
Homocysteine Reduction
Homocysteine Reduction
Last Section Update: 02/2026
Contributor(s): Maureen Williams, ND; Colleen Mazin, MS/MPH; Stephen Tapanes, PhD
Table of Contents
1 Overview
Summary and Quick Facts for Homocysteine Reduction
- Elevated homocysteine levels in the bloodstream have been linked with a wide range of health problems.
- A high-protein diet, especially one that includes red meat and dairy products, can increase blood levels of homocysteine.
- If you have or are at risk for high homocysteine, the lifestyle strategies and homocysteine-lowering nutrients discussed in this protocol may help you achieve and maintain healthy homocysteine levels.
- Supplementation with B-vitamins, including folate, vitamin B6 and B12 have been shown in numerous studies to help lower homocysteine levels.
Homocysteine is an amino acid made from a common dietary amino acid, methionine, that inflicts damage to the inner arterial lining (endothelium). Elevated levels of homocysteine have been associated with many diseases, including:
- cardiovascular disease
- congestive heart failure
- stroke
- migraines
- age-related macular degeneration
- hearing loss
- brain atrophy
- Alzheimer disease
Fortunately, B vitamins like folate, vitamins B6 and B12, and other integrative interventions can reduce homocysteine and counteract this destructive process.
Causes of High Homocysteine Levels (Hyperhomocysteinemia)
Many factors contribute to high homocysteine levels:
- Insufficient folate, vitamin B6, vitamin B12, betaine, vitamin B2, and magnesium
- Certain prescription drugs (including cholestyramine, colestipol, fenofibrate, levodopa, metformin, methotrexate, high-dose niacin, nitrous oxide, pemetrexed, phenytoin, sulfasalazine)
- High-methionine diet (including red meat and dairy products)
- Smoking
- High coffee consumption
- Alcohol consumption
- Advancing age
- Obesity
- Genetic variant that causes an impaired ability to metabolize active folate from folic acid
Note: Life Extension believes that the optimal range for homocysteine levels is <8 µmol/L, much lower than the currently accepted <15 µmol/L.
Dietary and Lifestyle Changes
Several dietary and lifestyle changes can help reduce chronic inflammation:
- Avoid methionine-rich foods like red meat and dairy products
- Exercise, as patients in a cardiac rehabilitation program showed a reduction in homocysteine from exercise alone
- Decrease or eliminate alcohol and smoking
Integrative Interventions
- B vitamins: Folate, along with vitamins B6 and B12, has been shown in numerous studies to help lower homocysteine levels. The active form of folate, L-methylfolate, can achieve plasma folate levels up to 700% higher than synthetic folic acid and therefore may be more effective at lowering homocysteine levels.
- Betaine (TMG) and choline: Higher intakes of TMG and choline (which is converted to TMG in the body) are related to lower circulating homocysteine concentrations.
- N-acetylcysteine (NAC): NAC may displace homocysteine from its protein carrier, which lowers homocysteine and promotes the formation of cysteine and glutathione, a powerful antioxidant.
- S-adenosylmethionine (SAMe): Supplementing with SAMe promotes the conversion of homocysteine to cysteine, which is then converted to glutathione and lowers homocysteine levels.
- Taurine: Research suggests taurine can block methionine absorption (which is converted to homocysteine in the body) and produce a significant decline in homocysteine levels in 4 weeks.
2 Introduction
Homocysteine is an amino acid made in the body through metabolism of the essential amino acid methionine. In healthy circumstances, homocysteine is rapidly broken down, but genetic factors, nutritional inadequacies, certain medications, and some medical conditions can lead to excess homocysteine accumulation, which can damage blood vessels.1 High homocysteine levels have been correlated with a range of health problems, including atherosclerosis, stroke, neurological diseases, diabetes complications, osteoporosis, depression, erectile dysfunction, and pregnancy complications.2,3 Although the degree of causality attributable to homocysteine in these conditions is debated, maintaining a healthy homocysteine level is an important part of an overall healthy lifestyle. This is especially true in the context of cardiovascular and neurological health.3
Adequate intake of the B vitamins folate (B9), cobalamin (B12), pyridoxine (B6), and riboflavin (B2) helps facilitate healthy breakdown of homocysteine.1 People with a genetic propensity for higher homocysteine levels may especially benefit from supplementing with B2, folate, B6, and B12.4 Omega-3 fatty acids may complement B vitamins in encouraging efficient metabolism of homocysteine.5 Betaine (also known as trimethylglycine, or TMG), magnesium, and the trace mineral lithium are also involved in maintaining homocysteine balance.6
In this protocol you will learn about methionine metabolism, factors that influence homocysteine regulation, and mechanisms by which excess homocysteine in the blood can cause harm. You will also learn the importance of monitoring homocysteine levels and effective methods for lowering high levels and protecting your long-term health.
3 Homocysteine Metabolism
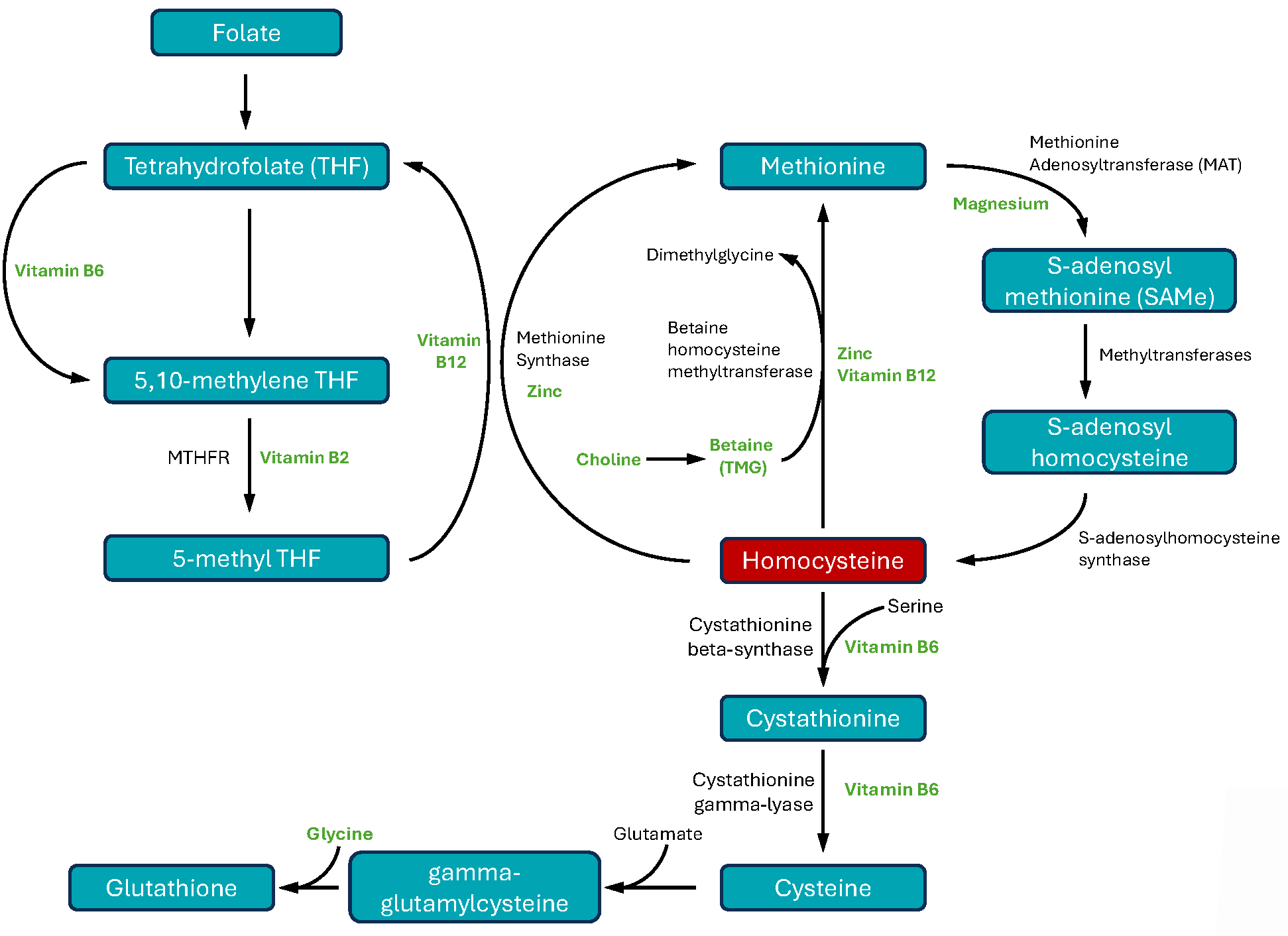
Although it is an amino acid, homocysteine is not derived from the diet. Instead, it is made inside cells from the essential dietary amino acid methionine.7 This process includes three steps:
- Methionine to SAMe. Methionine is first converted into S-adenosylmethionine (SAMe) by adding a chemical group called an adenosyl group. SAMe is an important cellular methyl donor: it contains a methyl group that can be transferred to other molecules through methylation reactions. Methylation is critical in biosynthetic processes such as synthesis of DNA, RNA, and many amino acids, proteins, and phospholipids. Methylation is also a critical mechanism for modifying the structure of chromatin, which forms the backbone of deoxyribonucleic acid (DNA)—and which in turn determines how genetic material is expressed. This type of change that regulates gene expression, without changing the genetic code itself, is called epigenetics.
- SAMe to S-adenosylhomocysteine. When SAMe gives up its methyl group, the result is S-adenosylhomocysteine.
- S-adenosylhomocysteine to homocysteine. Through removal of the adenosyl group, S-adenosylhomocysteine is converted to homocysteine.
