperidol (Haldol) risperidone (Risperdal) |
Antipsychotic drugs can cause hyperprolactinemia and related infertility.52 |
| Antidepressants | citalopram (Celexa) paroxetine (Paxil) venlafaxine (Effexor) mirtazapine (Remeron) |
Some studies suggest antidepressants are linked to reduced fertility in women, but a cause-and-effect relationship has yet to be established.53,54 |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | naproxen (Naprosyn, Aleve) diclofenac (Cambia) |
Some NSAIDs have been found to temporarily inhibit normal ovulation.55 |
| Corticosteroids | prednisone (Deltasone) | Corticosteroids appear to alter uterine tissue and interfere with implantation.56 |
| Immunosuppressants | tacrolimus (Prograf) sirolimus (Rapamune) |
Preclinical evidence suggests immunosuppressants, which are sometimes used as alternatives to anti-inflammatory drugs, may also impair ovarian function.57 |
| Opioids | hydromorphone (Dilaudid) oxycodone (Oxycontin and Percocet) hydrocodone (Vicodin, Lortab) oxymorphone (Opana) morphine (eg, Mitigo, MS Contin) fentanyl (Actiq, Duragesic) codeine (in Tylenol 3) |
Use of opioid pain relievers has been associated with reduced fertility and increased risk of pregnancy loss, even in healthy non-addicted women.58 |
| Anti-cancer drugs | cyclophosphamide (Cytoxan) ifosfamide (Ifex) busulfan (Busulfex) cisplatin (Platinol) doxorubicin (Adriamycin) methotrexate (Rasuvo) |
A number of drugs used in cancer therapy, some of which may also be used in treatment of autoimmune diseases, damage ovarian follicles, in some cases permanently.59 |
| Hormones | testosterone (Jatenzo) estradiol (Estrace) progesterone (Prometrium) ethinylestradiol (Estinyl) medroxyprogesterone (Provera) |
The use of anabolic steroids like testosterone can interfere with normal female hormone cycles and ovulation.60 Other hormone therapies, including estrogen, progesterone, and their analogs, can also disrupt HPO signaling and adversely impact female fertility.61 |
| Antihypertensives | Angiotensin-converting enzyme (ACE) inhibitors: enalapril (Vasotec) lisinopril (Prinivil) Angiotensin II receptor blockers (ARBs): candesartan (Atacand) losartan (Cozaar) |
ACE inhibitors and ARBs are known to reduce male fertility; although they have not been shown to affect female fertility, they are associated with an increased risk of birth defects, and women taking these drugs are encouraged to switch to safer therapies if they become pregnant.62 |
Environmental exposures. Exposure to a range of pollutants may contribute to female infertility.
- Heavy metals like lead, cadmium, and mercury appear to disrupt hormone balance and reduce female fertility, and are also linked to poor pregnancy outcomes. Exposure to heavy metals occurs mainly through drinking water. In addition, cigarette smoke is a source of cadmium, and high fish consumption of certain fish species can raise mercury levels.66
- Ionizing radiation exposure may occur occupationally or therapeutically (as part of cancer treatment), and can impact fertility by damaging the ovaries, hypothalamus or pituitary gland, or other reproductive tissues. Women preparing for radiation therapy targeting the pelvis or abdomen may want to consider egg harvesting and preservation for possible future in vitro fertilization.67,68
- Airborne pollutants can alter hormone signaling, increase levels of free radicals, induce inflammation, and interfere with normal cell division, all of which can contribute to reduced fertility.66 Although the effects of air purifiers on fertility are not yet known, one study that included 570 participants found regular use of home air purification systems during pregnancy resulted in increased birth weights of babies born at full term.69
- Bisphenols (like bisphenol A [BPA]), phthalates, dioxins, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) are examples of chemicals known as endocrine disruptors. They are used in a wide range of consumer products, including various plastics and epoxy resins, food and beverage containers, pesticides and herbicides, detergents, flame retardants, pharmaceuticals, dental sealants, toys, and cosmetics, and are widely dispersed environmental contaminants.66,70 Hairdressers; waste management workers; salesclerks in cosmetic, perfume, and clothing departments; and women who work in plastics manufacturing and recycling have been found to have especially high occupational exposure to endocrine disruptors.71 Because non-stick coated cookware is another source of PFAS, cast iron, glass, and stainless steel cookware are recommended as the safest choices for women concerned about fertility.72 In addition to disrupting hormone signaling, high levels of these compounds may reduce fertility by increasing ovarian oxidative stress and interfering with follicle development and function.66
5 Nutrients
L-carnitine and Acetyl-L-carnitine
L-carnitine is an amino acid that plays a critical role in cellular and mitochondrial metabolism. It also occurs in the form acetyl-L-carnitine. L-carnitine and its derivatives may improve female reproductive health through effects such as protecting DNA from oxidative damage, reducing inflammatory cytokine levels, supporting healthy lipid and glucose metabolism, improving cellular energy production, and stabilizing cell membranes.77-79
Acetyl-L-carnitine, at a dose of 1,500 mg twice daily, was found to add to the benefits of anti-diabetes medications in women with PCOS in a randomized controlled trial that included 147 participants. Those who received acetyl-L-carnitine in addition to metformin plus pioglitazone (Actos) had greater improvement in menstrual regularity as well as reductions in insulin and LH levels, waist circumference, and psychological stress scores.80 These positive effects may be due in part to L-carnitine’s ability to improve metabolic disturbances.82-84
L-carnitine and acetyl-L-carnitine have been proposed to improve function of the HPO axis.78 An uncontrolled trial in 27 patients with stress-induced pituitary suppression and amenorrhea (lack of menstrual periods) found 12 weeks of treatment with 500 mg L-carnitine plus 250 mg acetyl-L-carnitine daily decreased levels of markers of stress (cortisol and amylase) and increased LH levels in those with low baseline LH levels.85 Similar uncontrolled trials in women with stress-induced amenorrhea found 1,000–2,000 mg acetyl-L-carnitine daily for 16–24 weeks raised LH levels in women whose LH levels were suppressed.86,87 A combined supplement regime that included 500 mg L-carnitine, 250 mg acetyl-L-carnitine, 500 mg L-arginine, 50 mg NAC, and vitamins C and E, taken daily for 12 weeks, was also reported to improve hypothalamic LH release and reduce levels of stress markers (cortisol and amylase) in a trial in 29 women with stress-related amenorrhea.88
L- carnitine and its derivatives have also been shown to improve oocyte quality in animal studies and have the potential to be beneficial in women undergoing assisted reproductive therapies.79 In an uncontrolled trial, 214 women who had experienced an unsuccessful in vitro fertilization attempt were treated with 1,000 mg L-carnitine daily for an average of 82 days. Oocyte quality was improved, and 21 women (10%) delivered healthy newborns conceived through in vitro fertilization following L-carnitine treatment.89
Vitamin E
Vitamin E, a family of compounds that includes tocopherols and tocotrienols, is an essential fat-soluble antioxidant nutrient. Higher levels of vitamin E in the follicular fluid of women undergoing assisted reproductive techniques were associated with better outcomes in one study.90
A randomized placebo-controlled trial in 105 women with PCOS-related infertility undergoing in vitro fertilization with intracytoplasmic sperm injection found 400 mg vitamin E per day, along with 50,000 IU (1,250 mcg) vitamin D every second week, for eight weeks increased pregnancy rates compared with placebo.91
Vitamin E may exert its beneficial effects in part by promoting endometrial health. A controlled trial in 103 women with unexplained infertility undergoing ovulation induction via assisted reproductive methods found 400 IU (268 mg) of vitamin E per day, beginning between three and five days into a menstrual cycle until the day of ovarian stimulation, resulted in increased endometrial thickness.6 A retrospective cohort trial in 321 women with PCOS and infertility undergoing ovulation induction found the addition of vitamin E, at 100 mg per day for either one luteal phase or one full menstrual cycle, to the ovulation induction protocol reduced the amount of medication needed and increased endometrial thickness, although it did not alter likelihood of pregnancy.92 In an observational study, 200 mg vitamin E three times daily for a full menstrual cycle increased endometrial thickness in 13 of 25 women with a thin endometrium, a condition that may be caused by low uterine blood flow and can contribute to infertility.93 Another report described 19 cases of endometrial thinning that were unresponsive to hormone therapies. After supplementing with 1,000 IU (670 mg) vitamin E per day for an average of 8.1 months, endometrial thickness improved in 14 cases (approx. 74%) and pregnancy occurred in eight (42%).94
Inositol
Inositol is a family of isomers (compounds with the same composition but structured differently) found in cells of plants and animals. Myo-inositol is the most abundant inositol isomer occurring in nature and the body, followed by D-chiro-inositol as the second most abundant.95 Myo-inositol and D-chiro-inositol are present in high concentrations in the ovaries and, although their functions are different, are important for ovarian hormone production as well as oocyte maturation, fertilization, implantation, and embryo development. They also play a role in insulin signaling.96
Inositol has been found in numerous clinical trials to have beneficial effects on hormonal, metabolic, and reproductive health in women with PCOS. Supplementing with myo-inositol, or a combination of myo-inositol plus D-chiro-inositol at a ratio of 40:1, has been found in multiple clinical trials to improve menstrual cycles and ovulation rates in women with PCOS.96,97 Despite concerns that higher doses of D-chiro-inositol may have undesirable effects on hormone balance, one randomized controlled trial that included 60 women with PCOS undergoing intracytoplasmic sperm injection found a less than 4:1 ratio of myo-inositol to D-chiro-inositol (550 mg myo-inositol plus 150 mg D-chiro-inositol ) twice daily for 12 weeks prior to oocyte retrieval led to higher pregnancy and live birth rates than a 40:1 ratio (550 mg myo-inositol plus 13.8 mg D-chiro-inositol) twice daily. In addition, women given the higher dose of D-chiro-inositol were less likely to experience ovarian hyperstimulation, a dangerous side effect of ovulation-inducing medications.98
A growing body of research suggests myo-inositol may be beneficial in women with infertility undergoing assisted reproductive therapies, particularly those with PCOS.99 A meta-analysis of seven randomized controlled trials that included a total of 935 participants found myo-inositol supplementation increased pregnancy rate and decreased miscarriage rate in women with PCOS undergoing in vitro fertilization.100 In one randomized controlled trial, 60 women with PCOS-related infertility received a combination of 500 mg metformin plus 600 mg myo-inositol three times daily for three months prior to ovulation induction for intrauterine insemination, and 60 similar women received 500 mg metformin three times daily alone; those given myo-inositol had a live birth rate of 55% versus 26.67% in those not given myo-inositol.101
Myo-inositol therapy does not appear to be as beneficial in women without PCOS. A controlled trial in 112 women with infertility preparing for intracytoplasmic sperm injection found 4,000 mg myo-inositol plus 400 mcg folic acid (680 mcg DFE) daily for one month prior to ovulation induction led to improved fertilization rate and embryo quality, but did not significantly impact implantation or pregnancy rate compared with folic acid alone.102 In a randomized controlled trial in 23 women with infertility and obesity preparing for in vitro fertilization, those given 2,000 mg myo-inositol plus 800 mg alpha-lipoic acid and 400 mcg folic acid (680 mcg DFE) daily for two months before stimulated ovulation induction had a higher pregnancy rate than those given folic acid alone, with their pregnancy rate being similar to that of normal-weight women with infertility.103
Melatonin
Melatonin, a hormone produced mainly by the pineal gland in the brain, is needed to regulate the body’s circadian rhythms. It can also be produced by the ovaries and is found in high concentrations inside oocytes prior to ovulation where it acts as a free radical scavenger and is needed for oocyte growth and maturation, fertilization, and embryo development.104,105 Some evidence suggests supplemental melatonin may reduce loss of ovarian reserve in women undergoing chemotherapy.106
Fertility is strongly influenced by circadian rhythms, which are synchronized with changing levels of melatonin and cortisol. Disruption of circadian regulation due to stress or poor sleep has been implicated as a factor in infertility, increasing the risks of anovulation, loss of menstrual cycles, failed embryo implantation, and miscarriage.107,108 Supplementing with melatonin may help restore circadian cycles and has been found to reduce oxidative stress within ovarian follicles, preserve ovarian reserve, and increase oocyte quality, which may lead to improved in vitro fertilization outcomes.105,109,110 A meta-analysis of data from 10 randomized controlled trials with a total of 1,203 participants undergoing assisted reproductive techniques found treatment with melatonin, most commonly used at a dose of 3 mg per day, increased the number and quality of oocytes retrieved, number of good-quality embryos, and chance of pregnancy, but had no impact on miscarriage rate or the odds of live birth.7 Another meta-analysis of findings from seven randomized controlled trials found melatonin improved oocyte quality in women undergoing in vitro fertilization. Melatonin use was also found to increase the likelihood of pregnancy, but this effect was not statistically significant.111
Disturbances in melatonin production and circadian rhythms also may contribute to infertility in women with PCOS.112,113 A report from a pilot study that included data from 40 women with PCOS suggests melatonin supplementation may improve ovarian function, resulting in lower androgen levels and improved menstrual regularity.114
Coenzyme Q10
Coenzyme Q10 (CoQ10) is a lipid-soluble compound involved in mitochondrial energy production. CoQ10 also quenches free radicals and lowers oxidative stress.8 Preclinical research indicates CoQ10 supplementation may reduce age-related and oxidative stress-induced loss of ovarian reserve.116,117 One meta-analysis included findings from five randomized controlled trials with a combined total of 449 women being treated for infertility due to poor ovarian response or PCOS. Dosages of 600–1,200 mg CoQ10 per day for 8–12 weeks were used in trials in women with poor ovarian response, while a dose of 60 mg CoQ10 three times daily from the first day of a menstrual cycle until oocyte retrieval was used in trials in women with PCOS. The analysis found assisted reproductive procedures resulted in a higher pregnancy rate in those given CoQ10 (28.8%) than placebo or no treatment (14.1%).8
Probiotics
Lactobacillus species are common in both the lower and upper female reproductive tract and play an important role in protecting against bacterial vaginosis and urogenital infections that frequently contribute to infertility.74,118,119 The reproductive microbiome has been found to be altered in many women with infertility, with different patterns of disturbance seen in those with infertility due to an unknown versus known cause.120 Treatment with various probiotic supplements have been shown to promote positive changes in the vaginal microbiome and may potentially improve fertility.121
One probiotic strain that has been clinically shown to be effective for improving the vaginal microbiome by increasing lactobacillus levels is Lactobacillus plantarum P17630, also known as ROSELLA.122,123 When taken orally at a daily dose of 5 billion colony forming units (CFUs) for three cycles of 15 days followed by a 15-day washout (a study duration of 90 days), the probiotic was shown to improve vaginal Lactobacillus scores as well as vaginal tissue redness and swelling in women with recurrent vulvovaginal candidiasis.123 Probiotics also have the potential to improve microbiome composition in women with infertility. In a preliminary study, women with infertility treated with a probiotic containing four Lactobacillus strains (L. crispatus LBV88, L. rhamnosus LBV96, L. gasseri LBV150N, and L. jensenii LBV116) experienced positive changes in their vaginal microbiome.124 Findings from another preliminary study suggest a treatment regimen of antibiotics followed by a vaginal probiotic (as an inoculated tampon or suppository) containing two to three Lactobacillus strains (L. rhamnosus LN113, L. gasseri LN40, L. fermentum LN99) and oral lactoferrin at 300 mg daily may induce healthy Lactobacillus dominance in the endometrial microbiomes (as assessed by endometrial fluid sampling) of women with infertility and abnormal endometrial microbiomes.125
One clinical trial included 44 women, 21 of whom had experienced three or more miscarriages and 23 of whom had unexplained infertility and had not conceived after at least two cycles of in vitro fertilization. The participants received a daily probiotic supplement providing 1 billion CFUs of freeze-dried L. salivarius CECT5713 for six months or until the 15th week of pregnancy. At the end of six months, 29 pregnancies occurred out of 44 participants (66%), including 25 that resulted in successful pregnancies with full-term deliveries. Interestingly, women who had successful pregnancies had more improvement in vaginal health parameters after using the probiotic than women who did not become pregnant.126 A meta-analysis of nine randomized controlled trials in 587 women found probiotic and synbiotic (probiotic plus prebiotic) supplements improved hormonal, endocrine, and inflammatory disturbances in women with PCOS, a condition closely associated with infertility.127