The Fate of Homocysteine
About half of the homocysteine generated in cells is re-methylated, turning it back into methionine.8 In most of the body’s cells, this occurs via a folate-dependent pathway, in which a methylated form of folate (5-methyltetrahydofolate, or 5-MTHF) transfers its methyl group to homocysteine. The movement of a methyl group onto and off of folate involves vitamins B6, B2, and B12, as well as an important enzyme called methylenetetrahydrofolate reductase, or MTHFR.1,6,8
Homocysteine can also be re-methylated via a folate-independent pathway, in which betaine (also known as trimethylglycine, or TMG) donates the methyl group to homocysteine. This occurs mainly in liver and kidney cells.1,6,8
Most of the homocysteine that is not re-methylated is converted to cystathionine via a chemical process called transsulfuration, which requires the amino acid serine, two important enzymes called cystathionine beta-synthase (CBS) and cystathionine gamma-lyase (CSE), and vitamin B6. Cystathionine can then be converted into the amino acid cysteine, or metabolized into energy.6 In addition to being used to make proteins, cysteine is also incorporated into the antioxidant glutathione.18,9
4 Causes of High Homocysteine Levels
Normally, about 5–10% of the homocysteine generated inside the cells is not metabolized and makes its way into the bloodstream and is subsequently cleared by the kidneys.9 However, when either re-methylation or transsulfuration are interrupted, more homocysteine moves out of cells and blood levels rise.8
Nutritional and Genetic Causes
Deficiencies of vitamins B2, B6, or, more commonly, B12 or folate interrupt re-methylation of homocysteine into methionine. Genetic alterations that result in less-efficient variants of the MTHFR enzyme also prevent sufficient homocysteine re-methylation. Importantly, when homocysteine re-methylation is impaired, SAMe levels fall, leading to a lack of methyl donors for other cell functions.8
Homocysteine transsulfuration can be interrupted when vitamin B6 intake is deficient or in cases of genetic mutations in the code for the CBS enzymes.8 The impairment of transsulfuration also leads to dysregulated hydrogen sulfide production.9
Other Causes
High homocysteine levels can result from any condition that restricts the availability of nutrients and energy for processing homocysteine. These include6,10-13:
|
|
In addition, several medications have been implicated in raising homocysteine levels. For example, antacids (H2 blockers such as ranitidine [Zantac] and cimetidine [Tagamet]), proton pump inhibitors (such as omeprazole [Prilosec] and esomeprazole [Nexium]), and metformin (Glucophage) can reduce vitamin B12 absorption and may increase homocysteine levels.14 The cholesterol-lowering drug fenofibrate (Antara, and others) and the blood pressure-lowering diuretic hydrochlorothiazide (Apo-Hydro, and others) have also been found to raise homocysteine levels.15
5 Consequences of High Homocysteine Levels
Although the exact mechanism of injury is still being investigated, it is clear that homocysteine has toxic effects on the cells that line the blood vessels. These cells (endothelial cells) are crucial for maintaining vascular tone and function and regulating inflammatory signaling in the blood vessel wall.1,8 Several damaging effects of excess homocysteine on endothelial cells have been demonstrated. These include1,8,9,16,17:
- Inhibiting antioxidant enzyme activity and raising levels of free radicals
- Disrupting normal production of nitric oxide and hydrogen sulfide, which help keep blood vessels relaxed
- Triggering mitochondrial dysfunction
- Increasing production of inflammatory cytokines
- Impairing methylation reactions
- Damaging the structure and function of proteins
Through these mechanisms, high homocysteine levels contribute to widespread vascular injury. This increases the risk of atherosclerosis, heart attack, and stroke, as well as cerebrovascular disease, cognitive decline, and dementia.16
Heart Disease
Research performed over the past two decades has established a clear link between high homocysteine levels and coronary artery disease, as well as acute heart failure, heart attack, and death for any reason in those with existing heart disease and in the general population.18-20 Some evidence suggests homocysteine may be a better indicator of cardiac risk than cholesterol.21 In fact, one meta-analysis calculated that every 5 µmol/L increase in homocysteine was associated with a 52% increased risk of death from coronary artery disease, 32% from cardiovascular disease, and 27% from any cause.19 High homocysteine is a predictor of onset of coronary artery disease, and may be an especially important biomarker of heart disease severity and prognosis in those affected at a younger age.22,23
Stroke
The toxic effects of homocysteine can contribute to formation of blood clots, and high homocysteine levels are associated with increased risk of stroke.12,24 In particular, having elevated homocysteine levels increases the risk of stroke four-fold in patients with atrial fibrillation, the most common cause of stroke in those over 80 years old.25 Furthermore, those who experience stroke are more likely to suffer from neurological deterioration within the first three days if their homocysteine level is high.26 Multiple controlled trials have indicated homocysteine-lowering therapy with vitamin B12 and/or folate can reduce stroke risk by at least 10%, with greater effects seen in those with higher homocysteine levels and lower folate status at baseline.12,27
Neurological Diseases
By damaging the blood vessels that supply the brain, excess homocysteine in the blood can contribute to cerebrovascular cognitive decline, dementia, and Alzheimer disease. In addition, brain function depends on SAMe availability to run methylation reactions, and homocysteine accumulation is accompanied by SAMe depletion.26 Protein disruption and impaired epigenetic control of gene expression resulting from excess homocysteine further damages brain tissue.17
High homocysteine levels have been shown to be correlated with an increased risk of Alzheimer disease and Parkinson disease.2 Individuals with elevated homocysteine levels are more likely to have markers of Alzheimer disease progression in brain tissue: neurofibrillary tangles, dysfunctional protein (amyloid-beta and phosphorylated tau) accumulation, and brain atrophy (shrinkage).28 According to one meta-analysis, every 5 µmol/L increase in homocysteine level is associated with a 15% increase in Alzheimer disease risk.29 In patients with Parkinson disease, elevated homocysteine is associated with worse cognitive function.30 Even modest elevation of homocysteine within the normal range (>11 µmol/L) has been associated with a substantial increase in risk of dementia in the elderly.28 Furthermore, lowering homocysteine levels using vitamins B12, B6, and folate has been found to markedly slow brain atrophy and cognitive decline.28
Other Conditions
A number of other chronic conditions have been linked to high homocysteine levels. Importantly, the direction of causality between homocysteine and these conditions is not always clear, and more rigorous research is needed. Conditions associated with elevated homocysteine include:
- Cancer. Cancer patients have higher levels of homocysteine compared with healthy people, and levels are higher in late stages than early stages of cancer. It is thought that genetic, epigenetic, and environmental factors each play a role, but the exact nature of this relationship is still being explored.31
- Diabetes complications. Due to the toxic effects of homocysteine on blood vessels, high homocysteine is linked to increased risk of cardiovascular and microvascular diabetes complications. This includes diabetic retinopathy (eye damage) and nephropathy (kidney damage).32,33
- Erectile dysfunction. A meta-analysis of findings from nine studies found men with erectile dysfunction were more likely to have high homocysteine levels than men without. This connection is most likely related to vascular damage induced by homocysteine.34
- Pregnancy complications. High homocysteine levels have been linked to increased risk of pre-eclampsia in pregnancy, a dangerous condition marked by high blood pressure and organ damage.35 High maternal levels of homocysteine are also associated with a range of congenital disorders, such as neural tube defects, cleft lip and palate, and Down syndrome.36
- Osteoporosis. Excess homocysteine has been shown to reduce both bone density and bone quality by damaging cells involved in bone turnover and interfering with the functionality of collagen.37
- Hearing and vision loss. High homocysteine levels have been linked to sensorineural hearing loss, a common cause of hearing loss in the elderly.38 Other findings suggest a possible link between elevated homocysteine levels and age-related macular degeneration, a frequent cause of vision loss.39
6 Homocysteine: Finding the Right Level
Homocysteine levels are usually measured using a blood test. Typically, the total amount of homocysteine, which includes free and protein-bound homocysteine, is reported.21 Total homocysteine levels of 5–14.5 µmol/L are generally considered normal; levels of 15–30 µmol/L are considered mildly elevated; 30–100 µmol/L are intermediately elevated; and levels exceeding 100 µmol/L are seriously elevated.2
Optimal Level
The optimal level for homocysteine remains a topic of debate.40 Rather than there being a threshold above which disease occurs, the relationship between homocysteine levels and health may be more continuous.41-44 For this reason, Life Extension suggests most individuals strive to keep homocysteine levels below 12 µmol/L, with less than 8 µmol/L being considered ideal (although the latter may be difficult for some people to attain).
Early homocysteine research attempting to identify the relationship between homocysteine and health noted that incremental increases in homocysteine levels were accompanied by a higher risk of cardiovascular disease and death.41,44 In one report from the Hordaland (Norway) Homocysteine Study, 4,766 participants aged 65–67 years had their homocysteine levels measured and were followed for 4.1 years. Compared to participants with homocysteine levels below 9.0 µmol/L, those with levels of 9.0–11.9 µmol/L had a 30% increased risk of cardiovascular death and 40% increased odds of non-cardiovascular death, and those with levels of 12.0–14.9 µmol/L had a 110% increased risk of cardiovascular and 90% increased risk of non-cardiovascular death. Furthermore, the risks were more than two-fold higher in those with levels of 15–19.9 µmol/L and more than three-fold higher in those with levels of 20 µmol/L and above.44 In people with coronary artery disease, similar rising trends in risk of hospitalization and death have been associated with incremental increases in homocysteine levels.42,43 A prospective case-control study of Japanese individuals aged 40 to 85 years found that stroke risk was significantly greater among individuals with homocysteine levels of 11 µmol/L or higher compared with those whose levels were less than 7 µmol/L.45
Homocysteine’s relationship with brain health may be similar, and individuals whose homocysteine levels are within the currently accepted normal range may still benefit from homocysteine-lowering therapy. In an eight-year study, participants with baseline homocysteine levels higher than 14.5 µmol/L had nearly twice the risk of Alzheimer disease compared to those with lower levels.46 Another study found elderly subjects with homocysteine levels of 10 µmol/L experienced marked cognitive deterioration when levels doubled to 20 µmol/L over 10 years.47
A two-year randomized controlled trial assessed the rate of brain atrophy in 168 individuals over age 70 who had mild cognitive impairment and were given a placebo or B-vitamin supplementation (0.8 mg folic acid, 500 mcg B12, and 20 mg B6) daily. The researchers found that the rate of brain atrophy was considerably slower in the B-vitamin group compared with the control group. Importantly, the treatment response was related to baseline homocysteine levels such that individuals with levels above 13 μmol/L exhibited greater reductions in the rate of brain atrophy with B-vitamin supplementation than those with lower homocysteine levels. The researchers noted that homocysteine-lowering therapy with B vitamins was beneficial in those with baseline homocysteine levels of 9.5 µmol/L or higher.48 Furthermore, research from 1997 found that, in healthy men, folic acid supplementation reduced homocysteine levels in all tertiles except the lowest tertile, in which average baseline levels of homocysteine were 7.07 µmol/L.49 Cumulatively, these findings suggest supplementation with homocysteine-lowering B vitamins may confer benefits even among individuals whose homocysteine does not exceed normal lab reference ranges.
7 The Genetics of Homocycsteine Metabolism
Genetic variation (polymorphism) fundamentally impacts the activities of enzymes involved in homocysteine metabolism. As a result, some individuals are less efficient at metabolizing homocysteine, and are therefore prone to higher levels, due to genetic factors.
The most widely studied of these polymorphisms as it pertains to homocysteine are those that occur in the MTHFR gene; that is, the gene encoding the folate-metabolizing enzyme methylenetetrahydrofolate reductase (MTHFR). This key enzyme is active in the folate cycle, which, with help from vitamin B12, provides methyl groups for the re-methylation of homocysteine to produce methionine. One common variant, called the TT genotype, affects approximately 10% of the world population.2 People with this genotype have lower MTHFR activity, higher homocysteine levels, and a higher risk of cardiovascular and neurological diseases.50,51 They may be less responsive to folic acid therapy and may require higher supplemental folate doses; they also appear to benefit from supplemental riboflavin (B2).4,52,53
A less common but important genetic factor affecting homocysteine metabolism pertains to the CBS gene, which encodes the enzyme cystathionine beta-synthase (CBS). CBS catalyzes the transsulfuration of homocysteine to produce cystathionine. One variant of the CBS gene has been correlated with increased risk of stroke.54 A less common mutation of the CBS gene is the cause of a rare genetic disease called homocystinuria, marked by low or no activity of CBS, massive elevation of blood and urine homocysteine levels, and numerous serious complications.55
8 How to Lower Homocysteine Levels
B vitamins are the main therapeutic agents used to treat high homocysteine levels. Many studies confirm their ability, alone and in combinations, to lower high homocysteine levels, and some trials show clinical benefits in the form of reduced risk of stroke and dementia. In general, the combined use of vitamin B12 and folate is more effective than either alone. Benefits of including vitamins B6 and B2 in homocysteine-lowering treatment have also been reported.154,155
Note: In addition to the core approach (B vitamins) described in this section, refer to the Nutrients section later in this Life Extension Protocol for an overview of additional nutritional interventions that may beneficially affect homocysteine metabolism and related pathways.