Vitex
Vitex (Vitex agnus-castus, also known as chaste tree or chaste berry) has been used historically as an herbal medicine for improving menstrual regularity and treating disorders related to hormonal imbalance.128 Two clinical trials have examined the effect of a combination supplement containing vitex, along with L-arginine, green tea, folate, and other vitamins and minerals, on women’s fertility. In one placebo-controlled trial that included 30 women who had tried unsuccessfully to conceive for 6–36 months, taking the vitex-containing supplement for three months resulted in basal body temperature patterns suggestive of longer luteal phases; after five months, 33% of those in the supplement group and none in the placebo group had conceived.129 The same research group conducted a similar placebo-controlled trial with 93 participants. Not only did the vitex combination supplement increase the number of days with an elevated basal body temperature, but it also raised progesterone levels in those with low baseline progesterone levels. After three months, 26% of supplemented women and 10% of women given placebo were pregnant; after six months, three additional women in the treatment group conceived.130 An uncontrolled clinical trial investigated the effect of a combination of vitex plus maca (Lepidium meyenii) extracts and folate in 189 women with menstrual disorders trying to conceive. After six months of treatment, the percentage of women experiencing ovulatory cycles increased from 10% to 42.9%, and 37% of women had achieved pregnancy.131
L-arginine
L-arginine is an amino acid that acts as a precursor to nitric acid in the body. In addition to helping regulate blood vessel tone and function, L-arginine plays a role in adrenal and pituitary hormone activity.132 Some research suggests supplementing with L-arginine, in combination with other nutrients, may improve female sexual function.133 One controlled trial included 34 women with infertility attempting assisted reproductive techniques who had not responded to prior ovulation induction therapies. Those given 16 grams of L-arginine daily along with an ovulation induction protocol were more likely to have successful oocyte retrieval, had a greater number of oocytes collected, and had a higher chance of pregnancy than those who did not take arginine.134 In another controlled trial in 120 women preparing for in vitro fertilization, L-arginine, taken at doses of 1,000 or 2,000 mg per day for three months, improved pregnancy rates.135 Evidence from an uncontrolled trial suggests L-arginine, at 1,500 mg four times daily from the beginning of a menstrual cycle until ovulation induction, can increase endometrial thickness in women with a thin endometrium, a condition associated with low pregnancy rates that may be due to poor uterine blood flow.93
N-acetylcysteine
N-acetylcysteine (NAC), a form of the sulfur-containing amino acid cysteine, is a nutrient needed to produce glutathione, one of the body's most important antioxidants and detoxifiers. While cysteine is found in high-protein foods, NAC is not.136
Some clinical trials have found NAC, at doses of 1,200–1,800 mg per day, can improve fertility parameters such as menstrual cycle regularity and oocyte quality in women with PCOS being treated with the ovulation inducing medications clomiphene citrate (Serophene) and letrozole (Femara).138-140
Vitamin C
Vitamin C is needed to regulate oxidative stress, synthesize hormones, and produce collagen.141 Lower vitamin C levels have been noted in follicular fluid from women with, compared to those without, infertility.142 In one study that included data from 437 women with unexplained infertility, taking vitamin C supplements was associated with shorter time to pregnancy in those women who were not overweight and who were under 35 years old.143 In a controlled trial in 150 women with luteal phase deficiency marked by low peak progesterone levels, those who received 750 mg vitamin C per day for three menstrual cycles were more likely to have increased progesterone levels. In addition, pregnancy occurred in 25% of vitamin C-treated participants versus 11% of controls.141 Vitamin C, at 1,000 mg per day, along with vitamin E, at 800 IU per day, reduced oxidative stress and pain symptoms after eight weeks in a placebo-controlled trial in 60 women with endometriosis.144 In contrast, in a controlled trial in 245 patients with endometriosis undergoing in vitro fertilization, 1,000 mg vitamin C daily did not lower oxidative stress marker levels or affect any treatment outcomes after two months.145
Selenium
Selenium is a trace mineral nutrient needed for proper function of the glutathione system, which regulates reduction-oxidation balance in the body. Selenium is also needed for normal thyroid hormone production and may have a role in supporting heavy metal detoxification.146,147 Selenium deficiency impairs placental function and fetal development, and is associated with increased risk of miscarriage and preterm birth.147
Low selenium status has been associated with unexplained female infertility.146 In one study, women being treated for infertility were found to have lower selenium and higher mercury levels than a group of women not seeking fertility treatment.148 Another observational study that included data from 1,060 pregnant women found lower selenium levels were correlated with a longer time to pregnancy and a 46% higher risk of having been unable to conceive for more than one year.149
In a placebo-controlled trial in 70 women with unexplained early menopause, those who received 200 mcg selenium plus 400 IU vitamin E daily for 90 days had signs of improved ovarian function, including increased AMH levels, antral follicle counts, and ovarian volume, compared with placebo.150
Vitamin D
Vitamin D deficiency and insufficiency are common—especially during winter months and in people with darkly pigmented skin—and may be a factor in female infertility. One study that examined the vitamin D status of 500 women pursuing assisted reproductive techniques found only 16% of women had sufficient vitamin D levels.151
Findings from several observational studies suggest healthy vitamin D status may be correlated with better female reproductive function and fertility outcomes.152-155 However, a meta-analysis of observational data from 36 studies that included 7,882 participants found no overall association between vitamin D levels and indicators of ovarian reserve.156 It has also been reported that certain genetic patterns related to vitamin D receptor expression were correlated with risk of infertility, suggesting vitamin D receptors may play a role in modulating female reproductive function.157
An open trial in 30 women with low vitamin D levels and infertility found 1,250 mcg (50,000 IU) vitamin D weekly for three months increased levels of AMH, an indicator of ovarian reserve.158 Nevertheless, clinical trials have not yet been able to demonstrate a clear benefit from vitamin D supplementation on likelihood of pregnancy and live birth. In one placebo-controlled trial in 630 women with infertility, a single dose of 15,000 mcg (600,000 IU) vitamin D 2–12 weeks prior to undergoing in vitro fertilization had no impact on outcomes.159 In another placebo-controlled trial in 95 women with vitamin D deficiency undergoing in vitro fertilization, those given 0.5 mcg calcitriol (the active form of vitamin D3) daily for four weeks had a higher implantation rate but were no more likely than the placebo group to sustain pregnancy.160
Resveratrol
Resveratrol is a free radical-scavenging flavonoid found in grape skin, red wine, cocoa, and peanuts. Preclinical research suggests resveratrol can reduce oxidative stress, prevent oxidative damage, and enhance mitochondrial activity in ovarian cells that support oocyte growth and development.161,162 Furthermore, resveratrol appears to have anti-aging effects, such as promoting telomere repair and inhibiting AGE formation.23 Research in animals showed resveratrol may protect ovaries from chemotherapy-induced damage.163
Resveratrol may be beneficial in women with endometriosis: in a placebo-controlled trial in 34 women with endometriosis and infertility, 400 mg resveratrol daily for 12–14 weeks in addition to routine treatment was found to improve endometrial health.164 In a randomized controlled trial in 101 women undergoing intracytoplasmic sperm injection cycles, the number and quality of oocytes retrieved were higher in those treated for three months with a resveratrol-based multi-nutrient supplement (including 150 mg resveratrol, 400 mcg folic acid [680 mcg DFE], 25 mcg vitamin D, 2.5 mcg vitamin B12, and 1.4 mg vitamin B6). Resveratrol-treated women also had higher fertilization rates and numbers of viable embryos, but there were no significant differences in pregnancy, miscarriage, or live birth rates.165 A placebo-controlled trial in 62 women with PCOS and infertility undergoing intracytoplasmic sperm injection found resveratrol, at 800 mg daily for 40 days, increased the number of high-quality oocytes retrieved after ovulation induction but did not significantly influence pregnancy rate or outcomes.166
Folate
Folate, one of the B-complex vitamins, is needed for critical methylation reactions. Folic acid is a stable and well-absorbed form of folate commonly used in supplements. Folate deficiency during pregnancy has long been recognized as a cause of birth defects known as neural tube defects, and various guidelines recommend reproductive-aged women supplement with 400–800 mcg folic acid (680–1,360 mcg DFE) daily to reduce the risk of neural tube defects. A growing body of evidence further suggests folic acid supplementation may increase the likelihood of conception and healthy birth. Observational evidence indicates women who regularly take multivitamins with folic acid may have a shorter time to pregnancy and lower risk of infertility related to ovulation dysfunction, and supplementing with doses higher than those needed to prevent neural tube defects has been correlated with reduced risk of miscarriage.167,168
Folate, along with vitamins B12 (cobalamin) and B6 (pyridoxine), is needed for the metabolism of the amino acid compound homocysteine. High homocysteine levels are associated with a wide array of health problems, can trigger inflammation and oxidative stress in blood vessels, and have been correlated in some studies with increased risks of anovulation and recurrent miscarriages.169 One study in 269 women seeking in vitro fertilization reported 31% had high homocysteine levels (>15 µmol/L), 56% had low B12 status, and 88% had intracellular folate levels lower than those believed to be optimal for preventing neural tube defects.170 In an observational study in 100 women undergoing assisted reproductive treatment, those with higher blood levels of folate and B12 had higher pregnancy and live birth rates than those with lower levels.171
Certain mutations of the methylenetetrahydrofolate reductase (MTHFR) gene are known to cause abnormal homocysteine metabolism, resulting in high homocysteine levels, and some studies have found these mutations may be more common in women with infertility. Supplementing with 5-methyltetrahydrofolate (5-MTHF), an activated form of folate, and in some cases vitamin B12 may help women with these mutations, also known as single nucleotide polymorphisms or SNPs (pronounced “snips”), maintain lower homocysteine levels.172 In one study that included 92 women and 46 men undergoing assisted reproductive treatment and 161 controls, MTHFR mutations were correlated with poor in vitro fertilization outcomes and embryonic chromosomal abnormalities.173 However, it is important to note at least two studies have found no relationship between folate intake, blood folate levels, MTHFR mutations, and assisted reproductive outcomes.174,175
High homocysteine levels are also more common in women with than without PCOS. An open trial that included 32 women with PCOS found a multi-nutrient supplement providing 400 mcg (680 mcg DFE) 5-MTHF daily for three months decreased homocysteine levels compared with no treatment. In addition, in a subgroup of women with normal baseline homocysteine levels who received the supplement, AMH levels increased, suggesting improved ovarian function.176 The possible impact of folate or folic acid supplementation in women with PCOS still requires further study.
Omega-3 Fatty Acids
Omega-3 fatty acids from fish oil may improve fertility through their important role in steroid hormone production and anti-inflammatory effects.169 Some evidence suggests omega-3 fatty acids may lower the risk of anovulation, increase ovarian reserve, and improve oocyte quality and embryo development.169,177,178 In a preliminary trial in 17 healthy reproductive-aged women, taking 4,000 mg of an omega-3 fatty acid supplement (providing 1,860 mg eicosapentaenoic acid [EPA] and 1,500 mg docosahexaenoic acid [DHA]) daily for one month led to reductions in levels of FSH, a hormonal marker of diminished ovarian reserve and early menopause, in normal weight, but not obese, participants.179
Higher frequency of sexual intercourse and shorter time to pregnancy have been noted among couples in which either the male or female, and especially both partners, regularly consume seafood.180 In an observational study that included 1,290 North American women, higher intake of omega-3 fatty acids from fish was correlated with shorter time to pregnancy, while higher intake of trans fatty acids was linked to lower pregnancy rates181; however, another study that included data from 5,126 North American women did not find a relationship between omega-3 fatty acid intake and pregnancy rates, although higher intake of fried shellfish (a source of trans fatty acids) was associated with lower likelihood of pregnancy.182 Studies that examined blood levels of fatty acids have noted omega-6 and omega-3 fatty acid levels and omega-6:omega-3 ratios were unrelated to pregnancy or miscarriage rates.183,184
Observational studies examining the relationship between omega-3 fatty acids and assisted reproductive therapy outcomes have resulted in conflicting and inconclusive findings.185,186 On the other hand, trans fatty acid intake has consistently been associated with poorer assisted reproductive outcomes.187
Dehydroepiandrosterone (DHEA)
Dehydroepiandrosterone (DHEA) is a hormone produced mainly in the adrenal cortex, with smaller amounts produced in the testes, ovaries, and brain. Most DHEA is sulfated before being released into the bloodstream and circulates as DHEA-sulfate (DHEA-S).252 DHEA and DHEA-S have androgenic (male hormone) properties and are also used to synthesize estrogens (estradiol and estrone) and other androgens (androstenedione, testosterone, dihydrotestosterone) in the adrenal glands and other tissues.253 Although the role of DHEA in female reproductive function is still not fully understood,252 it appears to support fertility by increasing the growth, persistence, and mitochondrial function of ovarian follicles.254 Some evidence further suggests DHEA may increase AMH levels and improve oocyte and embryo quality, potentially increasing the odds of successful pregnancy.255
In an observational study, 277 women with infertility due to low ovarian reserve were treated with 75 mg DHEA daily and were followed for two years. After six months of treatment, 13.2% of the women experienced spontaneous pregnancy. The pregnancy rate increased to 21.3% after one year and 38.8% after two years.256
DHEA has been found in multiple clinical trials and meta-analyses to increase the success of assisted reproductive therapies. A meta-analysis of 18 clinical trials involving 1,883 women with poor ovarian response undergoing IVF or intracytoplasmic sperm injection found supplementing with 75 mg DHEA daily for two to four months reduced the risk of miscarriage by 50%, increased the pregnancy rate by 53%, and increased successful births by 87%.257 Another meta-analysis of five clinical studies with a total of 910 infertile women with low ovarian reserve undergoing IVF also found 25 mg DHEA three times daily increased the likelihood of pregnancy and decreased the risk of miscarriage.258 In a meta-analysis of randomized controlled trials investigating various interventions in women with poor ovarian response undergoing IVF, the pooled findings from two trials using DHEA therapy, at 25 mg three times daily, showed it increased the odds of pregnancy nearly 2.5-fold.259
Importantly, DHEA may be contraindicated in women with PCOS, who generally have elevated levels of androgens, including DHEA-S.260 Studies in animal models of PCOS suggest DHEA treatment may contribute to poor ovarian health and function in the context of this condition.261 Women with infertility and PCOS should therefore have their DHEA-S level checked before considering DHEA therapy.
For more information about DHEA, please see Life Extension’s DHEA Restoration Therapy protocol.
6 Dietary & Lifestyle Changes to Support Fertility
While some causes of female infertility are not preventable, mitigating the risks of other common contributors to infertility through lifestyle and dietary measures may improve the chance of conceiving.
Lifestyle
One of the most important ways to support fertility naturally is to avoid behaviors that can cause reproductive harm. This includes:
- Not smoking. As described in the “Risk Factors” section, smoking impairs fertility and leads to worse outcomes with assisted reproductive techniques.
- Limiting alcohol use. As even moderate drinking may impair fertility,48 women (and men) with fertility concerns should limit their alcohol use to amounts well within the moderate drinking levels defined by the 2020-2025 Dietary Guidelines for Americans. These levels are ≤ 1 drink daily for women and ≤ 2 drinks daily for men.