Vitamin B9: Folate
Reported dosage: 400–5,000 mcg (680–8,500 mcg DFE) per day
Folate, sometimes referred to as vitamin B9, is found in many plant foods, particularly leafy green vegetables, as well as eggs and liver.156 Because of its instability, it is often lost or degraded during storage, cooking, and processing.157,158 Folate deficiency causes a characteristic type of anemia called megaloblastic anemia, which can also be caused by B12 deficiency. Folate deficiency during pregnancy is a well-known cause of neural tube birth defects; in adults, it is associated with neurological disorders including cognitive decline, depression, and neuropathy, as well as Alzheimer and Parkinson diseases.159 Folic acid is the fully oxidized form of folate. It is a synthetic form of the vitamin that is more stable than folate and is commonly used in supplements and fortification programs.160 However, L-methylfolate can be an effective alternative to folic acid, with some potential advantages, particularly in people with MTHFR gene variants (see below).
In the United States and a number of other countries, particularly in North and South America, Australia, and New Zealand, folic acid fortification of white flour and cereal grain products is mandatory; and voluntary fortification of various other foods is encouraged as a measure to prevent neural tube birth defects.160-162 Folic acid fortification was implemented in the United States in 1996, with full implementation essentially completed in late 1997. After the initiation of folic acid fortification, mean plasma folate concentrations increased from 4.6 to 10 ng/mL, homocysteine concentrations fell from 10.1 to 9.4 µmol/L, and the prevalence of homocysteine levels above 13 μmol/L dropped from about 19% to about 10%. The prevalence of low folate concentrations also decreased from 22% to 2%.163 As of 2023, 32% of the world’s population lived in regions with mandatory folic acid fortification programs, 53% lived in countries with only voluntary programs, and 15% lived in regions without any folic acid fortification programs. Mandatory folic acid fortification has been associated with higher population-wide folate levels, as well as reduced prevalence of neural tube defects, while voluntary programs have been somewhat less effective.162
Certain people continue to be at increased risk of low folate/folic acid intake regardless of fortification programs, such as those on low-carbohydrate diets. In addition, factors that impair folate metabolism can cause functional folate deficiency (deficient folate activity, unrelated to folate intake). These include vitamin B12 deficiency, mutations of the MTHFR gene, and epigenetic modifications that alter expression of other key enzymes involved in folate metabolism.159,161
Folate is needed to convert homocysteine into methionine. People with low folate levels or impaired ability to use folate have been found to have higher homocysteine levels. Supplementing with folic acid has been shown to reduce homocysteine levels.159 A meta-analysis of findings from 16 randomized controlled trials found the optimal homocysteine-lowering folic acid dose was around 800 mcg daily. Folic acid in combination with other vitamins, such as 400 mcg folic acid and 400 mcg vitamin B12 or 1,000 mcg of folic acid plus 7.2 mg vitamin B6 and 20 mcg vitamin B12, was also found to reduce homocysteine levels. The trials in the analysis were conducted in countries across the globe, including some with mandatory folic acid fortification programs, like the United States and New Zealand.155 Likewise, a large clinical trial performed in China (where folic acid fortification is voluntary) tested different doses of folic acid in 2,163 patients with high blood pressure and homocysteine levels of 10 μmol/L or higher and found homocysteine lowering was maximized at 1,200 mcg of folic acid daily. The results also showed, relative to 1,200 mcg per day, only a small decline in benefit was seen at 800 mcg per day, and no additional benefit occurred with higher doses.164 One review examined the effects of folic acid on homocysteine levels in patients with various conditions that increase stroke risk. The review included 28 randomized controlled trials, almost half of which included some participants in countries with mandatory folic acid fortification policies. Overall, folic acid use, mainly at doses between 800 and 5,000 mcg daily, with or without other B vitamins, resulted in 15–46% reductions in homocysteine levels. In trials that only included subjects in regions with mandatory fortification, all but one used doses of 2,500−15,000 mcg daily and homocysteine levels decreased 15−30%.165 A systematic review that included eight studies with a total of 1,140 subjects with mild cognitive impairment living in countries without mandatory folic acid fortification showed folic acid interventions, with or without other B vitamins, decreased homocysteine levels by an average of almost 32%.166
Folic acid supplementation may enhance cognitive and cardiovascular health while lowering homocysteine levels. One meta-analysis that included findings from 22 randomized controlled trials with a total of 3,604 participants found treatment with folic acid not only lowered homocysteine levels but also improved cognitive function in those with mild cognitive impairment. Moreover, in patients with Alzheimer disease, doses of 3,000 mcg per day or higher had cognitive benefits. Conversely, folic acid was not effective at slowing cognitive loss in those with cognitive dysfunction caused by vascular disease.167 Another meta-analysis pooled findings from 21 randomized controlled trials with 115,559 participants and found lowering homocysteine levels with folic acid reduced stroke risk by an average of 10%. The benefits were strongest in those who had not previously had a stroke or heart attack, and the efficacy remained consistent regardless of baseline folate levels, folic acid dosage, inclusion of other B vitamins, or degree of homocysteine reduction. A subgroup analysis showed greater efficacy in areas without fortified food or with only partial fortification.168 A large meta-analysis of 65 randomized controlled trials with 7,887 hypertensive subjects found folic acid plus blood pressure-lowering medication was more effective than medication alone for lowering blood pressure, lowering homocysteine levels, and reducing risk of cardiovascular events and stroke by 13% compared with control groups. The clinical benefits were greatest in those who took folic acid for more than 12 weeks and those whose homocysteine levels dropped by more than 25%.169
It is important to note that folic acid supplementation can hide or mask a B12 deficiency by preventing or correcting megaloblastic anemia without resolving the neurotoxic effects of B12 deficiency.161 In addition, some evidence suggests the combination of excess folic acid plus B12 deficiency may cause more neurological harm than B12 deficiency alone.170
Vitamin B12: Cobalamin
Reported dosage: 500–2,000 mcg per day
Vitamin B12, or cobalamin, is found mainly in animal foods, but small amounts are present in mushrooms and fermented foods.183 After absorption, B12 is carried in the bloodstream by several different transport proteins, but only holotranscobalamin (holoTC) is biologically active since it can be transported into cells. Once inside the cell, it is used to make adenosylcobalamin, needed for energy production in mitochondria, or interacts with 5-MTHF to become methylcobalamin during homocysteine metabolism.184,185
The close relationship between vitamin B12 and folate can make it difficult to distinguish their independent deficiencies: B12 deficiency causes a functional folate deficiency by “trapping” folate as 5-MTHF, thereby blocking homocysteine metabolism, because B12is required for the conversion into functional folate; on the other hand, supplementing with functional folate can “mask” a B12 deficiency by normalizing changes to red blood cells that are often an early sign of B12 deficiency.161
Vitamin B12 deficiency is more common in older age. Infants, children, pregnant and lactating women, and people who eat a vegan diet are also at higher risk of deficiency.161,186 Over the long term, B12 deficiency can lead to a wide range of neurological disorders, including neuropathies, neuromuscular changes, cognitive impairment, depression, and neurodegenerative diseases. Furthermore, by interfering with folate metabolism and the one-carbon cycle, B12 deficiency raises homocysteine levels and risks of related health problems.187
The most common test for B12 status is total serum B12; however, because only about 6–20% of B12 in the blood is active holoTC, even people with total B12 levels in the normal range may have a functional B12 deficiency. Clinical research suggests serum holoTC levels are a more reliable indicator of B12 status than total B12 levels, especially in early-stage deficiency.188,189 Testing both serum total B12 levels and levels of indicators of B12 function such as methylmalonic acid, folate, and homocysteine—all of which increase with B12 deficiency—may also improve detection of deficiency.184
Supplemental B12, in the form of cyanocobalamin, hydroxocobalamin, or methylcobalamin, is often administered as intramuscular injections due to a long-held perception of poor absorption via the digestive tract; however, evidence now indicates sublingual and oral doses of 500–2,000 mcg per day can effectively raise B12 levels without significant difference between the route of administration.190 In one trial involving patients with pernicious anemia, a condition caused by B12 malabsorption, 1,000 mcg of oral cyanocobalamin daily for one month corrected B12 levels in 23 of the 26 participants.191
Clinical evidence shows supplementing with B12 can safely reduce homocysteine levels. A meta-analysis of 21 clinical trials with a total of 1,625 participants found B12 supplementation lowered homocysteine levels by an average of 4.15 μmol/L and the effect was enhanced when daily doses higher than 500 mcg were used for 12 weeks or longer. The analysis further showed hydroxocobalamin was more effective than other forms of B12.192 Including B12 with other B vitamins in homocysteine-lowering therapy may enhance efficacy.155 A meta-analysis of trials in elderly subjects with mild cognitive impairment found B12 plus folate lowered homocysteine and improved cognitive function more than either nutrient alone.154 Furthermore, observational evidence has indicated dietary B12 and folate may work together to reduce all-cause mortality in people with abdominal obesity.193
It is important to note that some evidence indicates repeated high doses of cyanocobalamin may be harmful in those with kidney disease; therefore, methylcobalamin or hydroxocobalamin are preferable forms for vitamin B12 therapy.194-200
Vitamin B6: Pyridoxal 5-Phosphate (P5P), Pyridoxine
Reported dosage: 7.2–50 mg per day, along with other B vitamins
Vitamin B6 (pyridoxine) is found in a wide array of plant and animal foods.156 It is a cofactor in more than 150 reactions in cells, including some involved in both folate and homocysteine metabolism.165 Vitamin B6 is widely available in foods and severe deficiency is rare; however, low intake and marginal deficiency have been shown to be relatively common around the world, and may be a contributing factor in cardiovascular disease, sarcopenia, frailty, and increased mortality in the elderly.201-203 Deficiency may be due to medications or conditions that interfere with B6 absorption or metabolism and is especially prevalent in older individuals.204 Symptoms of deficiency include neurological problems like neuropathy, cognitive dysfunction, and depression, as well as anemia, dermatitis, glossitis (inflamed tongue), and weakened immune function.205
In conditions of adequate methionine availability, conversion of homocysteine to methionine is suppressed, and an alternate metabolic pathway is engaged to regulate homocysteine levels. This other pathway requires B6 and results in production of cystathionine, followed by cysteine, which can be used to make the powerful antioxidant glutathione.165,206 Few studies have examined the role of vitamin B6 on homocysteine-related disorders independently of folate and B12, but a meta-analysis with a total of 369,746 participants determined higher intake of B6 was correlated with lower risk of coronary heart disease.207 In addition, a large study that followed 2,968 subjects with cardiovascular disease for a median of almost 10 years found lower B6 levels and higher homocysteine levels were each associated with increased mortality, higher levels of oxidative stress and inflammatory markers, and shorter telomere length for age, suggesting accelerated biological aging.206
At typical doses, pyridoxine is readily transported across cell membranes and converted to the active form of B6, pyridoxal 5-phosphate (P5P).208 Taking high doses of pyridoxine for prolonged periods results in abnormally high circulating pyridoxine levels. In some people, high intake of pyridoxine may contribute to peripheral neuropathy symptoms such as numbness, tingling, pain, and muscle weakness, mainly affecting the hands and feet. Although the U.S. Food and Nutrition Board recommends a safe upper limit of 100 mg per day, rare cases of pyridoxine-induced peripheral neuropathy have been reported in people taking doses as low as 24–40 mg per day, indicating important differences in individuals’ metabolism of pyridoxine. These effects typically subside following discontinuation.209 Some supplements contain P5P, which raises P5P levels and appears less likely to contribute to nerve problems than pyridoxine.210 In addition, supplementing with 50 mg of P5P has been shown to be effective in B-vitamin therapy for high homocysteine levels.211 Thus, P5P is generally the preferred form of supplemental B6.