- Avoiding heavy caffeine use. High caffeine intake (more than 500 mg or about five cups of coffee per day) reduces the chance of pregnancy by an estimated 45%.47
- Getting tested and treated for sexually transmitted infections. Sexually active women can protect reproductive health by using condoms when not trying to conceive and through regular visits with a healthcare professional who can assist with prevention, provide screening, and initiate prompt treatment of sexually transmitted infections.188
- Maintaining a healthy body weight. Healthy weight maintenance is important for reproductive health and fertility. Being either overweight or underweight has significant negative effects on the ability to conceive.36,189,190 In overweight or obese women with polycystic ovary syndrome, weight loss of 5% or more restored regular ovulatory menstrual cycling within six months in the majority of women.3,191
- Maintaining healthy sleep habits. A growing body of research connects poor sleep with impaired reproductive function and infertility in women. Menstrual irregularity, PCOS, premature ovarian insufficiency, difficulty conceiving and infertility, and pregnancy loss have been linked to sleep disturbances and disorders. Getting quality sleep that is adequate in duration, synchronous with circadian rhythms, and not interrupted or fragmented may help support conception and pregnancy.192
Sleep apnea, a condition in which breathing stops and restarts intermittently throughout sleep, has been associated with infertility in both men and women.246-248 In women, obstructive sleep apnea is associated with poor pregnancy outcomes, including gestational diabetes, preeclampsia, and fetal growth restriction.249 Some data also suggest sleep apnea is linked to higher rates of miscarriage.250 Additionally, sleep apnea is closely correlated with obesity and is more common in women with PCOS, both of which are associated with increased risk of female infertility.251
In addition to reducing harmful exposures, women’s fertility may be supported through positive measures such as getting regular exercise and stress reduction.13,193 Unfortunately, a diagnosis of infertility along with interventions to achieve pregnancy are frequently a source of stress and can deteriorate quality of life.193
Timing of Intercourse
For a couple trying to conceive, the timing of sexual intercourse is critical. Taking into account the three- to five-day lifespan of sperm and the 12–24 hours of receptiveness of an oocyte released at ovulation, the chance that intercourse will lead to conception is greatest from five days before until one day after ovulation. This is sometimes referred to as the fertile window.194 Thus, couples hoping for pregnancy may benefit from tracking menstrual cycles and predicting ovulation, although results from studies have been mixed.195
While tracking menstrual cycles to predict ovulation may potentially improve the likelihood of conception, it can also be stressful, triggering or aggravating sexual dysfunction and relationship difficulties.196,197 It is important to note that couples who have used timed intercourse unsuccessfully for five consecutive cycles are unlikely to become pregnant without other interventions.194
Diet
Diet has a substantial impact on fertility. Observational data suggest higher fruit intake and lower intake of fast food and sugar-sweetened beverages are linked to shorter time to pregnancy (a marker of fertility).198 In one study that included 113 women with infertility, adequate maternal vegetable intake was associated with increased likelihood of embryo implantation after in vitro fertilization with intracytoplasmic sperm injection.199
Maternal seafood consumption has also been correlated with higher fertility, with eight or more servings per menstrual cycle corresponding with a 60% shorter time to pregnancy than women who consumed ≤1 serving.180 Fish consumption has also been linked to increased live birth rate after assisted reproductive procedures.200 Of note, however, is that certain fish species may contain higher levels of harmful compounds like mercury and should be avoided, especially by women who are pregnant or seeking to become pregnant. These include king mackerel, marlin, orange roughy, shark, swordfish, tilefish from the Gulf of Mexico, and bigeye tuna.201
Higher intake of soy foods has also been correlated with improved assisted reproductive technology outcomes.202,203 In a study that included 239 women undergoing in vitro fertilization, increasing urinary levels of BPA correlated with diminished live birth rates in women who did not consume soy. However, the correlation between urinary BPA levels and lower birth rates was not apparent among women who regularly consumed soy foods. These findings suggest soy may mitigate the negative effect of BPA on fertility.203
Dietary patterns that emphasize fruits, vegetables, whole grains, fish, poultry, and healthy fats have been generally associated with better fertility.31 A study that looked at dietary patterns in 357 women undergoing assisted reproductive interventions found adherence to a pro-fertility diet, characterized by high intake of low-pesticide fruits and vegetables, seafood, soy foods, dairy, and whole grains; low intake of high-pesticide fruits and vegetables; and supplemental folic acid, vitamin B12, and vitamin D, was more closely related to live birth rate than other dietary patterns. In the study, foods and beverages with low pesticide residue included167:
|
|
|
| Foods with high pesticide residue included167: | ||
|
|
|
7 Diagnosing Female Infertility
Investigations into infertility causes are typically undertaken after one year of unsuccessful attempts to become pregnant, or after six months in women over age 35.3 However, if a couple has concerns about their fertility, a screening history and physical exam can be undertaken at any time. A thorough history can help ascertain whether a woman is likely to be ovulating regularly and identify possible contributors to reduced fertility such as genetics, stress, sexually transmitted infections, vaginal dysbiosis, endometriosis, smoking, high alcohol or caffeine intake, and other lifestyle factors. Counseling around proper timing of intercourse may be useful. A physical exam can help with preliminary screening for pelvic abnormalities or infections, androgen excess, obesity, thyroid disease, and other overt medical issues.3,33
Tests for Male Infertility
Once infertility has been diagnosed based on time trying to conceive, a more thorough investigation is appropriate. Since it is estimated that 30–50% of infertility cases are attributable at least in part to male factors, a semen analysis is recommended early in the evaluation process.12,268
Imaging Tests
Transvaginal ultrasound is used to screen for problems with the uterus, fallopian tubes, or ovaries. It can also be used to count antral follicles, also known as resting follicles, in the ovaries.33Antral follicle count detects if the ovarian follicle pool is depleted, which is considered a consequence of increased age. The antral follicle count is a reliable indicator of ovarian reserve, and a low count (less than or equal to 4) is associated with low responsiveness to ovulation induction methods.268
More advanced imaging of the uterus (saline infusion sonogram or hysteroscopy) and fallopian tubes (hysterosalpingogram or laparoscopy) may be recommended to rule out uterine problems, endometriosis, adhesions, or other blockages, especially in women with normal antral follicle counts and AMH levels.3,33
Lab Tests
Certain lab tests are often helpful in the diagnostic process, including3:
- Anti-Müllerian hormone (AMH). AMH is produced by growing follicles and is used as a measure of ovarian function. Very low levels may predict decreased responsiveness to ovulation induction medications.
- Progesterone. A low progesterone level approximately one week before the onset of menses suggests anovulation.
- Follicle stimulating hormone (FSH). A high FSH level on day three of the menstrual cycle may indicate low ovarian reserve, with less follicular hormone production leading to a lack of suppression of FSH release by the pituitary gland.
- Estradiol. High levels of estradiol, the predominant type of estrogen produced by the ovaries, early in the menstrual cycle can be an indicator of HPO axis dysfunction.
- Testosterone. A high testosterone level may be due to PCOS and can impair ovarian function and fertility.
- Prolactin. High prolactin levels inhibit gonadotropin release and can cause anovulation.
- Thyroid stimulating hormone (TSH). Abnormal TSH levels suggest a thyroid disorder may be an underlying factor.
- Urinary luteinizing hormone (LH). This test can be performed using an over-the-counter at-home testing kit and is used to confirm a mid-cycle surge in LH release, which suggests the occurrence of ovulatory cycles. Some women use urinary LH to time intercourse.
8 Female Infertility Treatment
Female infertility treatment is based on the cause. Some causes of infertility, such as certain genetic conditions, severe uterine disease, and substantial loss of ovarian reserve, are unfortunately not treatable.
Ovulation Induction
Women with infertility who have few or no ovulatory cycles may benefit from the use of medications that induce ovulation. Ovulation-inducing drugs can be used prior to timed intercourse, but are more likely to be effective when combined with intrauterine insemination.3,16,205 The combination of intrauterine insemination with ovulation-inducing drugs also may be used in the setting of male infertility due to low sperm counts. It is important to note ovulation-inducing drugs are associated with an increased likelihood of pregnancy with twins, triplets, and higher-order births due to the release of more than one oocyte. These pregnancies are associated with higher risk of complications and poorer outcomes.206,207 Medical ovulation induction, especially using gonadotropin hormone therapy, can also cause ovarian hyperstimulation syndrome, in which multiple follicles mature simultaneously, causing the ovaries to swell and become painful. In rare instances, ovarian hyperstimulation syndrome can lead to life-threatening outcomes.16,207-209
Clomiphene citrate, an oral antiestrogen medication and first-line infertility treatment for anovulatory female infertility, binds to estrogen receptors and reduces estrogenic activity. Through a negative feedback mechanism, this stimulates FSH and LH secretion by the pituitary gland, leading to follicular maturation and ovulation.16
Clomiphene citrate can be effective in women with normal estrogen levels and normal or suppressed FSH levels during the follicular phase (first half) of the menstrual cycle, women with luteal phase deficiency, PCOS, and unexplained infertility; however, it is not beneficial in women with low estrogen levels due to impaired ovarian function, such as those with premature menopause or problems with the HPO axis.3,210 Women treated with clomiphene citrate have been shown to have a 30–50% chance of becoming pregnant within six ovulatory cycles.211
Adverse side effects of clomiphene citrate include mood swings, hot flashes, breast tenderness, abdominal cramps, and nausea.207 In addition, clomiphene citrate is associated with a 5–7% increased risk of a multiple pregnancy, usually with twins.211
Letrozole, an oral antiestrogen medication used to treat breast cancer, has also been found to promote ovulation. Letrozole works by inhibiting aromatase, the enzyme needed for conversion of androgens to estrogens. Decreased estrogen signaling triggers increased FSH release from the pituitary gland, and increased FSH signaling increases the chance of ovulation.3,210
Multiple randomized controlled trials and meta-analyses have shown letrozole has a similar safety profile and is more effective than clomiphene citrate in women with PCOS.211-214 Although letrozole is not FDA approved for the treatment of infertility, it appears to be the most effective treatment available and is frequently recommended as a first-line therapy, particularly in PCOS patients.3,211
Known side effects of letrozole include digestive upset, low energy, hot flashes, headache, and back pain.207
Gonadotropin therapy is a second-line intensive infertility treatment approach that may be recommended to women who do not respond to multiple cycles of oral antiestrogen therapy, or women with anovulation due to premature menopause or dysregulation of the HPO axis.3 In this approach, synthetic or urine-derived FSH, sometimes with small amounts of LH, is injected to stimulate follicular maturation and increase the likelihood of ovulation.210
Gonadotropin therapy has been found to increase the odds of successful birth by 13–28% in women with PCOS who did not become pregnant using clomiphene citrate.215 Examples of gonadotropin medications are follitropin alfa (Gonal-f), follitropin beta (Follistim), urofollitropin (Bravelle, Fertinex), and menotropins (Menopur).215 These medications cause the ovaries to enlarge, which can lead to abdominal discomfort and in some cases nausea and vomiting. They also may be more likely than antiestrogens to cause multiple pregnancies, such as with twins, and ovarian hyperstimulation syndrome.209
Luteal phase support has been shown to be important for enhancing outcomes in women treated with gonadotropin therapy followed by intrauterine insemination or in vitro fertilization.17,216 Progesterone, as subcutaneous injections, vaginal formulations, and oral forms, are widely used for this purpose. Triptorelin (Decapeptyl), a drug that activates GnRH receptors, has also been shown to effectively provide luteal phase support, increasing rates of implantation, pregnancy, and live births. Human chorionic gonadotropin (HCG), a hormone that stimulates progesterone production during pregnancy, is sometimes used for luteal phase support but is used with caution since it is more likely than other methods to induce ovarian hyperstimulation syndrome.217
Laparoscopic ovarian drilling is a minor surgical procedure used to induce ovulation in women with infertility related to PCOS who have not responded to first-line measures.218 The procedure involves producing small holes in the ovary surface using a laser or heat. Although the exact mechanism by which this stimulates ovulation is not known, it is thought the reduction in ovary size and removal of some follicles results in reduced androgen production, enhanced ovarian blood flow, increased FSH secretion, and restored HPO signaling.210,218 Ovarian drilling can be highly effective, with reported pregnancy rates as high as 88% after one year; however, a potential side effect of the procedure is worsened infertility due to scarring or ovarian failure.218
Surgery
Women with infertility related to uterine or fallopian tube abnormalities or scarring may benefit from targeted surgical procedures. For example, surgical removal of uterine polyps, certain fibroid tumors, and uterine adhesions can increase pregnancy rates. In women with mild tubal disease affecting the portion of the tube closest to the ovary, surgery to open the tube can improve fertility, although it also increases the risk of ectopic (tubal) pregnancy. On the other hand, surgical removal of the fallopian tubes followed by assisted reproductive measures may be recommended to women with severe tubal disease affecting both tubes.3
Assisted Reproductive Techniques
Assisted reproductive techniques (also called assisted reproductive technology) are recommended to couples for whom other treatments are not indicated or are unsuccessful. Oocyte retrieval is an essential part of all assisted reproductive techniques. To harvest oocytes, an ultrasound-guided needle aspiration of the ovary is generally performed through the vaginal wall after ovulation stimulation, usually with an injection of gonadotropins.3
When considering whether to pursue assisted reproductive techniques, it is worth bearing in mind that these procedures come with known and theoretical risks. For example, assisted reproductive techniques are known to increase the chance of multiple pregnancies (such as with twins or triplets) and related pregnancy complications due to the use of ovulation-inducing medications. Although only 1.7% of all live births in the United States are attributable to assisted reproductive techniques, it is estimated 40% of twins and 77% of triplets and higher multiples are the result of assisted reproductive techniques. Importantly, multiple pregnancies are associated with much higher risks of pregnancy complications, preterm birth, and low birth weight.219 In addition, ovulation-inducing drugs can cause side effects, including potentially life-threatening ovarian hyperstimulation syndrome.220 Theoretical risks include health problems in offspring related to genetic and epigenetic effects of oxidative stress, which may occur as a result of the procedure or may be inherited.221 Observational studies have found a higher incidence of birth defects in babies conceived via assisted reproductive techniques, especially those conceived using intracytoplasmic sperm injection.222-224 In addition, assisted reproductive technique-conceived babies have been found to be more likely to experience perinatal problems (eg, low birth weight and preterm birth) compared with those conceived naturally, and some evidence suggests they may potentially be at higher risk of cardiovascular problems later in life.224
Another important consideration is the psychological toll to both partners of pursuing expensive, invasive procedures without a guarantee of success. Stress, anxiety, and depression are common in men and women being treated for infertility, and psychological support has been shown to improve couples’ mental health and well-being.225 While some research suggests psychosocial interventions may increase pregnancy rates in infertile couples, it is perhaps more important that psychosocial support improves acceptance of outcomes.225,226 This may help couples to adapt to a child-free life or begin exploring the possibility of adoption.