Vitamin B2: Riboflavin
Reported dosage: 1.6–8.4 mg per day
Vitamin B2 (riboflavin) is obtained through dairy foods and whole grains. It is a component of two essential cofactors used by numerous cellular enzymes, including methylenetetrahydrofolate reductase (MTHFR), a critical enzyme in folate and homocysteine metabolism. As a result, B2 deficiency can trigger functional folate deficiency, especially in carriers of MTHFR mutations associated with reduced activity of the MTHFR enzyme. B2-containing cofactors are also needed by enzymes that activate vitamins B12 and B6.212,213 In fact, blood levels of active B6 have been found to decrease in proportion to declining B2 status, independently of B6 intake.214
Vitamin B2 deficiency is thought to be relatively uncommon in the United States and other regions where white flour is routinely fortified; however, high rates of deficient intake have been reported in other parts of the world.212 For example, a study conducted in the United Kingdom, where B2 fortification is not mandatory, examined B2 intake in 407 healthy subjects aged 18–92 years old and found 29% were not achieving required intake levels. Furthermore, blood tests showed 37% of participants had a B2 deficiency which was correlated not only with low intake but also with MTHFR mutations.214 High homocysteine levels, high blood pressure, and microcytic anemia with low hemoglobin are among the numerous and wide-ranging signs of B2 deficiency.212
In a clinical trial involving 35 individuals with specific MTHFR gene mutations, 1.6 mg of vitamin B2 per day for 12 weeks lowered homocysteine levels significantly more than placebo and the effect was stronger in those with suboptimal B2 status.215 Another trial in 47 MTHFR mutation carriers found 1.6 mg of B2 per day for 16 weeks promoted conversion of homocysteine into cystathionine via a pathway that requires B6, but not folate.216 A randomized placebo-controlled trial that included 32 healthy Japanese men aged 20–29 years found 800 mcg of folic acid plus 8.4 mg of B2 daily for two weeks reduced homocysteine levels more than B2 alone and similarly to folic acid alone. The trial also found combining B2 and folic acid decreased levels of alanine transferase, a liver enzyme released during liver cell breakdown, while folic acid alone increased alanine transferase levels.217
In an observational study involving 20,725 participants in the 2007–2018 National Health and Nutrition Examination Survey (NHANES), higher vitamin B2 intake was correlated with lower likelihood of having coronary artery disease, and the effect plateaued at 10.5 mg per day.218 Another study that analyzed data from 10,480 NHANES (2005–2016) participants found those with the highest folate combined with the lowest B2 intake had the highest rate of death from cardiovascular disease during an average of 8.5 years of monitoring.219 Low B 2 status has also been correlated with higher risk of cognitive dysfunction in older adults.220
B Vitamin Combinations
Vitamins B2, B6, B12, and folate are inextricably linked through their interdependent roles in homocysteine metabolism and the fueling of methylation pathways.174 It is becoming increasingly evident that excess availability of individual B-complex vitamins can disturb the balance of interlinked pathways that depend on multiple B vitamins and other nutrients. Despite their interrelatedness, most research has examined the homocysteine-lowering abilities of folic acid alone, with much less emphasis on B12 and B6 and very little attention to B2.155,174
A meta-analysis of 16 randomized controlled trials conducted in countries around the world with varying fortification policies found combined B-vitamin therapy that included folic acid at doses close to 800 mcg daily was more effective for lowering homocysteine levels than single B-vitamin therapy. The analysis also found a combination of 1,000 mcg of folic acid, 20 mcg of vitamin B12, and 7.2 mg of vitamin B6 was the most effective B-vitamin regimen for lowering homocysteine levels.155
A randomized controlled trial conducted in China, where folic acid fortification of foods is voluntary, included 100 healthy adult participants with high homocysteine levels; results showed a combination supplement providing 400 mcg of folic acid, 6.4 mcg of B12, 8 mg of B6, and 1 mg of betaine per day for 12 weeks reduced homocysteine levels by about 3.9 μmol/L more than placebo.221 Other randomized controlled trials have also indicated B vitamin supplementation may benefit populations where food fortification is voluntary. For example, a randomized controlled trial conducted in the United Kingdom included 167 adults aged 50 years and older not consuming B-vitamin supplements or fortified foods greater than four times weekly. After two years, homocysteine levels in those who received a supplement with 200 mcg of folic acid, 10 mcg of B12, and 10 mg of B6 had decreased by 2.4 μmol/L, or about 17%, more than placebo, and bone mineral density loss had slowed in a subset of participants with low baseline B12 status.222 In a randomized controlled trial in elderly patients with mild cognitive impairment living in the United Kingdom, supplementing with 800 mcg folic acid, 500 mcg B12, and 20 mg B6 daily for two years reduced atrophy of gray matter (brain tissue most vulnerable to Alzheimer pathology) up to seven-fold in those with baseline homocysteine levels higher than 11 μmol/L.223
One controlled trial showed a combination B vitamin supplement had more benefits than folic acid alone in hypertension patients living in Italy—where there is no folic acid fortification policy. In the trial, 104 subjects with high blood pressure and high homocysteine levels (≥15 μmol/L) received either 5,000 mcg of folic acid per day or a daily supplement providing 400 mcg of folate (as 5-MTHF), 5 mcg of B12, 3 mg of B6, and 2.4 mg of B2, as well as 12.5 mg of zinc and 250 mg of betaine. Average homocysteine levels fell from 22.6 to 14.3 µmol/L in the folic acid group and from 21.5 to 10.0 µmol/L in the combination group. Furthermore, 56% of those taking the combination supplement attained homocysteine levels of <10 µmol/L.224
9 Diet and Lifestyle Factors
Diet
A healthy diet that includes an abundance of foods high in B vitamins has been associated with lower homocysteine levels and reduced risk of stroke.225,226 A Mediterranean diet, which balances high intake of olive oil, fruit, vegetables, whole grains, and plant proteins with modest consumption of dairy products and seafood, has been associated with healthy homocysteine levels.227 In a study in 237 women, adherence to a Mediterranean diet pattern was more closely correlated with homocysteine levels than either B12 or folate status.228 Similarly, in a study in 861 70-year-olds in Sweden, adherence to the EAT-Lancet diet, which was designed to support human health and environmental sustainability, was correlated with lower homocysteine levels. The EAT-Lancet diet includes ample whole grains, fruit, vegetables, legumes, nuts, and unsaturated fats, but limits added sugar, dairy, red meat, and other animal food.229 Eating habits that reflected a Prudent Diet, which includes fruit, vegetables, potato, cassava, cornmeal, fish, and chicken, were also associated with lower homocysteine levels in a study in 281 subjects in Brazil.230
The issue of plant versus animal protein is complex and likely depends on an individual’s age, digestive capacity, and genetic factors. Animal foods are good sources of B6, B12, and B2, and may help vulnerable individuals, including older individuals, meet their nutritional needs. One study in 29 older men with an average age of 74 years and unknown B vitamin status found homocysteine levels dropped more in those who increased their animal protein consumption to boost protein intake to 1.6 grams/kg/day compared with those whose protein intake was maintained at 0.8 grams/kg/day for 10 weeks.231 Nevertheless, in large observational studies, plant protein intake and plant-based diets have been associated with lower homocysteine levels.232,233 It is noteworthy that vegan diets, which exclude all animal protein, are frequently low in vitamin B12 and have been linked to high homocysteine levels.234
Observational research provides insights into specific foods that may help keep homocysteine levels from rising. In a study involving 7,521 healthy participants in China, higher fruit intake and better signs of metabolic health were correlated with normal (versus high) homocysteine levels.234 A study in 227 young Japanese women found higher intakes of fruit and mushrooms, as well as higher soluble, insoluble, and total fiber intake, were each associated with lower homocysteine levels.235 In a study in 4,175 Ethiopian adults, low fruit and/or vegetable intake was found to be a risk factor for high homocysteine levels.236 Another study that examined the diets of 252 subjects in Poland with two distinct dietary patterns indicated higher intake of foods such as legumes, whole milk, and whole grains were associated with normal (versus high) homocysteine levels.237
A study conducted in the Netherlands found high intake of ultra-processed food was associated with higher homocysteine levels.238 Ultra-processed foods are those formulated, typically using multiple food additives, for features like long shelf-life, palatability, convenience, low cost, and microbiological safety. They are typically calorie-dense but nutrient-poor.239 In clinical trials in healthy men, consuming the equivalent of two alcoholic drinks per day, in the form of wine or spirits, lowered B vitamin status and raised homocysteine levels, but a similar amount of alcohol as beer, which is a source of B vitamins, did not.240,241
Being overweight or obese may impair folate metabolism and cause homocysteine accumulation.242 A strong independent link between obesity and high homocysteine levels has been observed.243 Clinical studies have reported weight loss interventions, both dietary and surgical, increased homocysteine levels in the short term, though some evidence suggests levels may return to baseline in the long term.244,245
Exercise
Various types of exercise have been found to be associated with lower homocysteine levels. A 2017 analysis from the NHANES found participation in aerobic and strength-training exercise were each correlated with lower homocysteine levels.246 In a large observational study that included 110,551 adults in China aged 40 years and older, exercise was correlated with lower homocysteine levels.247 Another study found that, among older participants, those who were more physically active in general had lower homocysteine levels than those who were less physically active, independent of their B12 and folate status.248 In a study in 497 patients with hypertension, those who reported having a regular aerobic exercise habit had a lower likelihood of high homocysteine levels. In addition, greater intensity, frequency, and total energy expenditure through exercise were each independently linked to lower odds of high homocysteine levels.249
Clinical trials add to the evidence that exercise can reduce homocysteine levels. A randomized controlled trial in 44 women with type 2 diabetes found a 12-week aerobic exercise training program involving three sessions per week of mild- to moderate-intensity exercise lowered homocysteine levels and improved other markers of inflammation and metabolic health. Interestingly, beneficial changes in several biomarkers of metabolic health, including homocysteine levels, were found to be more pronounced in subjects who supplemented with 200 mg of saffron powder along with the exercise intervention.250 Similarly, in a randomized controlled trial in 30 metabolic syndrome patients, an eight-week exercise program that included aerobics and strength training lowered homocysteine levels and improved markers of metabolic and cardiovascular health.251
Mind-body exercise has also shown promise as a strategy for lowering homocysteine levels. In a randomized controlled trial involving 170 older adults at high risk of ischemic stroke, a 12-week Tai Chi (a mind-body exercise) program led to multiple benefits including reduced homocysteine levels.252 An observational study that included 326 participants who practiced Tai Chi at least three times per week and 326 matched participants who had no regular exercise habit found the Tai Chi group was nearly four times less likely to have homocysteine levels of 15 μmol/L or higher.253
Stress Management
The role of stress in cardiovascular disease is well documented; however, its effect on homocysteine metabolism has received little research attention. In one clinical trial, middle-aged and older participants experienced a rise in homocysteine after an experimentally-induced stress, but participants aged 20–30 years old did not.254 Another trial enrolled women with and without menstrual pain in an eight-week yoga intervention. Those with pain had higher baseline homocysteine levels, but both groups had dramatic reductions in homocysteine levels at the end of the yoga intervention: levels dropped by 51% in those with menstrual pain and 46% in those without pain.255
10 Nutrients
In addition to B-vitamin therapy described in the “How to Lower Homocysteine Levels” section of this Protocol, several nutrients exert a beneficial influence on various aspects of homocysteine metabolism and related pathways.