Intrauterine insemination. Intrauterine insemination involves the separation of motile sperm from semen and their placement directly in the uterus shortly after natural or induced ovulation.2,16 Medical ovulation induction prior to intrauterine insemination increases the likelihood of pregnancy when the cause of infertility is unknown or due to female factors.227 It also may be used if there is male infertility due to low sperm counts. The chance of pregnancy using intrauterine insemination is lower in women over 40 years old.2 In addition, most studies suggest pregnancy is unlikely using intrauterine insemination if male infertility is severe (<5 million total sperm count) but may be helpful if sperm counts are higher (10 to 30 million).228 In general, the successful pregnancy rate with intrauterine insemination is about 12% per attempt but decreases with each successive attempt. Although this pregnancy rate is lower than with in vitro fertilization and intracytoplasmic sperm injection, intrauterine insemination is often recommended first when feasible due to its lower invasiveness and cost.2
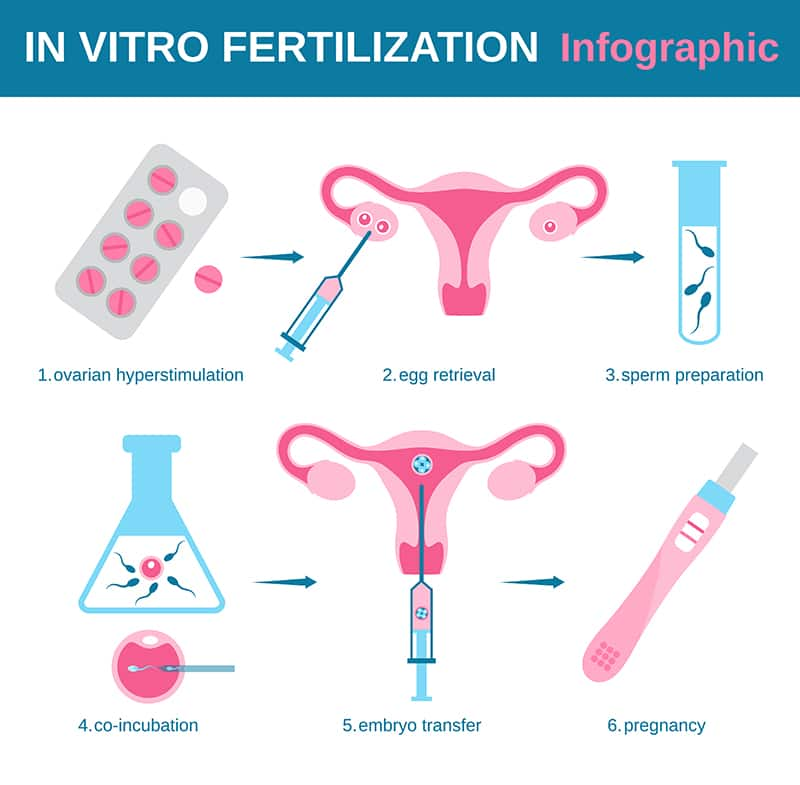
In vitro fertilization (IVF). In vitro fertilization (IVF) (Figure 3), which involves the fertilization of a mature oocyte outside of the female body, is available to infertile couples for whom intrauterine insemination is infeasible or unsuccessful. Typically, about 12 eggs are harvested after ovulation induction and placed in a fertilization medium with 50,000 to 500,000 motile sperm. If the number of healthy sperm is too low, intracytoplasmic sperm injection can be used. Several days later, 2‒4 embryos (successfully fertilized and growing oocytes) are transferred into the uterus and the rest are frozen.2,3 Genetic testing prior to embryo transfer may be recommended if one or both parents have known genetic mutations.3 IVF has been reported to be associated with a live birth rate of 3.1–47.6%, with greater success in younger women.220
Intracytoplasmic sperm injection. Intracytoplasmic sperm injection, a form of IVF in which a single mature sperm is injected directly into the cytoplasm of a mature oocyte in the laboratory, is the most widely used assisted reproductive technique in the world. The use of intracytoplasmic sperm injection has led to better IVF outcomes in couples with male infertility; however, its use in cases without a male infertility factor are still controversial.229,230 Intracytoplasmic sperm injection has been reported to result in successful pregnancy and birth in about 30% of cases overall.231
9 Novel & Emerging Treatments for Female Infertility
Mesenchymal stem cells (MSCs) are cells that have the ability to give rise to many types of cells, given the appropriate conditions. Emerging research suggests MSCs may be able to restore fertility to women with ovarian dysfunction, possibly by supporting the growth of immature ovarian follicles or generating new follicles.232-234 There are many sources of MSCs, including bone marrow, adipose (fat) tissue, amniotic fluid, umbilical cord tissue, placental tissue, and menstrual blood. Regardless of the source, MSCs have been shown in preclinical research to migrate and attach to injured ovarian tissue, where they then generate new ovarian cells in response to stimulation by growth factors and hormones.232,234 MSC therapy to promote healthy endometrial tissue has also been proposed as a future treatment for endometrial problems related to infertility, such as Asherman syndrome (intrauterine adhesions) and possibly even endometriosis.235,236
Early human clinical trials have yielded promising findings. In an uncontrolled trial, nine women with premature ovarian failure (also called premature menopause) had 5, 10, or 15 million MSCs derived from their own adipose tissue transplanted into their ovaries and were monitored for one year. Some participants experienced sporadic or short-term resumption of menstrual cycles and reduction in FSH levels, and the effects appeared to vary between individuals rather than number of MSCs used.237 In another clinical trial, 14 women with premature ovarian failure and 4‒5 years of infertility underwent injection of donor umbilical cord-derived MSCs into an ovary, resulting in increased ovarian volume, blood flow, and function in some women three months later. Two spontaneous pregnancies and one healthy birth occurred. The response to treatment appeared to be stronger in women who received MSCs loaded on a collagen scaffold, a fibrillary network that helps retain the stem cells in the ovary.238 Donor umbilical cord MSCs on a collagen scaffold were also found to improve endometrial health in a trial that included 26 women with recurrent uterine adhesions. In the trial, MSCs were transplanted into the uterus after adhesions were surgically separated. Endometrial thickening (a sign of endometrial health) and reduced adhesions were noted after three months, and after 30 months 10 women had become pregnant: eight pregnancies resulted in healthy childbirth, one pregnancy was ongoing, and one miscarriage had occurred.239
Findings from a small controlled trial further indicate MSC therapy may help women who have not responded to other treatments. In the trial, 15 women with poor ovarian response to ovulation-inducing therapies underwent transplantation of MSCs from their own menstrual blood into an ovary while 16 similar women underwent routine intracytoplasmic sperm injection (control). Four MSC-treated women and no control women became pregnant during the three months after treatment. Subsequent assisted reproductive techniques led to pregnancy in an additional three women in the MSC group and two in the control group. Ultimately, there were five live births (a rate of 33%) in the MSC group and one (a rate of 6.3%) in the control group.240
10 Living With Female Infertility
Infertility can be a devastating diagnosis that can trigger psychological, emotional, and social distress above and beyond the stress inherent in infertility testing and treatments. Many women experience feelings of grief and loss when they find themselves unable to conceive or following a miscarriage.241 Stigmatization of childless women can take many forms, depending on cultural norms and expectations, and may contribute to shame and diminished self-esteem.242 Infertility may also trigger sexual dysfunction and relationship tension.243 Individual psychotherapy and group support may help women cope with the trauma of infertility and begin making decisions to shape their families’ future.241,244 Some evidence suggests psychological tasks such as acknowledgement, acceptance, instilling meaning, and pursuing new life goals are key to healthy adjustment.244,245
After fully processing the grief of infertility and coming to a point of acceptance, some couples will find adoption is a fulfilling way to become parents and grow a family. If you are considering adoption, here are some resources that may be helpful:
11 Frequently Asked Questions About Female Infertility
When should I see my doctor about female infertility?
Generally, women 35 years old or younger who have not conceived after 12 months of trying (having regular unprotected sex) should seek medical care, while women over 35 should seek care sooner. Women with symptoms such as menstrual irregularity, abnormal pain or bleeding, vaginal soreness or itching, unusual vaginal discharge, fever, and pain with intercourse should speak with their doctor as soon as possible.
What happens if a woman is infertile?
A woman who is infertile has many options available to her, depending on the cause of her infertility. These range from non-invasive lifestyle and nutritional interventions to highly invasive assisted reproductive techniques, as well as adoption.
What are the signs of infertility in women?
The main sign of infertility is an inability to become pregnant. Especially short (less than 21 days) or long (35 days or more) menstrual cycles, irregular cycles, or absence of periods may be symptoms of reduced fertility. Other signs and symptoms of infertility can exist, depending on the cause.
What are the reasons why a woman cannot get pregnant?
A woman may have difficulty getting pregnant if she does not ovulate regularly or at all; has problems affecting her fallopian tubes or uterus; has endometriosis; or does not have many eggs in her ovaries due to aging, toxic exposures, or health problems. She may also have trouble conceiving if her partner does not have healthy sperm in adequate numbers. In some cases, no reason is found.
How can I check my fertility?
Women can use cervical mucous and basal body temperature records to track their menstrual cycle and identify their most fertile time of the month. At-home ovulation test kits can also help women see when they are ovulating.
2024
- Dec: Added section on classical and non-classical congenital adrenal hyperplasia (CAH and NC-CAH) to Causes of Female Infertility
- Jan: Added section on dehydroepiandrosterone (DHEA) to Nutrients
2023
- Apr: Updated Dietary & Lifestyle Changes section to include discussion of sleep apnea in “Maintaining healthy sleep habits”
2022
- Jul: Initial publication
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Kuohung W, Hornstein MD. Evaluation of female infertility. UpToDate. Updated 3/8/2021. Accessed 9/7/2021, https://www.uptodate.com/contents/evaluation-of-female-infertility?search=infertility%20female&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Leslie SW, Siref LE, Soon-Sutton TL, Khan MAB. Male Infertility. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Walker MH, Tobler KJ. Female Infertility. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Kuohung W, Hornstein MD. Female infertility: Causes. UpToDate. Last updated 7/22/2021. Accessed 9/7/2021. https://www.uptodate.com/contents/causes-of-female-infertility
- Cicek N, Eryilmaz OG, Sarikaya E, Gulerman C, Genc Y. Vitamin E effect on controlled ovarian stimulation of unexplained infertile women. J Assist Reprod Genet. Apr 2012;29(4):325-8. doi:10.1007/s10815-012-9714-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3309992/pdf/10815_2012_Article_9714.pdf
- Hu KL, Ye X, Wang S, Zhang D. Melatonin Application in Assisted Reproductive Technology: A Systematic Review and Meta-Analysis of Randomized Trials. Frontiers in endocrinology. 2020;11:160. doi:10.3389/fendo.2020.00160. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7118201/pdf/fendo-11-00160.pdf
- Florou P, Anagnostis P, Theocharis P, Chourdakis M, Goulis DG. Does coenzyme Q(10) supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J Assist Reprod Genet. Oct 2020;37(10):2377-2387. doi:10.1007/s10815-020-01906-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7550497/pdf/10815_2020_Article_1906.pdf
- Kuohung W, Hornstein MD. Treatments for female infertility. UpToDate. Updated 7/22/2021. Accessed 9/7/2021, https://www.uptodate.com/contents/treatments-for-female-infertility?search=infertility%20female&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=3
- Hart RJ. Physiological Aspects of Female Fertility: Role of the Environment, Modern Lifestyle, and Genetics. Physiol Rev. Jul 2016;96(3):873-909. doi:10.1152/physrev.00023.2015. https://journals.physiology.org/doi/pdf/10.1152/physrev.00023.2015
- Rosner J, Samardzic T, Sarao MS. Physiology, Female Reproduction. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Agarwal A, Baskaran S, Parekh N, et al. Male infertility. Lancet. Jan 23 2021;397(10271):319-333. doi:10.1016/S0140-6736(20)32667-2. https://www.ncbi.nlm.nih.gov/pubmed/33308486
- Hakimi O, Cameron LC. Effect of Exercise on Ovulation: A Systematic Review. Sports medicine (Auckland, NZ). Aug 2017;47(8):1555-1567. doi:10.1007/s40279-016-0669-8.
- Koyyada A, Orsu P. Role of hypothyroidism and associated pathways in pregnancy and infertility: Clinical insights. Tzu Chi Med J. Oct-Dec 2020;32(4):312-317. doi:10.4103/tcmj.tcmj_255_19. https://www.ncbi.nlm.nih.gov/pubmed/33163375
- Myneni R, Chawla HV, Grewal AS, et al. Thyroxine Replacement for Subfertile Females With Subclinical Hypothyroidism and Autoimmune Thyroiditis: A Systematic Review. Cureus. Aug 2021;13(8):e16872. doi:10.7759/cureus.16872. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8411998/pdf/cureus-0013-00000016872.pdf
- Carson SA, Kallen AN. Diagnosis and Management of Infertility: A Review. JAMA. 2021;326(1):65-76. doi:10.1001/jama.2021.4788. https://doi.org/10.1001/jama.2021.4788 https://jamanetwork.com/journals/jama/article-abstract/2781637
- Palomba S, Santagni S, La Sala GB. Progesterone administration for luteal phase deficiency in human reproduction: an old or new issue? Journal of ovarian research. Nov 19 2015;8:77. doi:10.1186/s13048-015-0205-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4653859/pdf/13048_2015_Article_205.pdf
- Diagnosis and treatment of luteal phase deficiency: a committee opinion. Fertility and sterility. Jun 2021;115(6):1416-1423. doi:10.1016/j.fertnstert.2021.02.010.
- Mesen TB, Young SL. Progesterone and the luteal phase: a requisite to reproduction. Obstetrics and gynecology clinics of North America. Mar 2015;42(1):135-51. doi:10.1016/j.ogc.2014.10.003. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4436586/pdf/nihms689536.pdf
- Guan J, Watrelot A. Fallopian tube subtle pathology. Best Pract Res Clin Obstet Gynaecol. Aug 2019;59:25-40. doi:10.1016/j.bpobgyn.2018.12.012. https://www.sciencedirect.com/science/article/abs/pii/S1521693418302219?via%3Dihub
- Mummert T, Gnugnoli DM. Ectopic Pregnancy. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Smikle C, Yarrarapu SNS, Khetarpal S. Asherman Syndrome. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Human reproduction update. Dec 16 2021;doi:10.1093/humupd/dmab038.
- Wang L, Tang J, Wang L, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. Jun 14 2021;doi:10.1002/jcp.30468. https://onlinelibrary.wiley.com/doi/10.1002/jcp.30468
- Shirasuna K, Iwata H. Effect of aging on the female reproductive function. Contracept Reprod Med. 2017;2:23. doi:10.1186/s40834-017-0050-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5683335/pdf/40834_2017_Article_50.pdf
- Mikwar M, MacFarlane AJ, Marchetti F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutation research Reviews in mutation research. Jul-Sep 2020;785:108320. doi:10.1016/j.mrrev.2020.108320.
- Seli E, Wang T, Horvath TL. Mitochondrial unfolded protein response: a stress response with implications for fertility and reproductive aging. Fertility and sterility. Feb 2019;111(2):197-204. doi:10.1016/j.fertnstert.2018.11.048.
- Homer HA. The Role of Oocyte Quality in Explaining "Unexplained" Infertility. Seminars in reproductive medicine. Jan 2020;38(1):21-28. doi:10.1055/s-0040-1721377.
- Rocca MS, Foresta C, Ferlin A. Telomere length: lights and shadows on their role in human reproduction. Biol Reprod. Feb 1 2019;100(2):305-317. doi:10.1093/biolre/ioy208.
- Mihalas BP, Redgrove KA, McLaughlin EA, Nixon B. Molecular Mechanisms Responsible for Increased Vulnerability of the Ageing Oocyte to Oxidative Damage. Oxid Med Cell Longev. 2017;2017:4015874. doi:10.1155/2017/4015874. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5664291/pdf/OMCL2017-4015874.pdf
- Aoun A, Khoury VE, Malakieh R. Can Nutrition Help in the Treatment of Infertility? Prev Nutr Food Sci. Jun 30 2021;26(2):109-120. doi:10.3746/pnf.2021.26.2.109.
- Rodichkina V, Kvetnoy I, Polyakova V, Arutjunyan A, Nasyrov R, Ivanov D. Inflammaging of Female Reproductive System: A Molecular Landscape. Current aging science. 2021;14(1):10-18. doi:10.2174/1874609813666200929112624.
- Garolla A, Pizzol D, Carosso AR, et al. Practical Clinical and Diagnostic Pathway for the Investigation of the Infertile Couple. Frontiers in endocrinology. 2020;11:591837. doi:10.3389/fendo.2020.591837. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7851076/pdf/fendo-11-591837.pdf
- Lewis TD, Malik M, Britten J, San Pablo AM, Catherino WH. A Comprehensive Review of the Pharmacologic Management of Uterine Leiomyoma. Biomed Res Int. 2018;2018:2414609. doi:10.1155/2018/2414609.
- Riazi H, Tehranian N, Ziaei S, Mohammadi E, Hajizadeh E, Montazeri A. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Womens Health. May 8 2015;15:39. doi:10.1186/s12905-015-0196-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4450847/pdf/12905_2015_Article_196.pdf
- Amiri M, Ramezani Tehrani F. Potential Adverse Effects of Female and Male Obesity on Fertility: A Narrative Review. International journal of endocrinology and metabolism. Jul 2020;18(3):e101776. doi:10.5812/ijem.101776. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7695350/pdf/ijem-18-3-101776.pdf
- Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause. Sep 2018;25(9):1046-1055. doi:10.1097/gme.0000000000001116.
- Mattsson K, Nilsson-Condori E, Elmerstig E, et al. Fertility outcomes in women with pre-existing type 2 diabetes-a prospective cohort study. Fertility and sterility. Aug 2021;116(2):505-513. doi:10.1016/j.fertnstert.2021.02.009. https://www.fertstert.org/article/S0015-0282(21)00127-8/pdf
- Morrison AE, Fleming S, Levy MJ. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin Endocrinol (Oxf). Aug 2021;95(2):229-238. doi:10.1111/cen.14399. https://onlinelibrary.wiley.com/doi/10.1111/cen.14399
- Frisch RE. The right weight: body fat, menarche and ovulation. Baillieres Clin Obstet Gynaecol. Sep 1990;4(3):419-39. doi:10.1016/s0950-3552(05)80302-5.
- Vanni VS, De Lorenzo R, Privitera L, Canti V, Viganò P, Rovere-Querini P. Safety of fertility treatments in women with systemic autoimmune diseases (SADs). Expert opinion on drug safety. Sep 2019;18(9):841-852. doi:10.1080/14740338.2019.1636964.
- Lasa JS, Zubiaurre I, Soifer LO. Risk of infertility in patients with celiac disease: a meta-analysis of observational studies. Arq Gastroenterol. Apr-Jun 2014;51(2):144-50. doi:10.1590/s0004-28032014000200014.
- Stentz NC, Koelper N, Barnhart KT, Sammel MD, Senapati S. Infertility and mortality. Am J Obstet Gynecol. Mar 2020;222(3):251.e1-251.e10. doi:10.1016/j.ajog.2019.09.007.
- Greydanus DE, Cabral MD, Patel DR. Pelvic inflammatory disease in the adolescent and young adult: An update. Disease-a-month : DM. Sep 11 2021:101287. doi:10.1016/j.disamonth.2021.101287.
- Dreisler E, Kjer JJ. Asherman's syndrome: current perspectives on diagnosis and management. International journal of women's health. 2019;11:191-198. doi:10.2147/ijwh.S165474.
- Saraf VS, Sheikh SA, Ahmad A, Gillevet PM, Bokhari H, Javed S. Vaginal microbiome: normalcy vs dysbiosis. Arch Microbiol. Sep 2021;203(7):3793-3802. doi:10.1007/s00203-021-02414-3.
- Fartushok TV, Semenyna HB, Yurchyshyn OM, Komissarova OS. WAYS TO IMPROVE NATURAL FERTILITY. Wiadomosci lekarskie (Warsaw, Poland : 1960). 2021;74(1):144-149.
- Anwar MY, Marcus M, Taylor KC. The association between alcohol intake and fecundability during menstrual cycle phases. Human reproduction (Oxford, England). Aug 18 2021;36(9):2538-2548. doi:10.1093/humrep/deab121. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8561243/pdf/deab121.pdf
- de Angelis C, Nardone A, Garifalos F, et al. Smoke, alcohol and drug addiction and female fertility. Reproductive biology and endocrinology : RB&E. Mar 12 2020;18(1):21. doi:10.1186/s12958-020-0567-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7069005/pdf/12958_2020_Article_567.pdf
- Vilar L, Vilar CF, Lyra R, Freitas MDC. Pitfalls in the Diagnostic Evaluation of Hyperprolactinemia. Neuroendocrinology. 2019;109(1):7-19. doi:10.1159/000499694. https://www.karger.com/Article/Pdf/499694
- Spears N, Lopes F, Stefansdottir A, et al. Ovarian damage from chemotherapy and current approaches to its protection. Human reproduction update. Nov 5 2019;25(6):673-693. doi:10.1093/humupd/dmz027. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6847836/pdf/dmz027.pdf
- Edinoff AN, Silverblatt NS, Vervaeke HE, et al. Hyperprolactinemia, Clinical Considerations, and Infertility in Women on Antipsychotic Medications. Psychopharmacol Bull. Mar 16 2021;51(2):131-148. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8146565/pdf/PB-51-2-131.pdf
- Evans-Hoeker EA, Eisenberg E, Diamond MP, et al. Major depression, antidepressant use, and male and female fertility. Fertil Steril. May 2018;109(5):879-887. doi:10.1016/j.fertnstert.2018.01.029. https://www.fertstert.org/article/S0015-0282(18)30029-3/pdf
- Casilla-Lennon MM, Meltzer-Brody S, Steiner AZ. The effect of antidepressants on fertility. Am J Obstet Gynecol. Sep 2016;215(3):314.e1-5. doi:10.1016/j.ajog.2016.01.170. https://www.ajog.org/article/S0002-9378(16)00220-9/pdf
- Salman S, Sherif B, Al-Zohyri A. OP0131 Effects of Some Non Steroidal Anti-Inflammatory Drugs on Ovulation in Women with Mild Musculoskeletal Pain. Annals of the Rheumatic Diseases. 06/01 2015;74:117.3-118. doi:10.1136/annrheumdis-2015-eular.1062. https://ard.bmj.com/content/74/Suppl_2/117.3
- Grbac E, So T, Varshney S, Williamson N, Dimitriadis E, Menkhorst E. Prednisolone Alters Endometrial Decidual Cells and Affects Decidual-Trophoblast Interactions. Original Research. Frontiers in Cell and Developmental Biology. 2021-April-09 2021;9doi:10.3389/fcell.2021.647496. https://www.frontiersin.org/article/10.3389/fcell.2021.647496
- Shivaswamy V, Ochsner L, Maroni D, et al. Tacrolimus and sirolimus induce reproductive abnormalities in female rats. Transplantation. Jun 27 2011;91(12):1333-9. doi:10.1097/TP.0b013e31821c1e8b.
- Flannagan KS, Mumford SL, Sjaarda LA, et al. Is opioid use safe in women trying to conceive? Epidemiology. Nov 2020;31(6):844-851. doi:10.1097/ede.0000000000001247. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7725439/pdf/nihms-1615537.pdf
- Salama M, Woodruff TK. Anticancer treatments and female fertility: clinical concerns and role of oncologists in oncofertility practice. Expert Rev Anticancer Ther. Aug 2017;17(8):687-692. doi:10.1080/14737140.2017.1335199. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6145143/pdf/nihms-1500870.pdf
- La Vignera S, Condorelli RA, Cannarella R, Duca Y, Calogero AE. Sport, doping and female fertility. Reprod Biol Endocrinol. Nov 19 2018;16(1):108. doi:10.1186/s12958-018-0437-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6241032/pdf/12958_2018_Article_437.pdf
- Buchanan JF, Davis LJ. Drug-induced infertility. Drug Intell Clin Pharm. Feb 1984;18(2):122-32. doi:10.1177/106002808401800205.
- Lu Y, Chen R, Cai J, Huang Z, Yuan H. The management of hypertension in women planning for pregnancy. Br Med Bull. Dec 1 2018;128(1):75-84. doi:10.1093/bmb/ldy035.
- Zamora-León P. Are the Effects of DES Over? A Tragic Lesson from the Past. International journal of environmental research and public health. Sep 30 2021;18(19)doi:10.3390/ijerph181910309. https://mdpi-res.com/d_attachment/ijerph/ijerph-18-10309/article_deploy/ijerph-18-10309-v2.pdf
- Al Jishi T, Sergi C. Current perspective of diethylstilbestrol (DES) exposure in mothers and offspring. Reprod Toxicol. Aug 2017;71:71-77. doi:10.1016/j.reprotox.2017.04.009.
- Conlon JL. Diethylstilbestrol: Potential health risks for women exposed in utero and their offspring. JAAPA : official journal of the American Academy of Physician Assistants. Feb 2017;30(2):49-52. doi:10.1097/01.Jaa.0000511800.91372.34.
- Canipari R, De Santis L, Cecconi S. Female Fertility and Environmental Pollution. International journal of environmental research and public health. Nov 26 2020;17(23)doi:10.3390/ijerph17238802. https://mdpi-res.com/d_attachment/ijerph/ijerph-17-08802/article_deploy/ijerph-17-08802-v2.pdf
- Marci R, Mallozzi M, Di Benedetto L, et al. Radiations and female fertility. Reprod Biol Endocrinol. Dec 16 2018;16(1):112. doi:10.1186/s12958-018-0432-0.
- Anderson M, Goldman RH. Occupational Reproductive Hazards for Female Surgeons in the Operating Room: A Review. JAMA Surg. Mar 1 2020;155(3):243-249. doi:10.1001/jamasurg.2019.5420.
- Barn P, Gombojav E, Ochir C, et al. The effect of portable HEPA filter air cleaner use during pregnancy on fetal growth: The UGAAR randomized controlled trial. Environ Int. Dec 2018;121(Pt 1):981-989. doi:10.1016/j.envint.2018.08.036.
- NIH. National Insititues of Health: National Institute of Environmental Health Sciences: Endocrine Disruptors. Available at https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm. Accessed 11/10/21. 2021;
- Caporossi L, Viganò P, Paci E, et al. Female Reproductive Health and Exposure to Phthalates and Bisphenol A: A Cross Sectional Study. Toxics. Nov 11 2021;9(11)doi:10.3390/toxics9110299. https://mdpi-res.com/d_attachment/toxics/toxics-09-00299/article_deploy/toxics-09-00299-v2.pdf
- EcologyCenter. What’s Cooking 2021 Update. Still cooking: An update on toxic PFAS in cookware products. Available at https://www.ecocenter.org/healthy-stuff/reports/whats-cooking-2021-update Published 10/06/2021. Accessed 03/24/2022. 2021;
- Tomaiuolo R, Veneruso I, Cariati F, D'Argenio V. Microbiota and Human Reproduction: The Case of Female Infertility. High Throughput. May 3 2020;9(2)doi:10.3390/ht9020012. https://www.ncbi.nlm.nih.gov/pubmed/32375241
- García-Velasco JA, Budding D, Campe H, et al. The reproductive microbiome - clinical practice recommendations for fertility specialists. Reprod Biomed Online. Sep 2020;41(3):443-453. doi:10.1016/j.rbmo.2020.06.014. https://www.rbmojournal.com/article/S1472-6483(20)30339-4/pdf
- Punzón-Jiménez P, Labarta E. The impact of the female genital tract microbiome in women health and reproduction: a review. J Assist Reprod Genet. Oct 2021;38(10):2519-2541. doi:10.1007/s10815-021-02247-5. https://link.springer.com/content/pdf/10.1007/s10815-021-02247-5.pdf
- Jiang I, Yong PJ, Allaire C, Bedaiwy MA. Intricate Connections between the Microbiota and Endometriosis. International journal of molecular sciences. May 26 2021;22(11)doi:10.3390/ijms22115644. https://mdpi-res.com/d_attachment/ijms/ijms-22-05644/article_deploy/ijms-22-05644.pdf
- Agarwal A, Sengupta P, Durairajanayagam D. Role of L-carnitine in female infertility. Reproductive biology and endocrinology : RB&E. Jan 26 2018;16(1):5. doi:10.1186/s12958-018-0323-4. https://www.ncbi.nlm.nih.gov/pubmed/29373970
- Petrillo T, Battipaglia C, Virmani MA, Genazzani AR, Genazzani AD. Neuroendocrine Effects of Carnitines on Reproductive Impairments. International journal of molecular sciences. Oct 5 2021;22(19)doi:10.3390/ijms221910781. https://mdpi-res.com/d_attachment/ijms/ijms-22-10781/article_deploy/ijms-22-10781-v2.pdf
- Li J, Liu L, Weng J, Yin TL, Yang J, Feng HL. Biological roles of l-carnitine in oocyte and early embryo development. Mol Reprod Dev. Oct 2021;88(10):673-685. doi:10.1002/mrd.23542. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/mrd.23542?download=true
- Tauqir S, Israr M, Rauf B, et al. Acetyl-L-Carnitine Ameliorates Metabolic and Endocrine Alterations in Women with PCOS: A Double-Blind Randomized Clinical Trial. Adv Ther. Jul 2021;38(7):3842-3856. doi:10.1007/s12325-021-01789-5. https://link.springer.com/article/10.1007%2Fs12325-021-01789-5
- Savic D, Hodson L, Neubauer S, Pavlides M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. Jul 22 2020;12(8)doi:10.3390/nu12082178. https://mdpi-res.com/d_attachment/nutrients/nutrients-12-02178/article_deploy/nutrients-12-02178-v2.pdf
- Bene J, Hadzsiev K, Melegh B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutrition & diabetes. Mar 7 2018;8(1):8. doi:10.1038/s41387-018-0017-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5856836/pdf/41387_2018_Article_17.pdf
- Liao D, Liu X, Yuan X, et al. Clinical evidence of the effects of carnitine supplementation on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a systematic review and meta-analysis. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Oct 11 2021:1-6. doi:10.1080/09513590.2021.1988559.
- Genazzani AD, Despini G, Czyzyk A, Podfigurna A, Simoncini T, Meczekalski B. Modulatory effects of l-carnitine plus l-acetyl-carnitine on neuroendocrine control of hypothalamic functions in functional hypothalamic amenorrhea (FHA). Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Dec 2017;33(12):963-967. doi:10.1080/09513590.2017.1332587.
- Genazzani AD, Lanzoni C, Ricchieri F, et al. Acetyl-L-carnitine (ALC) administration positively affects reproductive axis in hypogonadotropic women with functional hypothalamic amenorrhea. Journal of endocrinological investigation. Apr 2011;34(4):287-91. doi:10.1007/bf03347087. https://link.springer.com/content/pdf/10.1007/BF03347087.pdf
- Genazzani AD, Petraglia F, Algeri I, et al. Acetyl-l-carnitine as possible drug in the treatment of hypothalamic amenorrhea. Acta obstetricia et gynecologica Scandinavica. 1991;70(6):487-92. doi:10.3109/00016349109007165. https://obgyn.onlinelibrary.wiley.com/doi/abs/10.3109/00016349109007165?sid=nlm%3Apubmed
- Genazzani AD, Tomatis V, Manzo A, et al. Treatment with carnitines, L-arginine and N-acetylcysteine in patients affected by functional hypothalamic amenorrhea and metabolic changes. Eur Gynecol Obstet. 2020;2(4):239–245.
- Kitano Y, Hashimoto S, Matsumoto H, et al. Oral administration of l-carnitine improves the clinical outcome of fertility in patients with IVF treatment. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Aug 2018;34(8):684-688. doi:10.1080/09513590.2018.1431769. https://www.tandfonline.com/doi/full/10.1080/09513590.2018.1431769
- Ashraf M, Mustansir F, Baqir SM, Alam F, Rehman R. Changes in vitamin E levels as a marker of female infertility. J Pak Med Assoc. Oct 2020;70(10):1762-1766. doi:10.5455/jpma.40329.
- Fatemi F, Mohammadzadeh A, Sadeghi MR, et al. Role of vitamin E and D(3) supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clinical nutrition ESPEN. Apr 2017;18:23-30. doi:10.1016/j.clnesp.2017.01.002. https://clinicalnutritionespen.com/article/S2405-4577(17)30011-6/fulltext
- Chen J, Guo Q, Pei YH, et al. Effect of a short-term vitamin E supplementation on oxidative stress in infertile PCOS women under ovulation induction: a retrospective cohort study. BMC Womens Health. Apr 6 2020;20(1):69. doi:10.1186/s12905-020-00930-w. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7137506/pdf/12905_2020_Article_930.pdf
- Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertility and sterility. Apr 2010;93(6):1851-8. doi:10.1016/j.fertnstert.2008.12.062. https://www.fertstert.org/article/S0015-0282(08)04783-3/pdf
- Acharya S, Yasmin E, Balen AH. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies--a report of 20 cases. Hum Fertil (Camb). Dec 2009;12(4):198-203. doi:10.3109/14647270903377178. https://www.ncbi.nlm.nih.gov/pubmed/19938908 https://www.tandfonline.com/doi/full/10.3109/14647270903377178
- Gambioli R, Montanino Oliva M, Nordio M, Chiefari A, Puliani G, Unfer V. New Insights into the Activities of D-Chiro-Inositol: A Narrative Review. Biomedicines. Oct 2 2021;9(10)doi:10.3390/biomedicines9101378. https://mdpi-res.com/d_attachment/biomedicines/biomedicines-09-01378/article_deploy/biomedicines-09-01378.pdf
- Kamenov Z, Gateva A. Inositols in PCOS. Molecules (Basel, Switzerland). Nov 27 2020;25(23)doi:10.3390/molecules25235566. https://www.ncbi.nlm.nih.gov/pubmed/33260918 https://res.mdpi.com/d_attachment/molecules/molecules-25-05566/article_deploy/molecules-25-05566.pdf
- Unfer V, Dinicola S, Laganà AS, Bizzarri M. Altered Ovarian Inositol Ratios May Account for Pathological Steroidogenesis in PCOS. International journal of molecular sciences. Sep 28 2020;21(19)doi:10.3390/ijms21197157. https://mdpi-res.com/d_attachment/ijms/ijms-21-07157/article_deploy/ijms-21-07157.pdf
- Mendoza N, Diaz-Ropero MP, Aragon M, et al. Comparison of the effect of two combinations of myo-inositol and D-chiro-inositol in women with polycystic ovary syndrome undergoing ICSI: a randomized controlled trial. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Aug 2019;35(8):695-700. doi:10.1080/09513590.2019.1576620. https://www.tandfonline.com/doi/pdf/10.1080/09513590.2019.1576620?needAccess=true
- Facchinetti F, Espinola MSB, Dewailly D, et al. Breakthroughs in the Use of Inositols for Assisted Reproductive Treatment (ART). Trends in endocrinology and metabolism: TEM. Aug 2020;31(8):570-579. doi:10.1016/j.tem.2020.04.003. https://www.cell.com/trends/endocrinology-metabolism/fulltext/S1043-2760(20)30080-1?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1043276020300801%3Fshowall%3Dtrue
- Zheng X, Lin D, Zhang Y, et al. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine. Dec 2017;96(49):e8842. doi:10.1097/md.0000000000008842. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5728865/pdf/medi-96-e8842.pdf
- Agrawal A, Mahey R, Kachhawa G, Khadgawat R, Vanamail P, Kriplani A. Comparison of metformin plus myoinositol vs metformin alone in PCOS women undergoing ovulation induction cycles: randomized controlled trial. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Jun 2019;35(6):511-514. doi:10.1080/09513590.2018.1549656. https://www.ncbi.nlm.nih.gov/pubmed/30614289 https://www.tandfonline.com/doi/full/10.1080/09513590.2018.1549656
- Nazari L, Salehpour S, Hosseini S, et al. Effect of myo-inositol supplementation on ICSI outcomes among poor ovarian responder patients: A randomized controlled trial. J Gynecol Obstet Hum Reprod. May 2020;49(5):101698. doi:10.1016/j.jogoh.2020.101698.
- Novielli C, Anelli GM, Lisso F, et al. Effects of α-lipoic acid and myo-inositol supplementation on the oocyte environment of infertile obese women: A preliminary study. Reprod Biol. Dec 2020;20(4):541-546. doi:10.1016/j.repbio.2020.10.002.