Betaine
Reported dosage: 1,000–6,000 mg per day
Choline is a nutrient found in many foods, with particularly high amounts in egg yolks, dairy products, fish, beef, chicken, cruciferous vegetables, and soybeans. It is an important structural component of cell membranes, a precursor for the neurotransmitter acetylcholine, and an integral part of brain tissue.256 Choline is also used to make betaine, or trimethylglycine, an important methyl donor that acts as an alternative to folate in metabolizing homocysteine into methionine. Despite its presence in many and various foods, the NHANES found approximately 11% of Americans achieve the recommended intake for choline, which is 550 mg per day for men and 425 mg per day for women.257 In addition to being made in the body from choline, betaine occurs in foods like seafood, wheat germ and bran, beets, and spinach.258
Betaine plays a critical role in regulating homocysteine levels, especially in low folate, low B12, and high methionine conditions.259,260 Inadequate intake of choline can result in low betaine availability, increased accumulation of homocysteine, and reduced production of SAMe, a critical methyl donor needed for a host of cellular activities.261,262 Well-regulated homocysteine metabolism depends on a balance between the betaine-dependent and folate-dependent pathways for homocysteine’s conversion to methionine, and deficiencies of either cofactor has been shown to cause depletion of the other.262 Some evidence indicates choline may have a similar relationship with vitamin B12.263
Betaine supplementation, at doses of 1,000–6,000 mg per day, has been shown to lower blood homocysteine levels and moderate the increase in homocysteine that follows ingestion of methionine.264-266 A randomized controlled crossover trial in 20 healthy active men found betaine supplementation, at 2,500 mg or 5,000 mg daily for three weeks, lowered homocysteine levels more than placebo.267 In a placebo-controlled trial in 23 athletes, those given 2,500 mg betaine per day during a six-week exercise training program had a lower exercise-induced rise in urinary homocysteine thiolactone, a form of homocysteine known to have toxic effects on blood vessels and interfere with normal protein production and function.268,269
Choline appears to have a cardioprotective role, which may be related to its homocysteine-lowering effect. In a large observational study that analyzed seven years of data from 14,289 NHANES participants, higher intake of choline was associated with lower risk of cardiovascular disease. The analysis found that every 100 mg increase in choline intake per day was linked to a 9% reduction in cardiovascular disease risk. Furthermore, those with moderate choline intake (those in the third of five intake level categories) had the lowest risk of all-cause mortality. Higher choline intake has also been associated with reduced risk of various cancers.256,270 Another study examined 11 years of data from 7,341 NHANES participants aged 65 years and older and found choline was one of eight nutrients independently associated with lower likelihood of cardiovascular disease.271
It is important to note that excessively high dietary choline intake results in increased trimethylamine N-oxide (TMAO) production by intestinal microbes. TMAO promotes atherosclerosis and increases the risk of major cardiovascular events such as heart attack and stroke. 256
Omega-3 Fatty Acids
Reported dosage: 2,400–3,000 mg per day
Omega-3 fatty acids appear to work synergistically with B vitamins to promote healthy homocysteine metabolism and reduce risks of conditions associated with high homocysteine levels, including cardiovascular and neurological diseases.5 Multiple randomized controlled trials have shown that fish oil and its omega-3 polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) can reduce homocysteine levels, and their effect is augmented by the addition of vitamins B12, B6, and folic acid.272 In one randomized controlled trial involving 80 subjects with metabolic syndrome, 800 mg of omega-3 fatty acids three times daily for three months not only improved measures of endothelial function but also decreased apolipoprotein B levels and reduced homocysteine levels from an average of 19.3 μmol/L to 13.8 μmol/L.273 Furthermore, a meta-analysis of findings from 20 randomized controlled trials with a combined total of 2,676 participants found supplementing with omega-3 fatty acids reduced homocysteine levels, and the effects were strongest with daily doses of 3 grams or more for less than 12 weeks and in those with higher baseline homocysteine levels.274
The beneficial effects of omega-3 fatty acids on cardiovascular and neurological health may depend on adequate homocysteine metabolism, which requires healthy B vitamin status. For example, an analysis from one clinical trial in Alzheimer disease patients found treatment with 1,700 mg DHA and 600 mg EPA daily for six months improved cognitive function only in those with homocysteine levels below 11.7 µmol/L.275 Likewise, analysis of data from 782 participants in a randomized placebo-controlled trial found higher omega-3 fatty acid status was correlated with better cognitive performance in subjects with homocysteine levels of 16.8 μmol/L or lower at baseline, and those with high homocysteine levels experienced more cognitive decline than those with low levels during five years of omega-3 fatty acid therapy.276
Healthy omega-3 fatty acid status may also enhance the benefits of B vitamins. In one study, high homocysteine levels were linked to increased brain beta-amyloid (a marker of Alzheimer disease risk) in elderly subjects with low omega-3 status, but not those with high omega-3 status.277 Another study analyzed data from 191 participants aged 65 years or older whose baseline homocysteine levels were 12 μmol/L or higher in a randomized controlled trial and found the positive effects of two years of 400 mcg folic acid and 500 mcg B12 therapy daily on cognitive function were stronger in those with higher DHA levels.278 Clinical trials have also indicated B vitamins’ ability to slow brain tissue atrophy and improve cognitive function in individuals with mild cognitive impairment depends on adequate blood levels of omega-3 fatty acids, especially DHA.279,280
N-acetylcysteine
Reported dosages: 600–1,800 mg per day
N-acetylcysteine (NAC) is a source of cysteine that can be used in the body to manufacture the important antioxidant compound glutathione. By increasing glutathione production and lowering oxidative stress, it is thought NAC might help mitigate some toxic effects of excess homocysteine. In addition, NAC appears to lower homocysteine levels.281,282
A meta-analysis of 28 controlled trials found supplementing with NAC not only reduced oxidative stress but also lowered homocysteine levels.283 In an eight-week, randomized, controlled trial involving 60 participants with high homocysteine levels and coronary artery disease, 600 mg NAC daily was as effective as 5 mg per day of folic acid for reducing homocysteine levels compared with placebo.284 In another trial, 30 patients with high homocysteine levels and Alzheimer disease or a related disorder were treated with a supplement providing undisclosed doses of folate (as 5-MTHF), vitamin B12 (as methylcobalamin), and NAC for periods of time ranging from 2.5 to 34.6 months. Compared to similar patients who received no supplements, brain tissue atrophy in those receiving the B vitamin/NAC combination was substantially reduced. This reduction was proportional to the degree of homocysteine lowering.285 An analysis of data from two placebo-controlled trials in middle-aged men found supplementing with 1,800 mg NAC daily for four weeks decreased homocysteine levels by an average of almost 12%. NAC also lowered blood pressure, particularly in those with high cholesterol and triglyceride levels.286
Taurine
Reported dosages: 0.5–3 grams per day
Taurine is a sulfur-containing non-essential amino acid that is closely related to methionine, cysteine, and homocysteine. Taurine can be made from cysteine or homocysteine in the body and has beneficial effects on vascular, neurological, metabolic, and musculoskeletal health.287 Taurine supplementation has been shown to lower blood pressure and improve cardiac function in heart failure patients.288 It has also been found to improve metabolic health in individuals with metabolic syndrome and those with overweight and obesity.289,290 Preclinical evidence suggests taurine supplementation may reduce high homocysteine levels and protect heart and blood vessel cells from homocysteine-induced damage.291-293
In a preliminary trial, 22 middle-aged women (aged 33–54 years) were given 3 grams taurine daily for four weeks. This resulted in a drop in average homocysteine level from 8.5 to 7.6 µmol/L.294 Even in subjects with blood homocysteine levels >125 µmol/L due to a genetic disorder called homocystinuria, taurine supplementation was found to improve vascular function.295
S-adenosylmethionine (SAMe)
Reported dosage: 800–1,600 mg per day
S-adenosylmethionine (SAMe) is a crucial methyl donor in many cell processes, including epigenetic gene modification and neurotransmitter synthesis. Since high homocysteine levels are often the result of poor conversion of homocysteine to methionine, which is a precursor of SAMe, SAMe depletion and homocysteine accumulation usually go hand-in-hand.8,262 This may be a contributing factor in the relationship between high homocysteine levels and psycho-emotional conditions such as depression, as well as the higher risk of depression seen in carriers of the MTHFR gene variant associated with impaired folate metabolism.296,297
Clinical evidence suggests SAMe may be helpful in treating depression, including in people with elevated homocysteine levels.296,298 One case report described the benefit of SAMe in a 32 year old patient with anxiety who was found to have a MTHFR gene mutation: treatment with methylated B12 and folate was not effective for relieving symptoms until SAMe, 400 mg twice daily, was added.299 Although concerns have been raised about the possibility that SAMe supplementation might increase homocysteine production, a trial in subjects with major depressive disorder found that 800–1,600 mg SAMe per day for six weeks did not raise homocysteine levels.300
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Esse R, Barroso M, Tavares de Almeida I, Castro R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. International journal of molecular sciences. 2019;20(4).
- Tinelli C, Di Pino A, Ficulle E, Marcelli S, Feligioni M. Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies. Frontiers in nutrition. 2019;6:49.
- Zaric BL, Obradovic M, Bajic V, Haidara MA, Jovanovic M, Isenovic ER. Homocysteine and Hyperhomocysteinaemia. Curr Med Chem. 2019;26(16):2948-2961.
- Troesch B, Weber P, Mohajeri MH. Potential Links between Impaired One-Carbon Metabolism Due to Polymorphisms, Inadequate B-Vitamin Status, and the Development of Alzheimer's Disease. Nutrients. 2016;8(12).
- Rizzo G, Lagana AS. The Link between Homocysteine and Omega-3 Polyunsaturated Fatty Acid: Critical Appraisal and Future Directions. Biomolecules. 2020;10(2).
- Komorniak N, Szczuko M, Kowalewski B, Stachowska E. Nutritional Deficiencies, Bariatric Surgery, and Serum Homocysteine Level: Review of Current Literature. Obesity surgery. 2019;29(11):3735-3742.
- Froese DS, Fowler B, Baumgartner MR. Vitamin B12 , folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. Journal of inherited metabolic disease. 2019;42(4):673-685.
- Fu Y, Wang X, Kong W. Hyperhomocysteinaemia and vascular injury: advances in mechanisms and drug targets. Br J Pharmacol. 2018;175(8):1173-1189.
- Yang Q, He GW. Imbalance of Homocysteine and H2S: Significance, Mechanisms, and Therapeutic Promise in Vascular Injury. Oxid Med Cell Longev. 2019;2019:7629673.
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6.
- Ledda C, Cannizzaro E, Lovreglio P, et al. Exposure to Toxic Heavy Metals Can Influence Homocysteine Metabolism? Antioxidants (Basel, Switzerland). 2019;9(1).
- Hankey GJ. B vitamins for stroke prevention. Stroke Vasc Neurol. 2018;3(2):51-58.
- Urgert R, van Vliet T, Zock PL, Katan MB. Heavy coffee consumption and plasma homocysteine: a randomized controlled trial in healthy volunteers. Am J Clin Nutr. 2000;72(5):1107-1110.
- Miller JW. Proton Pump Inhibitors, H2-Receptor Antagonists, Metformin, and Vitamin B-12 Deficiency: Clinical Implications. Adv Nutr. 2018;9(4):511s-518s.
- Dierkes J, Luley C, Westphal S. Effect of lipid-lowering and anti-hypertensive drugs on plasma homocysteine levels. Vasc Health Risk Manag. 2007;3(1):99-108.
- Djuric D, Jakovljevic V, Zivkovic V, Srejovic I. Homocysteine and homocysteine-related compounds: an overview of the roles in the pathology of the cardiovascular and nervous systems. Canadian journal of physiology and pharmacology. 2018;96(10):991-1003.
- Jakubowski H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol Rev. 2019;99(1):555-604.
- Zhu M, Mao M, Lou X. Elevated homocysteine level and prognosis in patients with acute coronary syndrome: a meta-analysis. Biomarkers. 2019;24(4):309-316.
- Peng HY, Man CF, Xu J, Fan Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: a meta-analysis of prospective studies. Journal of Zhejiang University Science B. 2015;16(1):78-86.
- Ma Y, Peng D, Liu C, Huang C, Luo J. Serum high concentrations of homocysteine and low levels of folic acid and vitamin B12 are significantly correlated with the categories of coronary artery diseases. BMC cardiovascular disorders. 2017;17(1):37.
- Alam SF, Kumar S, Ganguly P. Measurement of homocysteine: a historical perspective. Journal of clinical biochemistry and nutrition. 2019;65(3):171-177.
- Li S, Pan G, Chen H, Niu X. Determination of Serum Homocysteine and Hypersensitive C-reactive Protein and Their Correlation with Premature Coronary Heart Disease. Heart Surg Forum. 2019;22(3):E215-e217.
- Wei M, Wang L, Liu YS, et al. Homocysteine as a potential predictive factor for high major adverse cardiovascular events risk in female patients with premature acute coronary syndrome. Medicine. 2019;98(47):e18019.
- Moretti R, Peinkhofer C. B Vitamins and Fatty Acids: What Do They Share with Small Vessel Disease-Related Dementia? International journal of molecular sciences. 2019;20(22).
- Spence JD. Cardioembolic stroke: everything has changed. Stroke Vasc Neurol. 2018;3(2):76-83.
- Moretti R, Caruso P. The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. International journal of molecular sciences. 2019;20(1).
- Spence JD. Homocysteine lowering for stroke prevention: Unravelling the complexity of the evidence. Int J Stroke. 2016;11(7):744-747.
- Smith AD, Refsum H, Bottiglieri T, et al. Homocysteine and Dementia: An International Consensus Statement. J Alzheimers Dis. 2018;62(2):561-570.
- Zhou F, Chen S. Hyperhomocysteinemia and risk of incident cognitive outcomes: An updated dose-response meta-analysis of prospective cohort studies. Ageing Res Rev. 2019;51:55-66.
- Licking N, Murchison C, Cholerton B, et al. Homocysteine and cognitive function in Parkinson's disease. Parkinsonism Relat Disord. 2017;44:1-5.
- Hasan T, Arora R, Bansal AK, Bhattacharya R, Sharma GS, Singh LR. Disturbed homocysteine metabolism is associated with cancer. Experimental & molecular medicine. 2019;51(2):21.
- Lei X, Zeng G, Zhang Y, et al. Association between homocysteine level and the risk of diabetic retinopathy: a systematic review and meta-analysis. Diabetol Metab Syndr. 2018;10:61.
- Mao S, Xiang W, Huang S, Zhang A. Association between homocysteine status and the risk of nephropathy in type 2 diabetes mellitus. Clin Chim Acta. 2014;431:206-210.
- Sansone A, Cignarelli A, Sansone M, et al. Serum Homocysteine Levels in Men with and without Erectile Dysfunction: A Systematic Review and Meta-Analysis. International journal of endocrinology. 2018;2018:7424792.
- Gaiday AN, Tussupkaliyev AB, Bermagambetova SK, et al. Effect of homocysteine on pregnancy: A systematic review. Chem Biol Interact. 2018;293:70-76.
- Iacobazzi V, Infantino V, Castegna A, Andria G. Hyperhomocysteinemia: related genetic diseases and congenital defects, abnormal DNA methylation and newborn screening issues. Molecular genetics and metabolism. 2014;113(1-2):27-33.
- Saito M, Marumo K. The Effects of Homocysteine on the Skeleton. Current osteoporosis reports. 2018;16(5):554-560.
- Partearroyo T, Vallecillo N, Pajares MA, Varela-Moreiras G, Varela-Nieto I. Cochlear Homocysteine Metabolism at the Crossroad of Nutrition and Sensorineural Hearing Loss. Frontiers in molecular neuroscience. 2017;10:107.
- Pinna A, Zaccheddu F, Boscia F, Carru C, Solinas G. Homocysteine and risk of age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. 2018;96(3):e269-e276.
- Pizzorno J. Homocysteine: Friend or Foe? Integrative medicine (Encinitas, Calif). 2014;13(4):8-14.
- Refsum H, Nurk E, Smith AD, et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136(6 Suppl):1731s-1740s.
- Nurk E, Tell GS, Vollset SE, Nygard O, Refsum H, Ueland PM. Plasma total homocysteine and hospitalizations for cardiovascular disease: the Hordaland Homocysteine Study. Arch Intern Med. 2002;162(12):1374-1381.
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. The New England journal of medicine. 1997;337(4):230-236.
- Vollset SE, Refsum H, Tverdal A, et al. Plasma total homocysteine and cardiovascular and noncardiovascular mortality: the Hordaland Homocysteine Study. Am J Clin Nutr. 2001;74(1):130-136.
- Iso H, Moriyama Y, Sato S, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109(22):2766-2772.
- Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. The New England journal of medicine. 2002;346(7):476-483.
- Clarke R, Birks J, Nexo E, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86(5):1384-1391.
- Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010;5(9):e12244.
- Ward M, McNulty H, McPartlin J, Strain JJ, Weir DG, Scott JM. Plasma homocysteine, a risk factor for cardiovascular disease, is lowered by physiological doses of folic acid. Qjm. 1997;90(8):519-524.
- Rai V. Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism and Alzheimer Disease Risk: a Meta-Analysis. Molecular neurobiology. 2017;54(2):1173-1186.
- Reilly R, McNulty H, Pentieva K, Strain JJ, Ward M. MTHFR 677TT genotype and disease risk: is there a modulating role for B-vitamins? The Proceedings of the Nutrition Society. 2014;73(1):47-56.
- Du B, Tian H, Tian D, et al. Genetic polymorphisms of key enzymes in folate metabolism affect the efficacy of folate therapy in patients with hyperhomocysteinaemia. The British journal of nutrition. 2018;119(8):887-895.
- Garcia-Minguillan CJ, Fernandez-Ballart JD, Ceruelo S, et al. Riboflavin status modifies the effects of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) polymorphisms on homocysteine. Genes Nutr. 2014;9(6):435.
- Ding R, Lin S, Chen D. The association of cystathionine β synthase (CBS) T833C polymorphism and the risk of stroke: a meta-analysis. J Neurol Sci. 2012;312(1-2):26-30.
- Bublil EM, Majtan T. Classical homocystinuria: From cystathionine beta-synthase deficiency to novel enzyme therapies. Biochimie. 2019.
- Zhao Y, Ge Y, Zhang Z, et al. The effects of methyl nutrients on cognition and one carbon metabolism in older adults with mild cognitive impairment, A systematic review and meta-analysis. Geriatr Nurs . May-Jun 2025;63:395-406. doi:10.1016/j.gerinurse.2025.03.038 https://pubmed.ncbi.nlm.nih.gov/40249981/ https://www.sciencedirect.com/science/article/abs/pii/S0197457225001569?via%3Dihub
- Liu C, Yao H, Wang F. Effect of Nutritional Supplements for Reducing Homocysteine Levels in Healthy Adults: A Systematic Review and Network Meta-Analysis of Randomized Trials. Nutr Rev. Jul 1 2025;83(7):e1533-e1543. doi:10.1093/nutrit/nuae191 https://www.ncbi.nlm.nih.gov/pubmed/39960689
- Chungchunlam SMS, Moughan PJ. Comparative bioavailability of vitamins in human foods sourced from animals and plants. Critical Reviews in Food Science and Nutrition . 2024/12/09 2024;64(31):11590-11625. doi:10.1080/10408398.2023.2241541 https://doi.org/10.1080/10408398.2023.2241541 https://pubmed.ncbi.nlm.nih.gov/37522617/
- Czarnowska-Kujawska M, Draszanowska A, Starowicz M. Effect of different cooking methods on the folate content, organoleptic and functional properties of broccoli and spinach. LWT. 2022/09/15/ 2022;167:113825. doi:https://doi.org/10.1016/j.lwt.2022.113825 https://www.sciencedirect.com/science/article/pii/S0023643822007605
- Liang Q, Wang K, Shariful I, Ye X, Zhang C. Folate content and retention in wheat grains and wheat-based foods: Effects of storage, processing, and cooking methods. Food Chem. Dec 15 2020;333:127459. doi:10.1016/j.foodchem.2020.127459
- Sobral AF, Cunha A, Silva V, Gil-Martins E, Silva R, Barbosa DJ. Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration. Int J Mol Sci . Aug 28 2024;25(17)doi:10.3390/ijms25179339 https://mdpi-res.com/d_attachment/ijms/ijms-25-09339/article_deploy/ijms-25-09339-v2.pdf?version=1725242242
- Li J, Duan H, Ramaswamy H, Wang C. A Comprehensive Review of Fortification, Bioavailability, and Health Benefits of Folate. Int J Mol Sci . Aug 9 2025;26(16)doi:10.3390/ijms26167703 https://mdpi-res.com/d_attachment/ijms/ijms-26-07703/article_deploy/ijms-26-07703.pdf?version=1754712297
- Castillo LF, Pelletier CM, Heyden KE, Field MS. New Insights into Folate-Vitamin B(12) Interactions. Annu Rev Nutr. Aug 2025;45(1):23-39. doi:10.1146/annurev-nutr-120524-043056
- Quinn M, Halsey J, Sherliker P, et al. Global heterogeneity in folic acid fortification policies and implications for prevention of neural tube defects and stroke: a systematic review. eClinicalMedicine . 2024;67doi:10.1016/j.eclinm.2023.102366 https://doi.org/10.1016/j.eclinm.2023.102366 https://www.thelancet.com/pdfs/journals/eclinm/PIIS2589-5370(23)00543-6.pdf