- Ivanov D, Mazzoccoli G, Anderson G, et al. Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression. International journal of molecular sciences. May 30 2021;22(11)doi:10.3390/ijms22115885. https://mdpi-res.com/d_attachment/ijms/ijms-22-05885/article_deploy/ijms-22-05885-v2.pdf
- Tamura H, Jozaki M, Tanabe M, et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. International journal of molecular sciences. Feb 8 2020;21(3)doi:10.3390/ijms21031135. https://mdpi-res.com/d_attachment/ijms/ijms-21-01135/article_deploy/ijms-21-01135.pdf
- Jang H, Hong K, Choi Y. Melatonin and Fertoprotective Adjuvants: Prevention against Premature Ovarian Failure during Chemotherapy. International journal of molecular sciences. Jun 7 2017;18(6)doi:10.3390/ijms18061221. https://mdpi-res.com/d_attachment/ijms/ijms-18-01221/article_deploy/ijms-18-01221-v2.pdf
- Sciarra F, Franceschini E, Campolo F, et al. Disruption of Circadian Rhythms: A Crucial Factor in the Etiology of Infertility. International journal of molecular sciences. May 30 2020;21(11)doi:10.3390/ijms21113943. https://www.ncbi.nlm.nih.gov/pubmed/32486326 https://mdpi-res.com/d_attachment/ijms/ijms-21-03943/article_deploy/ijms-21-03943-v2.pdf
- Lateef OM, Akintubosun MO. Sleep and Reproductive Health. J Circadian Rhythms. Mar 23 2020;18:1. doi:10.5334/jcr.190. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7101004/pdf/jcr-18-190.pdf
- Yang L, Xu H, Chen Y, et al. Melatonin: Multi-Target Mechanism Against Diminished Ovarian Reserve Based on Network Pharmacology. Frontiers in endocrinology. 2021;12:630504. doi:10.3389/fendo.2021.630504. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8095380/pdf/fendo-12-630504.pdf
- Jiang Y, Shi H, Liu Y, Zhao S, Zhao H. Applications of Melatonin in Female Reproduction in the Context of Oxidative Stress. Oxid Med Cell Longev. 2021;2021:6668365. doi:10.1155/2021/6668365. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8342146/pdf/OMCL2021-6668365.pdf
- Mejlhede MAB, Jepsen JB, Knudsen UB. Oral melatonin supplementation during in vitro fertilization treatment: a systematic PRISMA review and meta-analysis of randomized controlled trials. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. Dec 2021;37(12):1079-1085. doi:10.1080/09513590.2021.1974378. https://www.tandfonline.com/doi/full/10.1080/09513590.2021.1974378
- Simon SL, McWhirter L, Diniz Behn C, et al. Morning Circadian Misalignment Is Associated With Insulin Resistance in Girls With Obesity and Polycystic Ovarian Syndrome. J Clin Endocrinol Metab. Aug 1 2019;104(8):3525-3534. doi:10.1210/jc.2018-02385. https://academic.oup.com/jcem/article/104/8/3525/5381916
- Lim AJR, Indran IR, Kramer MS, Yong EL. Phenotypic spectrum of polycystic ovary syndrome and their relationship to the circadian biomarkers, melatonin and cortisol. Endocrinol Diabetes Metab. Jul 2019;2(3):e00047. doi:10.1002/edm2.47. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6613235/pdf/EDM2-2-e00047.pdf
- Tagliaferri V, Romualdi D, Scarinci E, et al. Melatonin Treatment May Be Able to Restore Menstrual Cyclicity in Women With PCOS: A Pilot Study. Reprod Sci. Feb 2018;25(2):269-275. doi:10.1177/1933719117711262. https://journals.sagepub.com/doi/10.1177/1933719117711262
- Özcan P, Fıçıcıoğlu C, Kizilkale O, et al. Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. Sep 2016;33(9):1223-30. doi:10.1007/s10815-016-0751-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5010809/pdf/10815_2016_Article_751.pdf
- Ben-Meir A, Burstein E, Borrego-Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. Oct 2015;14(5):887-95. doi:10.1111/acel.12368. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4568976/pdf/acel0014-0887.pdf
- Zhang F, Dai J, Chen T. Role of Lactobacillus in Female Infertility Via Modulating Sperm Agglutination and Immobilization. Frontiers in cellular and infection microbiology. 2020;10:620529. doi:10.3389/fcimb.2020.620529. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7868545/pdf/fcimb-10-620529.pdf
- Mastromarino P, Hemalatha R, Barbonetti A, et al. Biological control of vaginosis to improve reproductive health. The Indian journal of medical research. Nov 2014;140 Suppl(Suppl 1):S91-7.
- Campisciano G, Florian F, D'Eustacchio A, et al. Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J Cell Physiol. Jul 2017;232(7):1681-1688. doi:10.1002/jcp.25806.
- López-Moreno A, Aguilera M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients. Mar 13 2020;12(3)doi:10.3390/nu12030757. https://mdpi-res.com/d_attachment/nutrients/nutrients-12-00757/article_deploy/nutrients-12-00757.pdf
- Montella R, Malfa P, Giuliano A, Brustia G, Coïsson JD, Arlorio M. Vaginal adhesion of Lactobacillus plantarum P17630 after probiotic food supplement oral administration: a preliminary in vivo study. Nutrafoods. 2013;12(2):35-42.
- Vladareanu R, Mihu D, Mitran M, et al. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): a randomized double-blind placebo-controlled study. European review for medical and pharmacological sciences. Jan 2018;22(1):262-267. doi:10.26355/eurrev_201801_14128. https://www.ncbi.nlm.nih.gov/pubmed/29364495
- Schenk M, Grumet L, Sternat J, Reinschissler N, Weiss G. Effect of probiotics on vaginal Ureaplasma parvum in women suffering from unexplained infertility. Reprod Biomed Online. Sep 2021;43(3):503-514. doi:10.1016/j.rbmo.2021.06.004. https://www.rbmojournal.com/article/S1472-6483(21)00288-1/pdf
- Kyono K, Hashimoto T, Kikuchi S, Nagai Y, Sakuraba Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod Med Biol. Jan 2019;18(1):72-82. doi:10.1002/rmb2.12250. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6332758/pdf/RMB2-18-72.pdf
- Fernández L, Castro I, Arroyo R, Alba C, Beltrán D, Rodríguez JM. Application of Ligilactobacillus salivarius CECT5713 to Achieve Term Pregnancies in Women with Repetitive Abortion or Infertility of Unknown Origin by Microbiological and Immunological Modulation of the Vaginal Ecosystem. Nutrients. Jan 6 2021;13(1)doi:10.3390/nu13010162. https://mdpi-res.com/d_attachment/nutrients/nutrients-13-00162/article_deploy/nutrients-13-00162-v2.pdf
- Cozzolino M, Vitagliano A, Pellegrini L, et al. Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. European journal of nutrition. Oct 2020;59(7):2841-2856. doi:10.1007/s00394-020-02233-0. https://link.springer.com/article/10.1007/s00394-020-02233-0
- Rani A, Sharma A. The genus Vitex: A review. Pharmacognosy reviews. Jul 2013;7(14):188-98. doi:10.4103/0973-7847.120522.
- Westphal LM, Polan ML, Trant AS, Mooney SB. A nutritional supplement for improving fertility in women: a pilot study. J Reprod Med. Apr 2004;49(4):289-93. https://www.ncbi.nlm.nih.gov/pubmed/15134155
- Westphal LM, Polan ML, Trant AS. Double-blind, placebo-controlled study of Fertilityblend: a nutritional supplement for improving fertility in women. Clinical and experimental obstetrics & gynecology. 2006;33(4):205-8. https://www.ncbi.nlm.nih.gov/pubmed/17211965
- Antoine E, Chirila S, Teodorescu C. A Patented Blend Consisting of a Combination of Vitex agnus-castus Extract, Lepidium meyenii (Maca) Extract and Active Folate, a Nutritional Supplement for Improving Fertility in Women. Maedica (Bucur). Sep 2019;14(3):274-279. doi:10.26574/maedica.2019.14.3.274. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6861720/pdf/maedica-14-274.pdf
- Appleton J. Arginine: Clinical potential of a semi-essential amino acid. Alternative medicine review : a journal of clinical therapeutic. Dec 2002;7(6):512-22. https://www.ncbi.nlm.nih.gov/pubmed/12495375
- Cieri-Hutcherson NE, Jaenecke A, Bahia A, et al. Systematic Review of l-Arginine for the Treatment of Hypoactive Sexual Desire Disorder and Related Conditions in Women. Pharmacy (Basel). Mar 27 2021;9(2)doi:10.3390/pharmacy9020071. https://mdpi-res.com/d_attachment/pharmacy/pharmacy-09-00071/article_deploy/pharmacy-09-00071.pdf
- Battaglia C, Salvatori M, Maxia N, Petraglia F, Facchinetti F, Volpe A. Adjuvant L-arginine treatment for in-vitro fertilization in poor responder patients. Human reproduction (Oxford, England). Jul 1999;14(7):1690-7. doi:10.1093/humrep/14.7.1690.
- So S, Yamaguchi W, Murabayashi N, Miyano N, Tawara F, Kanayama N. Beneficial effect of l-arginine in women using assisted reproductive technologies: a small-scale randomized controlled trial. Nutr Res. Oct 2020;82:67-73. doi:10.1016/j.nutres.2020.08.008.
- Parcell S. Sulfur in human nutrition and applications in medicine. Alternative medicine review : a journal of clinical therapeutic. Feb 2002;7(1):22-44.
- Nemati M, Nemati S, Taheri AM, Heidari B. Comparison of metformin and N-acetyl cysteine, as an adjuvant to clomiphene citrate, in clomiphene-resistant women with polycystic ovary syndrome. J Gynecol Obstet Hum Reprod. Sep 2017;46(7):579-585. doi:10.1016/j.jogoh.2017.07.004.
- Mostajeran F, Tehrani HG, Rahbary B. N-Acetylcysteine as an Adjuvant to Letrozole for Induction of Ovulation in Infertile Patients with Polycystic Ovary Syndrome. Advanced biomedical research. 2018;7:100. doi:10.4103/abr.abr_157_17. https://www.ncbi.nlm.nih.gov/pubmed/30050888 https://www.advbiores.net/article.asp?issn=2277-9175;year=2018;volume=7;issue=1;spage=100;epage=100;aulast=Mostajeran https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6036781/pdf/ABR-7-100.pdf
- Cheraghi E, Soleimani Mehranjani M, Shariatzadeh SMA, Nasr Esfahani MH, Alani B. N-Acetylcysteine Compared to Metformin, Improves The Expression Profile of Growth Differentiation Factor-9 and Receptor Tyrosine Kinase c-Kit in The Oocytes of Patients with Polycystic Ovarian Syndrome. Int J Fertil Steril. Jan 2018;11(4):270-278. doi:10.22074/ijfs.2018.5142. https://www.ncbi.nlm.nih.gov/pubmed/29043702 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5641458/pdf/Int-J-Fertil-Steril-11-270.pdf
- Henmi H, Endo T, Kitajima Y, Manase K, Hata H, Kudo R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertility and sterility. Aug 2003;80(2):459-61. doi:10.1016/s0015-0282(03)00657-5. https://www.fertstert.org/article/S0015-0282(03)00657-5/pdf
- Prieto L, Quesada JF, Cambero O, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertility and sterility. Jul 2012;98(1):126-30. doi:10.1016/j.fertnstert.2012.03.052. https://www.fertstert.org/article/S0015-0282(12)00390-1/pdf
- Ruder EH, Hartman TJ, Reindollar RH, Goldman MB. Female dietary antioxidant intake and time to pregnancy among couples treated for unexplained infertility. Fertility and sterility. Mar 2014;101(3):759-66. doi:10.1016/j.fertnstert.2013.11.008. https://www.fertstert.org/article/S0015-0282(13)03261-5/pdf
- Amini L, Chekini R, Nateghi MR, et al. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res Manag. 2021;2021:5529741. doi:10.1155/2021/5529741. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8172324/pdf/PRM2021-5529741.pdf
- Lu X, Wu Z, Wang M, Cheng W. Effects of vitamin C on the outcome of in vitro fertilization-embryo transfer in endometriosis: A randomized controlled study. The Journal of international medical research. Nov 2018;46(11):4624-4633. doi:10.1177/0300060518786918.
- Mintziori G, Mousiolis A, Duntas LH, Goulis DG. Evidence for a manifold role of selenium in infertility. Hormones (Athens, Greece). Mar 2020;19(1):55-59. doi:10.1007/s42000-019-00140-6. https://www.ncbi.nlm.nih.gov/pubmed/31701489
- Mojadadi A, Au A, Salah W, Witting P, Ahmad G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients. Sep 18 2021;13(9)doi:10.3390/nu13093256. https://mdpi-res.com/d_attachment/nutrients/nutrients-13-03256/article_deploy/nutrients-13-03256-v2.pdf
- Maeda E, Murata K, Kumazawa Y, et al. Associations of environmental exposures to methylmercury and selenium with female infertility: A case-control study. Environ Res. Jan 2019;168:357-363. doi:10.1016/j.envres.2018.10.007.
- Grieger JA, Grzeskowiak LE, Wilson RL, et al. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients. Jul 16 2019;11(7)doi:10.3390/nu11071609. https://mdpi-res.com/d_attachment/nutrients/nutrients-11-01609/article_deploy/nutrients-11-01609.pdf
- Safiyeh FD, Mojgan M, Parviz S, Sakineh MA, Behnaz SO. The effect of selenium and vitamin E supplementation on anti-Mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: A randomized controlled clinical trial. Complementary therapies in medicine. Jan 2021;56:102533. doi:10.1016/j.ctim.2020.102533.
- Chu J, Gallos I, Tobias A, et al. Vitamin D and assisted reproductive treatment outcome: a prospective cohort study. Reprod Health. Jul 15 2019;16(1):106. doi:10.1186/s12978-019-0769-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6631833/pdf/12978_2019_Article_769.pdf
- Jukic AMZ, Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Steiner AZ. Pre-conception 25-hydroxyvitamin D (25(OH)D) and fecundability. Human reproduction (Oxford, England). Nov 1 2019;34(11):2163-2172. doi:10.1093/humrep/dez170. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7209776/pdf/dez170.pdf
- Jensen A, Nielsen ML, Guleria S, Kjaer SK, Heitmann BL, Kesmodel US. Chances of live birth after exposure to vitamin D-fortified margarine in women with fertility problems: results from a Danish population-based cohort study. Fertility and sterility. Feb 2020;113(2):383-391. doi:10.1016/j.fertnstert.2019.09.017. https://www.fertstert.org/article/S0015-0282(19)32371-4/pdf
- Jukic AMZ, Wilcox AJ, McConnaughey DR, Weinberg CR, Steiner AZ. 25-Hydroxyvitamin D and Long Menstrual Cycles in a Prospective Cohort Study. Epidemiology (Cambridge, Mass). May 2018;29(3):388-396. doi:10.1097/ede.0000000000000804. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5882585/pdf/nihms909897.pdf
- Butts SF, Seifer DB, Koelper N, et al. Vitamin D Deficiency Is Associated With Poor Ovarian Stimulation Outcome in PCOS but Not Unexplained Infertility. J Clin Endocrinol Metab. Feb 1 2019;104(2):369-378. doi:10.1210/jc.2018-00750.