- Jacques PF, Selhub J, Bostom AG, Wilson PWF, Rosenberg IH. The Effect of Folic Acid Fortification on Plasma Folate and Total Homocysteine Concentrations. New England Journal of Medicine. 1999;340(19):1449-1454. doi:doi:10.1056/NEJM199905133401901 https://www.nejm.org/doi/full/10.1056/NEJM199905133401901
- Huang X, Bao H, Ding C, et al. Optimal folic acid dosage in lowering homocysteine: Precision Folic Acid Trial to lower homocysteine (PFAT-Hcy). Eur J Nutr. Aug 2024;63(5):1513-1528. doi:10.1007/s00394-024-03344-8
- Li M, Ren R, Wang K, et al. Effects of B Vitamins on Homocysteine Lowering and Thrombotic Risk Reduction-A Review of Randomized Controlled Trials Published Since January 1996. Nutrients. Mar 24 2025;17(7)doi:10.3390/nu17071122 https://www.ncbi.nlm.nih.gov/pubmed/40218880
- Olaso-Gonzalez G, Inzitari M, Bellelli G, Morandi A, Barcons N, Vina J. Impact of supplementation with vitamins B(6) , B(12) , and/or folic acid on the reduction of homocysteine levels in patients with mild cognitive impairment: A systematic review. IUBMB Life. Jan 2022;74(1):74-84. doi:10.1002/iub.2507 https://www.ncbi.nlm.nih.gov/pubmed/34058062
- Xu M, Zhu Y, Chen J, et al. Effects of folic acid supplementation on cognitive impairment: A meta-analysis of randomized controlled trials. J Evid Based Med. Mar 2024;17(1):134-144. doi:10.1111/jebm.12588
- Zhang N, Zhou Z, Chi X, et al. Folic acid supplementation for stroke prevention: A systematic review and meta-analysis of 21 randomized clinical trials worldwide. Clin Nutr. Jul 2024;43(7):1706-1716. doi:10.1016/j.clnu.2024.05.034
- Wang WW, Wang XS, Zhang ZR, He JC, Xie CL. A Meta-Analysis of Folic Acid in Combination with Anti-Hypertension Drugs in Patients with Hypertension and Hyperhomocysteinemia. Front Pharmacol . 2017;8:585. doi:10.3389/fphar.2017.00585 https://www.ncbi.nlm.nih.gov/pubmed/28912716
- Reynolds EH. Folate, vitamin B12, one carbon metabolism and the nervous system: Excess folic acid is potentially harmful. J Neurol Sci . Sep 15 2025;476:123627. doi:10.1016/j.jns.2025.123627
- NIH. National Institutes of Health: Office of Dietary Supplements. Folate. Last updated 11/30/2022. Accessed 10/30/2025. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- CDC. Centers for Disease Control and Prevention. Neural Tube Defects. Updated 5/20/2025. Accessed 11/4/2025, https://www.cdc.gov/birth-defects/about/neural-tube-defects.html
- Menezo Y, Elder K, Clement A, Clement P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules . Jan 24 2022;12(2)doi:10.3390/biom12020197 https://mdpi-res.com/d_attachment/biomolecules/biomolecules-12-00197/article_deploy/biomolecules-12-00197-v2.pdf?version=1643074627
- Łoboś P, Regulska-Ilow B. Link between methyl nutrients and the DNA methylation process in the course of selected diseases in adults. Rocz Panstw Zakl Hig. 2021;72(2):123-136. doi:10.32394/rpzh.2021.0157
- Araszkiewicz AF, Jańczak K, Wójcik P, et al. MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications-A Review. Genes. Apr 8 2025;16(4)doi:10.3390/genes16040441 https://mdpi-res.com/d_attachment/genes/genes-16-00441/article_deploy/genes-16-00441-v2.pdf?version=1744177062
- Gunnala S, Buhlman LM, Jadavji NM. How Increased Dietary Folic Acid Intake Impacts Health Outcomes Through Changes in Inflammation, Angiogenesis, and Neurotoxicity. Nutrients. Apr 7 2025;17(7)doi:10.3390/nu17071286 https://mdpi-res.com/d_attachment/nutrients/nutrients-17-01286/article_deploy/nutrients-17-01286.pdf?version=1744016586
- Maruvada P, Stover PJ, Mason JB, et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr . Nov 11 2020;112(5):1390-1403. doi:10.1093/ajcn/nqaa259 https://www.sciencedirect.com/science/article/pii/S0002916522009091?via%3Dihub
- Liu F, Edelmann M, Piironen V, Kariluoto S. The bioaccessibility of folate in breads and the stability of folate vitamers during in vitro digestion. 10.1039/D1FO03352B. Food & Function. 2022;13(6):3220-3233. doi:10.1039/D1FO03352B http://dx.doi.org/10.1039/D1FO03352B
- Henderson AM, Aleliunas RE, Loh SP, et al. l-5-Methyltetrahydrofolate Supplementation Increases Blood Folate Concentrations to a Greater Extent than Folic Acid Supplementation in Malaysian Women. J Nutr. Jun 1 2018;148(6):885-890. doi:10.1093/jn/nxy057 https://www.ncbi.nlm.nih.gov/pubmed/29878267 https://www.sciencedirect.com/science/article/pii/S0022316622163388?via%3Dihub
- beid R, Schön C, Pietrzik K, et al. Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts. Nutrients. Nov 25 2020;12(12)doi:10.3390/nu12123623 https://mdpi-res.com/d_attachment/nutrients/nutrients-12-03623/article_deploy/nutrients-12-03623-v2.pdf?version=1606376364
- Sicinska E, Brzozowska A, Roszkowski W, Finglas PM. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces serum homocysteine concentrations in older adults. Int J Food Sci Nutr . Feb 2018;69(1):64-73. doi:10.1080/09637486.2017.1320536 https://www.ncbi.nlm.nih.gov/pubmed/28460586
- Ayoub G. Vitamins, Vascular Health and Disease. Nutrients . Sep 15 2025;17(18)doi:10.3390/nu17182955 https://mdpi-res.com/d_attachment/nutrients/nutrients-17-02955/article_deploy/nutrients-17-02955.pdf?version=1757925960
- Zhou Y, He A, Xu B. Natural resources, quantification, microbial bioconversion, and bioactivities of vitamin B(12) for vegetarian diet. Food Chem. Jan 15 2025;463(Pt 1):140849. doi:10.1016/j.foodchem.2024.140849 https://www.sciencedirect.com/science/article/abs/pii/S0308814624024993?via%3Dihub
- Özdemir S, Demirtaş S. Are vitamin B-12 measurements adequate for evaluating its deficiency in individuals? Asia Pac J Clin Nutr . Apr 2025;34(2):232-239. doi:10.6133/apjcn.202504_34(2).0010
- Harrington DJ, Stevenson E, Sobczyńska-Malefora A. The application and interpretation of laboratory biomarkers for the evaluation of vitamin B12 status. Annals of clinical biochemistry. Jan 2025;62(1):22-33. doi:10.1177/00045632241292432 https://journals.sagepub.com/doi/10.1177/00045632241292432?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
- Niklewicz A, Hannibal L, Warren M, Ahmadi KR. A systematic review and meta-analysis of functional vitamin B12 status among adult vegans. Nutrition Bulletin / Bnf. Dec 2024;49(4):463-479. doi:10.1111/nbu.12712
- Mathew AR, Di Matteo G, La Rosa P, et al. Vitamin B12 Deficiency and the Nervous System: Beyond Metabolic Decompensation-Comparing Biological Models and Gaining New Insights into Molecular and Cellular Mechanisms. Int J Mol Sci. Jan 2 2024;25(1)doi:10.3390/ijms25010590 https://mdpi-res.com/d_attachment/ijms/ijms-25-00590/article_deploy/ijms-25-00590.pdf?version=1704183914
- Dastidar R, Sikder K. Diagnostic reliability of serum active B12 (holo-transcobalamin) in true evaluation of vitamin B12 deficiency: Relevance in current perspective. BMC Res Notes. Oct 22 2022;15(1):329. doi:10.1186/s13104-022-06224-8 https://bmcresnotes.biomedcentral.com/counter/pdf/10.1186/s13104-022-06224-8.pdf
- Stürmer P, Strathmann EA, Liedtke TP, et al. Correlates of serum holo-Transcobalamin in the elderly general population. Eur J Nutr . Aug 31 2025;64(6):270. doi:10.1007/s00394-025-03789-5
- Abdelwahab OA, Abdelaziz A, Diab S, et al. Efficacy of different routes of vitamin B12 supplementation for the treatment of patients with vitamin B12 deficiency: A systematic review and network meta-analysis. Ir J Med Sci. Jun 2024;193(3):1621-1639. doi:10.1007/s11845-023-03602-4 https://www.ncbi.nlm.nih.gov/pubmed/38231320
- Lacombe V, Vinatier E, Roquin G, et al. Oral vitamin B12 supplementation in pernicious anemia: a prospective cohort study. Am J Clin Nutr . Jul 2024;120(1):217-224. doi:10.1016/j.ajcnut.2024.05.019 https://www.sciencedirect.com/science/article/pii/S0002916524004842?via%3Dihub
- Sohouli MH, Almuqayyid F, Alfardous Alazm A, et al. A comprehensive review and meta-regression analysis of randomized controlled trials examining the impact of vitamin B12 supplementation on homocysteine levels. Nutr Rev. May 10 2024;82(6):726-737. doi:10.1093/nutrit/nuad091 https://www.ncbi.nlm.nih.gov/pubmed/37495210
- Wang X, Peng H, Xia C, et al. Association of B vitamin intake and total homocysteine levels with all-cause and cause-specific mortality in central obesity. Nutrition. Dec 2023;116:112189. doi:10.1016/j.nut.2023.112189 https://www.ncbi.nlm.nih.gov/pubmed/37689015
- Spence JD. Cardioembolic stroke: everything has changed. Stroke Vasc Neurol. Jun 2018;3(2):76-83. doi:10.1136/svn-2018-000143 https://www.ncbi.nlm.nih.gov/pubmed/30022801
- Spence JD, Hankey GJ. Problem in the Recent American Heart Association Guideline on Secondary Stroke Prevention: B Vitamins to Lower Homocysteine Do Prevent Stroke. Stroke. Aug 2022;53(8):2702-2708. doi:10.1161/STROKEAHA.122.038640 https://www.ncbi.nlm.nih.gov/pubmed/35748292
- Heinz J, Kropf S, Domrose U, et al. B vitamins and the risk of total mortality and cardiovascular disease in end-stage renal disease: results of a randomized controlled trial. Circulation. Mar 30 2010;121(12):1432-8. doi:10.1161/CIRCULATIONAHA.109.904672 https://www.ncbi.nlm.nih.gov/pubmed/20231532
- Rafeq Z, Roh JD, Guarino P, Kaufman J, Joseph J. Adverse myocardial effects of B-vitamin therapy in subjects with chronic kidney disease and hyperhomocysteinaemia. Nutr Metab Cardiovasc Dis. Sep 2013;23(9):836-42. doi:10.1016/j.numecd.2012.07.002 https://www.ncbi.nlm.nih.gov/pubmed/22902185
- Spence JD. B vitamin therapy for homocysteine: renal function and vitamin B12 determine cardiovascular outcomes. Clinical chemistry and laboratory medicine : CCLM / FESCC . Mar 01 2013;51(3):633-7. doi:10.1515/cclm-2012-0465
- Spence JD. Reducing the Risk of Stroke in Patients with Impaired Renal Function: Nutritional Issues. J Stroke Cerebrovasc Dis . Sep 2021;30(9):105376. doi:10.1016/j.jstrokecerebrovasdis.2020.105376 https://www.ncbi.nlm.nih.gov/pubmed/33214054