- Karimi E, Arab A, Rafiee M, Amani R. A systematic review and meta-analysis of the association between vitamin D and ovarian reserve. Sci Rep. Aug 6 2021;11(1):16005. doi:10.1038/s41598-021-95481-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8346573/pdf/41598_2021_Article_95481.pdf
- Djurovic J, Stamenkovic G, Todorovic J, Aleksic N, Stojkovic O. Polymorphisms and haplotypes in VDR gene are associated with female idiopathic infertility. Hum Fertil (Camb). Jun 2020;23(2):101-110. doi:10.1080/14647273.2018.1515503. https://www.tandfonline.com/doi/full/10.1080/14647273.2018.1515503
- Naderi Z, Kashanian M, Chenari L, Sheikhansari N. Evaluating the effects of administration of 25-hydroxyvitamin D supplement on serum anti-mullerian hormone (AMH) levels in infertile women. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. May 2018;34(5):409-412. doi:10.1080/09513590.2017.1410785. https://www.tandfonline.com/doi/full/10.1080/09513590.2017.1410785
- Somigliana E, Sarais V, Reschini M, et al. Single oral dose of vitamin D(3) supplementation prior to in vitro fertilization and embryo transfer in normal weight women: the SUNDRO randomized controlled trial. Am J Obstet Gynecol. Sep 2021;225(3):283.e1-283.e10. doi:10.1016/j.ajog.2021.04.234. https://www.ajog.org/article/S0002-9378(21)00464-6/fulltext
- Doryanizadeh L, Morshed-Behbahani B, Parsanezhad ME, Dabbaghmanesh MH, Jokar A. Calcitriol Effect on Outcomes of in Vitro Fertilization in Infertile Women with Vitamin D Deficiency: A Double-Blind Randomized Clinical Trial. Z Geburtshilfe Neonatol. Jun 2021;225(3):226-231. doi:10.1055/a-1206-1064. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/a-1206-1064
- Moreira-Pinto B, Costa L, Felgueira E, Fonseca BM, Rebelo I. Low Doses of Resveratrol Protect Human Granulosa Cells from Induced-Oxidative Stress. Antioxidants (Basel, Switzerland). Apr 4 2021;10(4)doi:10.3390/antiox10040561. https://mdpi-res.com/d_attachment/antioxidants/antioxidants-10-00561/article_deploy/antioxidants-10-00561-v2.pdf
- Ragonese F, Monarca L, De Luca A, et al. Resveratrol depolarizes the membrane potential in human granulosa cells and promotes mitochondrial biogenesis. Fertility and sterility. Apr 2021;115(4):1063-1073. doi:10.1016/j.fertnstert.2020.08.016. https://www.fertstert.org/article/S0015-0282(20)30770-6/fulltext
- Atli M, Engin-Ustun Y, Tokmak A, Caydere M, Hucumenoglu S, Topcuoglu C. Dose dependent effect of resveratrol in preventing cisplatin-induced ovarian damage in rats: An experimental study. Reprod Biol. Sep 2017;17(3):274-280. doi:10.1016/j.repbio.2017.07.001.
- Khodarahmian M, Amidi F, Moini A, et al. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J Reprod Immunol. Feb 2021;143:103248. doi:10.1016/j.jri.2020.103248.
- Gerli S, Della Morte C, Ceccobelli M, et al. Biological and clinical effects of a resveratrol-based multivitamin supplement on intracytoplasmic sperm injection cycles: a single-center, randomized controlled trial. J Matern Fetal Neonatal Med. Aug 1 2021:1-9. doi:10.1080/14767058.2021.1958313. https://www.ncbi.nlm.nih.gov/pubmed/34338114
- Bahramrezaie M, Amidi F, Aleyasin A, et al. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: a triple-blind randomized clinical trial. J Assist Reprod Genet. Aug 2019;36(8):1701-1712. doi:10.1007/s10815-019-01461-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6708036/pdf/10815_2019_Article_1461.pdf
- Gaskins AJ, Nassan FL, Chiu YH, et al. Dietary patterns and outcomes of assisted reproduction. Am J Obstet Gynecol. Jun 2019;220(6):567.e1-567.e18. doi:10.1016/j.ajog.2019.02.004. https://www.ajog.org/article/S0002-9378(19)30345-X/fulltext
- Chiu YH, Chavarro JE, Souter I. Diet and female fertility: doctor, what should I eat? Fertility and sterility. Sep 2018;110(4):560-569. doi:10.1016/j.fertnstert.2018.05.027. https://www.fertstert.org/article/S0015-0282(18)30428-X/pdf
- Skoracka K, Ratajczak AE, Rychter AM, Dobrowolska A, Krela-Kaźmierczak I. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Adv Nutr. Jun 17 2021;doi:10.1093/advances/nmab068.
- La Vecchia I, Paffoni A, Castiglioni M, et al. Folate, homocysteine and selected vitamins and minerals status in infertile women. Eur J Contracept Reprod Health Care. Feb 2017;22(1):70-75. doi:10.1080/13625187.2016.1263292. https://www.tandfonline.com/doi/full/10.1080/13625187.2016.1263292
- Gaskins AJ, Chiu YH, Williams PL, et al. Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. Am J Clin Nutr. Oct 2015;102(4):943-50. doi:10.3945/ajcn.115.112185. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4588741/pdf/ajcn112185.pdf
- Cirillo M, Coccia ME, Attanasio M, Fatini C. Homocysteine, vitamin B status and MTHFR polymorphisms in Italian infertile women. European journal of obstetrics, gynecology, and reproductive biology. Aug 2021;263:72-78. doi:10.1016/j.ejogrb.2021.06.003. https://www.ejog.org/article/S0301-2115(21)00274-8/fulltext
- Enciso M, Sarasa J, Xanthopoulou L, et al. Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Human genetics. May 2016;135(5):555-568. doi:10.1007/s00439-016-1652-z. https://link.springer.com/content/pdf/10.1007/s00439-016-1652-z.pdf
- Murto T, Kallak TK, Hoas A, et al. Folic acid supplementation and methylenetetrahydrofolate reductase (MTHFR) gene variations in relation to in vitro fertilization pregnancy outcome. Acta obstetricia et gynecologica Scandinavica. Jan 2015;94(1):65-71. doi:10.1111/aogs.12522. https://obgyn.onlinelibrary.wiley.com/doi/pdfdirect/10.1111/aogs.12522?download=true
- Murto T, Skoog Svanberg A, Yngve A, et al. Folic acid supplementation and IVF pregnancy outcome in women with unexplained infertility. Reprod Biomed Online. Jun 2014;28(6):766-72. doi:10.1016/j.rbmo.2014.01.017. https://www.rbmojournal.com/article/S1472-6483(14)00073-X/pdf
- Schiuma N, Costantino A, Bartolotti T, et al. Micronutrients in support to the one carbon cycle for the modulation of blood fasting homocysteine in PCOS women. Journal of endocrinological investigation. Jun 2020;43(6):779-786. doi:10.1007/s40618-019-01163-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7230049/pdf/40618_2019_Article_1163.pdf
- Mínguez-Alarcón L, Williams PL, Souter I, et al. Hair mercury levels, intake of omega-3 fatty acids and ovarian reserve among women attending a fertility center. Int J Hyg Environ Health. Aug 2021;237:113825. doi:10.1016/j.ijheh.2021.113825.
- Nehra D, Le HD, Fallon EM, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. Dec 2012;11(6):1046-54. doi:10.1111/acel.12006. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/acel.12006?download=true
- Bauer JL, Kuhn K, Bradford AP, et al. Reduction in FSH Throughout the Menstrual Cycle After Omega-3 Fatty Acid Supplementation in Young Normal Weight but not Obese Women. Reprod Sci. Aug 2019;26(8):1025-1033. doi:10.1177/1933719119828099. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6974596/pdf/10.1177_1933719119828099.pdf
- Gaskins AJ, Sundaram R, Buck Louis GM, Chavarro JE. Seafood Intake, Sexual Activity, and Time to Pregnancy. J Clin Endocrinol Metab. Jul 1 2018;103(7):2680-2688. doi:10.1210/jc.2018-00385.
- Wise LA, Wesselink AK, Tucker KL, et al. Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. American journal of epidemiology. Jan 1 2018;187(1):60-74. doi:10.1093/aje/kwx204. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5860620/pdf/kwx204.pdf
- Wise LA, Willis SK, Mikkelsen EM, et al. The Association between Seafood Intake and Fecundability: Analysis from Two Prospective Studies. Nutrients. Jul 29 2020;12(8)doi:10.3390/nu12082276. https://mdpi-res.com/d_attachment/nutrients/nutrients-12-02276/article_deploy/nutrients-12-02276-v2.pdf
- Stanhiser J, Jukic AMZ, Steiner AZ. Serum omega-3 and omega-6 fatty acid concentrations and natural fertility. Human reproduction (Oxford, England). Apr 28 2020;35(4):950-957. doi:10.1093/humrep/dez305. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8411851/pdf/dez305.pdf
- Mumford SL, Browne RW, Kim K, et al. Preconception Plasma Phospholipid Fatty Acids and Fecundability. J Clin Endocrinol Metab. Dec 1 2018;103(12):4501-4510. doi:10.1210/jc.2018-00448.
- Zarezadeh R, Mehdizadeh A, Leroy J, Nouri M, Fayezi S, Darabi M. Action mechanisms of n-3 polyunsaturated fatty acids on the oocyte maturation and developmental competence: Potential advantages and disadvantages. J Cell Physiol. Feb 2019;234(2):1016-1029. doi:10.1002/jcp.27101. https://onlinelibrary.wiley.com/doi/10.1002/jcp.27101
- Lass A, Belluzzi A. Omega-3 polyunsaturated fatty acids and IVF treatment. Reprod Biomed Online. Jan 2019;38(1):95-99. doi:10.1016/j.rbmo.2018.10.008. https://www.rbmojournal.com/article/S1472-6483(18)30586-8/pdf
- Eskew AM, Wormer KC, Matthews ML, Norton HJ, Papadakis MA, Hurst BS. The association between fatty acid index and in vitro fertilization outcomes. J Assist Reprod Genet. Dec 2017;34(12):1627-1632. doi:10.1007/s10815-017-1032-1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5714824/pdf/10815_2017_Article_1032.pdf
- Llata E, Cuffe KM, Picchetti V, Braxton JR, Torrone EA. Demographic, Behavioral, and Clinical Characteristics of Persons Seeking Care at Sexually Transmitted Disease Clinics - 14 Sites, STD Surveillance Network, United States, 2010-2018. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). Nov 5 2021;70(7):1-20. doi:10.15585/mmwr.ss7007a1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8575410/pdf/ss7007a1.pdf
- Hunter E, Avenell A, Maheshwari A, Stadler G, Best D. The effectiveness of weight-loss lifestyle interventions for improving fertility in women and men with overweight or obesity and infertility: A systematic review update of evidence from randomized controlled trials. Obes Rev. Aug 13 2021:e13325. doi:10.1111/obr.13325. https://www.ncbi.nlm.nih.gov/pubmed/34390109
- Boutari C, Pappas PD, Mintziori G, et al. The effect of underweight on female and male reproduction. Metabolism: clinical and experimental. Jun 2020;107:154229. doi:10.1016/j.metabol.2020.154229. https://www.metabolismjournal.com/article/S0026-0495(20)30093-7/fulltext
- Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Human reproduction (Oxford, England). Sep 2003;18(9):1928-32. doi:10.1093/humrep/deg367.
- Beroukhim G, Esencan E, Seifer DB. Impact of sleep patterns upon female neuroendocrinology and reproductive outcomes: a comprehensive review. Reprod Biol Endocrinol. Jan 18 2022;20(1):16. doi:10.1186/s12958-022-00889-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8764829/pdf/12958_2022_Article_889.pdf
- Palomba S, Daolio J, Romeo S, Battaglia FA, Marci R, La Sala GB. Lifestyle and fertility: the influence of stress and quality of life on female fertility. Reproductive Biology and Endocrinology. 2018/12/02 2018;16(1):113. doi:10.1186/s12958-018-0434-y. https://doi.org/10.1186/s12958-018-0434-y https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6275085/pdf/12958_2018_Article_434.pdf
- Ahn SH, Lee I, Cho S, et al. Predictive Factors of Conception and the Cumulative Pregnancy Rate in Subfertile Couples Undergoing Timed Intercourse With Ultrasound. Original Research. Frontiers in endocrinology. 2021-April-15 2021;12(363)doi:10.3389/fendo.2021.650883. https://www.frontiersin.org/article/10.3389/fendo.2021.650883
- Manders M, McLindon L, Schulze B, Beckmann MM, Kremer JA, Farquhar C. Timed intercourse for couples trying to conceive. The Cochrane database of systematic reviews. Mar 17 2015;(3):Cd011345. doi:10.1002/14651858.CD011345.pub2.
- Patel A, Sharma P, Kumar P. "When Love Does not bear a Fruit": Patterns and Prevalence of Sexual Difficulties in Infertile Men and Women as Predictors of Emotional Distress. J Hum Reprod Sci. Jul-Sep 2021;14(3):307-312. doi:10.4103/jhrs.jhrs_70_21. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8527079/pdf/JHRS-14-307.pdf
- Byun JS, Lyu SW, Seok HH, Kim WJ, Shim SH, Bak CW. Sexual dysfunctions induced by stress of timed intercourse and medical treatment. BJU Int. Apr 2013;111(4 Pt B):E227-34. doi:10.1111/j.1464-410X.2012.11577.x.
- Grieger JA. Preconception diet, fertility, and later health in pregnancy. Curr Opin Obstet Gynecol. Jun 2020;32(3):227-232. doi:10.1097/gco.0000000000000629.
- Hoek J, Schoenmakers S, Baart EB, et al. Preconceptional Maternal Vegetable Intake and Paternal Smoking Are Associated with Pre-implantation Embryo Quality. Reprod Sci. Nov 2020;27(11):2018-2028. doi:10.1007/s43032-020-00220-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7522074/pdf/43032_2020_Article_220.pdf
- Nassan FL, Chiu YH, Vanegas JC, et al. Intake of protein-rich foods in relation to outcomes of infertility treatment with assisted reproductive technologies. Am J Clin Nutr. Nov 1 2018;108(5):1104-1112. doi:10.1093/ajcn/nqy185. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6692709/pdf/nqy185.pdf
- U. S. FDA. Advice about Eating Fish For Those Who Might Become or Are Pregnant or Breastfeeding and Children Ages 1 - 11 Years. Updated Jun. 8, 2022. Accessed Jun. 13, 2022, https://www.fda.gov/food/consumers/advice-about-eating-fish#:~:text=The%20Dietary%20Guidelines%20for%20Americans%20states%20that%20to%20consume%20those,%2C%20plaice%2C%20pollock%2C%20salmon%2C
- Vanegas JC, Afeiche MC, Gaskins AJ, et al. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertility and sterility. Mar 2015;103(3):749-55.e2. doi:10.1016/j.fertnstert.2014.12.104. https://www.fertstert.org/article/S0015-0282(14)02529-1/pdf
- Chavarro JE, Mínguez-Alarcón L, Chiu YH, et al. Soy Intake Modifies the Relation Between Urinary Bisphenol A Concentrations and Pregnancy Outcomes Among Women Undergoing Assisted Reproduction. J Clin Endocrinol Metab. Mar 2016;101(3):1082-90. doi:10.1210/jc.2015-3473.
- Rushing JS, Santoro N. Fertility Issues in Polycystic Ovarian Disease: A Systematic Approach. Endocrinology and metabolism clinics of North America. Mar 2021;50(1):43-55. doi:10.1016/j.ecl.2020.10.004. https://www.sciencedirect.com/science/article/abs/pii/S0889852920300815?via%3Dihub
- Kulkarni AD, Jamieson DJ, Jones HW, Jr., et al. Fertility treatments and multiple births in the United States. The New England journal of medicine. Dec 5 2013;369(23):2218-25. doi:10.1056/NEJMoa1301467. https://www.nejm.org/doi/pdf/10.1056/NEJMoa1301467?articleTools=true
- Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Human reproduction update. Nov 2016;22(6):687-708. doi:10.1093/humupd/dmw025. https://www.ncbi.nlm.nih.gov/pubmed/27511809
- Timmons D, Montrief T, Koyfman A, Long B. Ovarian hyperstimulation syndrome: A review for emergency clinicians. The American journal of emergency medicine. Aug 2019;37(8):1577-1584. doi:10.1016/j.ajem.2019.05.018.
- Fauser BC, Barbieri RL, Martin KA. UpToDate: Infertility treatment with gonadotropins (Beyond the Basics). Available at https://www.uptodate.com/contents/infertility-treatment-with-gonadotropins-beyond-the-basics . Last reviewed 11/2021. Accessed 12/14/2021. 2021;
- Sharma M, Balasundaram P. Ovulation Induction Techniques. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Costello M, Garad R, Hart R, et al. A Review of First Line Infertility Treatments and Supporting Evidence in Women with Polycystic Ovary Syndrome. Med Sci (Basel). Sep 10 2019;7(9)doi:10.3390/medsci7090095. https://www.ncbi.nlm.nih.gov/pubmed/31510088 https://mdpi-res.com/d_attachment/medsci/medsci-07-00095/article_deploy/medsci-07-00095.pdf
- Hu S, Yu Q, Wang Y, Wang M, Xia W, Zhu C. Letrozole versus clomiphene citrate in polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Archives of gynecology and obstetrics. May 2018;297(5):1081-1088. doi:10.1007/s00404-018-4688-6. https://www.ncbi.nlm.nih.gov/pubmed/29392438 https://link.springer.com/article/10.1007%2Fs00404-018-4688-6
- Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. The Cochrane database of systematic reviews. May 24 2018;5(5):Cd010287. doi:10.1002/14651858.CD010287.pub3.
- Tsiami AP, Goulis DG, Sotiriadis AI, Kolibianakis EM. Higher ovulation rate with letrozole as compared with clomiphene citrate in infertile women with polycystic ovary syndrome: a systematic review and meta-analysis. Hormones (Athens, Greece). May 25 2021;doi:10.1007/s42000-021-00289-z.
- Weiss NS, Kostova E, Nahuis M, Mol BWJ, van der Veen F, van Wely M. Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. The Cochrane database of systematic reviews. Jan 16 2019;1:CD010290. doi:10.1002/14651858.CD010290.pub3. https://www.ncbi.nlm.nih.gov/pubmed/30648738 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6353048/pdf/CD010290.pdf
- Quaas AM, Hansen KR. The role of steroid hormone supplementation in non-assisted reproductive technology treatments for unexplained infertility. Fertility and sterility. Dec 2016;106(7):1600-1607. doi:10.1016/j.fertnstert.2016.09.012. https://www.fertstert.org/article/S0015-0282(16)62802-9/pdf
- Dashti S, Eftekhar M. Luteal-phase support in assisted reproductive technology: An ongoing challenge. Int J Reprod Biomed. Sep 2021;19(9):761-772. doi:10.18502/ijrm.v19i9.9708. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8548747/pdf/ijrb-19-761.pdf
- Mitra S, Nayak PK, Agrawal S. Laparoscopic ovarian drilling: An alternative but not the ultimate in the management of polycystic ovary syndrome. Journal of natural science, biology, and medicine. Jan-Jun 2015;6(1):40-8. doi:10.4103/0976-9668.149076. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4367066/pdf/JNSBM-6-40.pdf
- Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol. Sep 2017;217(3):270-281. doi:10.1016/j.ajog.2017.03.012.
- Choe J, Archer JS, Shanks AL. In Vitro Fertilization. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- Yang H, Kuhn C, Kolben T, et al. Early Life Oxidative Stress and Long-Lasting Cardiovascular Effects on Offspring Conceived by Assisted Reproductive Technologies: A Review. International journal of molecular sciences. Jul 22 2020;21(15)doi:10.3390/ijms21155175. https://mdpi-res.com/d_attachment/ijms/ijms-21-05175/article_deploy/ijms-21-05175.pdf
- Zhang L, Zhang W, Xu H, Liu K. Birth defects surveillance after assisted reproductive technology in Beijing: a whole of population-based cohort study. BMJ open. Jun 23 2021;11(6):e044385. doi:10.1136/bmjopen-2020-044385. https://www.ncbi.nlm.nih.gov/pubmed/34162637 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8231031/pdf/bmjopen-2020-044385.pdf
- Luke B, Brown MB, Wantman E, et al. The risk of birth defects with conception by ART. Human reproduction (Oxford, England). Jan 1 2021;36(1):116-129. doi:10.1093/humrep/deaa272. https://www.ncbi.nlm.nih.gov/pubmed/33251542
- Lu YH, Wang N, Jin F. Long-term follow-up of children conceived through assisted reproductive technology. Journal of Zhejiang University Science B. May 2013;14(5):359-71. doi:10.1631/jzus.B1200348. https://www.ncbi.nlm.nih.gov/pubmed/23645173 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3650450/pdf/JZUSB14-0359.pdf
- Paraskevi L, Antigoni S, Kleanthi G. Stress and Anxiety Levels in Couples who Undergo Fertility Treatment: a Review of Systematic Reviews. Mater Sociomed. Mar 2021;33(1):60-64. doi:10.5455/msm.2021.33.60-64. https://www.ncbi.nlm.nih.gov/pubmed/34012353 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8116083/pdf/MSM-33-60.pdf
- Frederiksen Y, Farver-Vestergaard I, Skovgård NG, Ingerslev HJ, Zachariae R. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ open. Jan 28 2015;5(1):e006592. doi:10.1136/bmjopen-2014-006592. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4316425/pdf/bmjopen-2014-006592.pdf
- Ayeleke RO, Asseler JD, Cohlen BJ, Veltman-Verhulst SM. Intra-uterine insemination for unexplained subfertility. The Cochrane database of systematic reviews. Mar 3 2020;3(3):CD001838. doi:10.1002/14651858.CD001838.pub6. https://www.ncbi.nlm.nih.gov/pubmed/32124980 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7059962/pdf/CD001838.pdf
- Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: a review. Fertil Res Pract. Dec 11 2020;6(1):23. doi:10.1186/s40738-020-00092-1. https://www.ncbi.nlm.nih.gov/pubmed/33308319 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7731622/pdf/40738_2020_Article_92.pdf
- Bosch E, Espinós JJ, Fabregues F, et al. ALWAYS ICSI? A SWOT analysis. J Assist Reprod Genet. Sep 2020;37(9):2081-2092. doi:10.1007/s10815-020-01836-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7492350/pdf/10815_2020_Article_1836.pdf
- Haddad M, Stewart J, Xie P, et al. Thoughts on the popularity of ICSI. J Assist Reprod Genet. Jan 2021;38(1):101-123. doi:10.1007/s10815-020-01987-0. https://www.ncbi.nlm.nih.gov/pubmed/33155089 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7823003/pdf/10815_2020_Article_1987.pdf
- Ambar RF, Agarwal A, Majzoub A, et al. The Use of Testicular Sperm for Intracytoplasmic Sperm Injection in Patients with High Sperm DNA Damage: A Systematic Review. World J Mens Health. Jul 2021;39(3):391-398. doi:10.5534/wjmh.200084. https://www.ncbi.nlm.nih.gov/pubmed/32648379 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8255394/pdf/wjmh-39-391.pdf
- Esfandyari S, Chugh RM, Park HS, Hobeika E, Ulin M, Al-Hendy A. Mesenchymal Stem Cells as a Bio Organ for Treatment of Female Infertility. Cells. Oct 8 2020;9(10)doi:10.3390/cells9102253. https://www.ncbi.nlm.nih.gov/pubmed/33050021
- Zhao YX, Chen SR, Su PP, et al. Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases. Stem cells international. 2019;2019:9071720. doi:10.1155/2019/9071720. https://www.ncbi.nlm.nih.gov/pubmed/31885630
- Ulin M, Cetin E, Hobeika E, et al. Human Mesenchymal Stem Cell Therapy and Other Novel Treatment Approaches for Premature Ovarian Insufficiency. Reprod Sci. Jun 2021;28(6):1688-1696. doi:10.1007/s43032-021-00528-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8144118/pdf/43032_2021_Article_528.pdf
- Lv Q, Wang L, Luo X, Chen X. Adult stem cells in endometrial regeneration: Molecular insights and clinical applications. Mol Reprod Dev. Jun 2021;88(6):379-394. doi:10.1002/mrd.23476. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8362170/pdf/MRD-88-379.pdf
- Chen JM, Huang QY, Zhao YX, Chen WH, Lin S, Shi QY. The Latest Developments in Immunomodulation of Mesenchymal Stem Cells in the Treatment of Intrauterine Adhesions, Both Allogeneic and Autologous. Front Immunol. 2021;12:785717. doi:10.3389/fimmu.2021.785717. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8634714/pdf/fimmu-12-785717.pdf
- Mashayekhi M, Mirzadeh E, Chekini Z, et al. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: non-randomized clinical trial, phase I, first in human. Journal of ovarian research. Jan 6 2021;14(1):5. doi:10.1186/s13048-020-00743-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7786909/pdf/13048_2020_Article_743.pdf
- Ding L, Yan G, Wang B, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Science China Life sciences. Dec 2018;61(12):1554-1565. doi:10.1007/s11427-017-9272-2. https://link.springer.com/content/pdf/10.1007/s11427-017-9272-2.pdf
- Cao Y, Sun H, Zhu H, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. Jul 11 2018;9(1):192. doi:10.1186/s13287-018-0904-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6042450/pdf/13287_2018_Article_904.pdf
- Zafardoust S, Kazemnejad S, Darzi M, Fathi-Kazerooni M, Rastegari H, Mohammadzadeh A. Improvement of Pregnancy Rate and Live Birth Rate in Poor Ovarian Responders by Intraovarian Administration of Autologous Menstrual Blood Derived- Mesenchymal Stromal Cells: Phase I/II Clinical Trial. Stem Cell Rev Rep. Aug 2020;16(4):755-763. doi:10.1007/s12015-020-09969-6.
- Jaffe J. Reproductive trauma: Psychotherapy for pregnancy loss and infertility clients from a reproductive story perspective. Psychotherapy (Chic). Dec 2017;54(4):380-385. doi:10.1037/pst0000125.
- Taebi M, Kariman N, Montazeri A, Alavi Majd H. Infertility Stigma: A Qualitative Study on Feelings and Experiences of Infertile Women. Int J Fertil Steril. Jul 2021;15(3):189-196. doi:10.22074/ijfs.2021.139093.1039.
- Luca G, Parrettini S, Sansone A, Calafiore R, Jannini EA. The Inferto-Sex Syndrome (ISS): sexual dysfunction in fertility care setting and assisted reproduction. Journal of endocrinological investigation. Oct 2021;44(10):2071-2102. doi:10.1007/s40618-021-01581-w. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8421318/pdf/40618_2021_Article_1581.pdf
- Bhat A, Byatt N. Infertility and Perinatal Loss: When the Bough Breaks. Curr Psychiatry Rep. Mar 2016;18(3):31. doi:10.1007/s11920-016-0663-8. https://link.springer.com/content/pdf/10.1007/s11920-016-0663-8.pdf
- Gameiro S, Finnigan A. Long-term adjustment to unmet parenthood goals following ART: a systematic review and meta-analysis. Human reproduction update. May 1 2017;23(3):322-337. doi:10.1093/humupd/dmx001.
- Jhuang YH, Chung CH, Wang ID, et al. Association of Obstructive Sleep Apnea With the Risk of Male Infertility in Taiwan. JAMA Netw Open. Jan 4 2021;4(1):e2031846. doi:10.1001/jamanetworkopen.2020.31846. https://www.ncbi.nlm.nih.gov/pubmed/33475753
- Lim ZW, Wang ID, Wang P, et al. Obstructive sleep apnea increases risk of female infertility: A 14-year nationwide population-based study. PLoS One. 2021;16(12):e0260842. doi:10.1371/journal.pone.0260842. https://www.ncbi.nlm.nih.gov/pubmed/34910749
- Wang ID, Tsai P-Y, Peng C-K, et al. Association of obstructive sleep apnea with female infertility - A 13-year nationwide population-based retrospective study. European Respiratory Journal. 2019;54(suppl 63):PA901. doi:10.1183/13993003.congress-2019.PA901. http://erj.ersjournals.com/content/54/suppl_63/PA901.abstract
- Bui LM, Bazalakova M, Antony KM, Cooney LG. Obstructive Sleep Apnea: Another Condition to Screen for in Women with Infertility. Women. 2022;2(1):56-63. https://www.mdpi.com/2673-4184/2/1/6
- Ibrahim S, Mehra R, Tantibhedhyangkul J, Bena J, Flyckt RL. Sleep and obstructive sleep apnea in women with infertility. Sleep & breathing = Schlaf & Atmung. Jan 7 2023;doi:10.1007/s11325-022-02770-4. https://pubmed.ncbi.nlm.nih.gov/36609819/
- Shrivastava S, Conigliaro RL. Polycystic Ovarian Syndrome. The Medical clinics of North America. Mar 2023;107(2):227-234. doi:10.1016/j.mcna.2022.10.004. https://pubmed.ncbi.nlm.nih.gov/36759093/
- Alemany M. The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health. Int J Mol Sci. Oct 8 2022;23(19)doi:10.3390/ijms231911952. https://www.ncbi.nlm.nih.gov/pubmed/36233256
- Papadopoulou-Marketou N, Kassi E, Chrousos GP. Adrenal Androgens and Aging. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext . MDText.com, Inc. Copyright © 2000-2023, MDText.com, Inc.; 2000.
- Wang J, Pan X, Zhou J, Li X, Sun Y, Wang L. Advances in understanding the effect and mechanism of dehydroepiandrosterone on diminished ovarian reserve. Drug Discov Ther. May 15 2023;17(2):87-94. doi:10.5582/ddt.2022.01109. https://www.ncbi.nlm.nih.gov/pubmed/37019659
- Naredi N, Sandeep K, Jamwal VD, Nagraj N, Rai S. Dehydroepiandrosterone: A panacea for the ageing ovary? Med J Armed Forces India . Jul 2015;71(3):274-7. doi:10.1016/j.mjafi.2014.12.022. https://www.ncbi.nlm.nih.gov/pubmed/26288496
- Glachant S, Salle B, Langlois-Jacques C, et al. [Predictive factors of spontaneous pregnancies among women with diminished ovarian reserve patients treated with DHEA]. Gynecol Obstet Fertil Senol. Sep 2023;51(9):400-407. Facteurs predictifs de grossesse spontanee chez les femmes presentant une reserve ovarienne diminuee traitees par DHEA. doi:10.1016/j.gofs.2023.06.001. https://www.ncbi.nlm.nih.gov/pubmed/37331511
- Zhang M, Niu W, Wang Y, et al. Dehydroepiandrosterone treatment in women with poor ovarian response undergoing IVF or ICSI: a systematic review and meta-analysis. J Assist Reprod Genet. Aug 2016;33(8):981-91. doi:10.1007/s10815-016-0713-5. https://www.ncbi.nlm.nih.gov/pubmed/27094195
- Schwarze JE, Canales J, Crosby J, Ortega-Hrepich C, Villa S, Pommer R. DHEA use to improve likelihood of IVF/ICSI success in patients with diminished ovarian reserve: A systematic review and meta-analysis. JBRA Assist Reprod. Nov 1 2018;22(4):369-374. doi:10.5935/1518-0557.20180046. https://www.ncbi.nlm.nih.gov/pubmed/30125071
- Zhang Y, Zhang C, Shu J, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Human reproduction update. Feb 28 2020;26(2):247-263. doi:10.1093/humupd/dmz046. https://www.ncbi.nlm.nih.gov/pubmed/32045470
- Benjamin JJ, K M, Koshy T, K NM, R P. DHEA and polycystic ovarian syndrome: Meta-analysis of case-control studies. PLoS One. 2021;16(12):e0261552. doi:10.1371/journal.pone.0261552. https://www.ncbi.nlm.nih.gov/pubmed/34932604
- Christodoulaki C, Trakakis E, Pergialiotis V, et al. Dehydroepiandrosterone-Sulfate, Insulin Resistance and Ovarian Volume Estimation in Patients With Polycystic Ovarian Syndrome. J Family Reprod Health . Mar 2017;11(1):24-29. https://pubmed.ncbi.nlm.nih.gov/29114265/
- Papadakis G, Kandaraki EA, Tseniklidi E, Papalou O, Diamanti-Kandarakis E. Polycystic Ovary Syndrome and NC-CAH: Distinct Characteristics and Common Findings. A Systematic Review. Frontiers in endocrinology. 2019;10:388. doi:10.3389/fendo.2019.00388. https://pubmed.ncbi.nlm.nih.gov/31275245/
- New MI, Ghizzoni L, Meyer-Bahlburg H, Khattab A, Reichman D, Rosenwaks Z. Fertility in patients with nonclassical congenital adrenal hyperplasia. Fertility and sterility. 2019;111(1):13-20. doi:10.1016/j.fertnstert.2018.11.023. https://doi.org/10.1016/j.fertnstert.2018.11.023. https://pubmed.ncbi.nlm.nih.gov/30611403/
- US Department of Health and Human Services. Eunice Kennedy Shriver National Institute of Child Health and Human Development. How common is infertility? 2/8/2018. Accessed 2/26/2025. https://www.nichd.nih.gov/health/topics/infertility/conditioninfo/common
- Pinkerton JV. Polycystic Ovary Syndrome (PCOS). (Hyperandrogenic Chronic Anovulation; Stein-Leventhal Syndrome). Merck Manual: Professional Version. Last updated 1/2023. Accessed 2/26/2025. https://www.merckmanuals.com/professional/gynecology-and-obstetrics/menstrual-abnormalities/polycystic-ovary-syndrome-pcos
- Barbieri RL, Ehrmann DA. Diagnosis of polycystic ovary syndrome in adults. UpToDate. Last updated 11/21/2024. Accessed 2/26/2025. https://www.uptodate.com/contents/diagnosis-of-polycystic-ovary-syndrome-in-adults
- Hornstein MD, Young SL. Endometriosis: Treatment of infertility in females. UpToDate. Last updated 11/22/2024. Accessed 2/27/2025. https://www.uptodate.com/contents/endometriosis-treatment-of-infertility-in-females
- Brady PC and Barbieri R. Female Infertility. In: Yen & Jaffe’s Reproductive Endocrinology. Ch. 23: pp. 548 – 574.e9.