- Wu HHL, Wang AY. Vitamin B12 and chronic kidney disease. Vitam Horm. 2022;119:325-353. doi:10.1016/bs.vh.2022.01.011 https://www.ncbi.nlm.nih.gov/pubmed/35337625
- Kumrungsee T, Peipei Z, Yanaka N, Suda T, Kato N. Emerging cardioprotective mechanisms of vitamin B6: a narrative review. Eur J Nutr. Mar 2022;61(2):605-613. doi:10.1007/s00394-021-02665-2 https://link.springer.com/article/10.1007/s00394-021-02665-2
- Kato N, Kimoto A, Zhang P, et al. Relationship of Low Vitamin B6 Status with Sarcopenia, Frailty, and Mortality: A Narrative Review. Nutrients. Jan 4 2024;16(1)doi:10.3390/nu16010177 https://mdpi-res.com/d_attachment/nutrients/nutrients-16-00177/article_deploy/nutrients-16-00177.pdf?version=1704377272
- Stach K, Stach W, Augoff K. Vitamin B6 in Health and Disease. Nutrients. Sep 17 2021;13(9)doi:10.3390/nu13093229 https://mdpi-res.com/d_attachment/nutrients/nutrients-13-03229/article_deploy/nutrients-13-03229.pdf?version=1631853526
- Gana W, De Luca A, Debacq C, et al. Analysis of the Impact of Selected Vitamins Deficiencies on the Risk of Disability in Older People. Nutrients. Sep 10 2021;13(9)doi:10.3390/nu13093163 https://mdpi-res.com/d_attachment/nutrients/nutrients-13-03163/article_deploy/nutrients-13-03163-v2.pdf?version=1631803549
- Muhamad R, Akrivaki A, Papagiannopoulou G, Zavridis P, Zis P. The Role of Vitamin B6 in Peripheral Neuropathy: A Systematic Review. Nutrients. Jun 21 2023;15(13)doi:10.3390/nu15132823 https://mdpi-res.com/d_attachment/nutrients/nutrients-15-02823/article_deploy/nutrients-15-02823.pdf?version=1687326148
- Pusceddu I, Herrmann W, Kleber ME, et al. Subclinical inflammation, telomere shortening, homocysteine, vitamin B6, and mortality: the Ludwigshafen Risk and Cardiovascular Health Study. Eur J Nutr. Jun 2020;59(4):1399-1411. doi:10.1007/s00394-019-01993-8 https://www.ncbi.nlm.nih.gov/pubmed/31129702
- Jayedi A, Zargar MS. Intake of vitamin B6, folate, and vitamin B12 and risk of coronary heart disease: a systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr . 2019;59(16):2697-2707. doi:10.1080/10408398.2018.1511967 https://www.ncbi.nlm.nih.gov/pubmed/30431328
- Calderon-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS neuroscience & therapeutics . Jan 2020;26(1):5-13. doi:10.1111/cns.13207 https://www.ncbi.nlm.nih.gov/pubmed/31490017
- Hadtstein F, Vrolijk M. Vitamin B-6-Induced Neuropathy: Exploring the Mechanisms of Pyridoxine Toxicity. Adv Nutr. Oct 1 2021;12(5):1911-1929. doi:10.1093/advances/nmab033 https://pubmed.ncbi.nlm.nih.gov/33912895/
- Reddy P. Preventing Vitamin B6-Related Neurotoxicity. American journal of therapeutics. Nov-Dec 01 2022;29(6):e637-e643. doi:10.1097/mjt.0000000000001460
- Pokushalov E, Ponomarenko A, Bayramova S, et al. Effect of Methylfolate, Pyridoxal-5'-Phosphate, and Methylcobalamin (Soloways(TM)) Supplementation on Homocysteine and Low-Density Lipoprotein Cholesterol Levels in Patients with Methylenetetrahydrofolate Reductase, Methionine Synthase, and Methionine Synthase Reductase Polymorphisms: A Randomized Controlled Trial. Nutrients. May 21 2024;16(11)doi:10.3390/nu16111550 https://www.ncbi.nlm.nih.gov/pubmed/38892484
- McNulty H, Pentieva K, Ward M. Causes and Clinical Sequelae of Riboflavin Deficiency. Annu Rev Nutr. Aug 21 2023;43:101-122. doi:10.1146/annurev-nutr-061121-084407
- Jarrett H, McNulty H, Hughes CF, et al. Vitamin B-6 and riboflavin, their metabolic interaction, and relationship with MTHFR genotype in adults aged 18-102 years. Am J Clin Nutr. Dec 19 2022;116(6):1767-1778. doi:10.1093/ajcn/nqac240 https://www.sciencedirect.com/science/article/pii/S0002916523037097?via%3Dihub
- Jungert A, McNulty H, Hoey L, et al. Riboflavin Is an Important Determinant of Vitamin B-6 Status in Healthy Adults. J Nutr . Oct 12 2020;150(10):2699-2706. doi:10.1093/jn/nxaa225 https://www.sciencedirect.com/science/article/pii/S0022316622023392?via%3Dihub
- McNulty H, Dowey le RC, Strain JJ, et al. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C->T polymorphism. Circulation. Jan 3 2006;113(1):74-80. doi:10.1161/CIRCULATIONAHA.105.580332 https://www.ncbi.nlm.nih.gov/pubmed/16380544
- Rooney M, Bottiglieri T, Wasek-Patterson B, et al. Impact of the MTHFR C677T polymorphism on one-carbon metabolites: Evidence from a randomised trial of riboflavin supplementation. Biochimie. Jun 2020;173:91-99. doi:10.1016/j.biochi.2020.04.004 https://www.sciencedirect.com/science/article/abs/pii/S0300908420300742?via%3Dihub
- Araki R, Maruyama C, Igarashi S, et al. Effects of short-term folic acid and/or riboflavin supplementation on serum folate and plasma total homocysteine concentrations in young Japanese male subjects. Eur J Clin Nutr. May 2006;60(5):573-9. doi:10.1038/sj.ejcn.1602351 https://www.ncbi.nlm.nih.gov/pubmed/16391577
- Jin Q, Chen S, Ji X. Associations of dietary riboflavin intake with coronary heart disease in US adults: a cross-sectional study of NHANES 2007-2018. Front Nutr. 2024;11:1467889. doi:10.3389/fnut.2024.1467889 https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2024.1467889/pdf
- Li M, Shi Z. Riboflavin Intake Inversely Associated with Cardiovascular-Disease Mortality and Interacting with Folate Intake: Findings from the National Health and Nutrition Examination Survey (NHANES) 2005-2016. Nutrients. Dec 16 2022;14(24)doi:10.3390/nu14245345 https://mdpi-res.com/d_attachment/nutrients/nutrients-14-05345/article_deploy/nutrients-14-05345.pdf?version=1671175204
- Gordon S, Hoey L, McNulty H, et al. Associations of one-carbon metabolism, related B-vitamins and ApoE genotype with cognitive function in older adults: identification of a novel gene-nutrient interaction. BMC Med. Jul 28 2025;23(1):440. doi:10.1186/s12916-025-04276-8 https://bmcmedicine.biomedcentral.com/counter/pdf/10.1186/s12916-025-04276-8.pdf
- Lu XT, Wang YN, Mo QW, et al. Effects of low-dose B vitamins plus betaine supplementation on lowering homocysteine concentrations among Chinese adults with hyperhomocysteinemia: a randomized, double-blind, controlled preliminary clinical trial. Eur J Nutr. Jun 2023;62(4):1599-1610. doi:10.1007/s00394-023-03087-y https://www.ncbi.nlm.nih.gov/pubmed/36717385
- Clements M, Heffernan M, Ward M, et al. A 2-Year Randomized Controlled Trial With Low-Dose B-Vitamin Supplementation Shows Benefits on Bone Mineral Density in Adults With Lower B12 Status. J Bone Miner Res . Dec 2022;37(12):2443-2455. doi:10.1002/jbmr.4709 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/jbmr.4709?download=true
- Douaud G, Refsum H, de Jager CA, et al. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A. Jun 4 2013;110(23):9523-8. doi:10.1073/pnas.1301816110 https://www.ncbi.nlm.nih.gov/pubmed/23690582
- Mazza A, Cicero AF, Ramazzina E, et al. Nutraceutical approaches to homocysteine lowering in hypertensive subjects at low cardiovascular risk: a multicenter, randomized clinical trial. J Biol Regul Homeost Agents . Jul-Sep 2016;30(3):921-927. https://www.ncbi.nlm.nih.gov/pubmed/27655522
- Chen L, Li Q, Fang X, Wang X, Min J, Wang F. Dietary Intake of Homocysteine Metabolism-Related B-Vitamins and the Risk of Stroke: A Dose-Response Meta-Analysis of Prospective Studies. Adv Nutr. Nov 16 2020;11(6):1510-1528. doi:10.1093/advances/nmaa061 https://www.ncbi.nlm.nih.gov/pubmed/32503038
- Osadnik T, Pawlas N, Lejawa M, et al. Genetic and environmental factors associated with homocysteine concentrations in a population of healthy young adults. Analysis of the MAGNETIC study. Nutr Metab Cardiovasc Dis . Jun 9 2020;30(6):939-947. doi:10.1016/j.numecd.2020.01.012 https://www.ncbi.nlm.nih.gov/pubmed/32404292
- Foscolou A, Rallidis LS, Tsirebolos G, et al. The association between homocysteine levels, Mediterranean diet and cardiovascular disease: a case-control study. Int J Food Sci Nutr. Aug 2019;70(5):603-611. doi:10.1080/09637486.2018.1547688 https://www.ncbi.nlm.nih.gov/pubmed/30501542
- Visekruna I, Rumbak I, Samarin IR, Keser I, Ranilovic J. Homocysteine Levels Show Significant Differences among Mediterranean Dietary Quality Index Variables Compared to Folate and Vitamin B(12) Status in Women. Int J Vitam Nutr Res. 2015;85(3-4):202-10. doi:10.1024/0300-9831/a000237 https://www.ncbi.nlm.nih.gov/pubmed/26780397
- Stubbendorff A, Kern S, Rydén L, Skoog I, Samuelsson J. The EAT-Lancet diet in relation nutrient intake among older adults: insights from the Gothenburg H70 birth cohort study. Nutr J. Aug 8 2025;24(1):124. doi:10.1186/s12937-025-01193-7 https://nutritionj.biomedcentral.com/counter/pdf/10.1186/s12937-025-01193-7.pdf
- Teixeira JA, Steluti J, Gorgulho BM, et al. Prudent dietary pattern influences homocysteine level more than folate, vitamin B12, and docosahexaenoic acid: a structural equation model approach. Eur J Nutr . Feb 2020;59(1):81-91. doi:10.1007/s00394-018-1886-8 https://www.ncbi.nlm.nih.gov/pubmed/30649595
- Gillies NA, Milan AM, Chia PHP, et al. Responsiveness of one-carbon metabolites to a high-protein diet in older men: Results from a 10-wk randomized controlled trial. Nutrition. Sep 2021;89:111231. doi:10.1016/j.nut.2021.111231
- Park S, Kang S. Interaction of Genetics and Dietary Patterns Scored by the High Healthy Eating Index in Hyperhomocysteinaemia Influencing Cardiovascular Disease Risk. Nutrition Bulletin / Bnf. Jun 2025;50(2):311-325. doi:10.1111/nbu.70007
- Tore EC, Eussen S, Bastani NE, et al. The Associations of Habitual Intake of Sulfur Amino Acids, Proteins and Diet Quality with Plasma Sulfur Amino Acid Concentrations: The Maastricht Study. J Nutr. Jul 2023;153(7):2027-2040. doi:10.1016/j.tjnut.2023.05.008 https://www.sciencedirect.com/science/article/pii/S0022316623376016?via%3Dihub
- Han L, Liu Y, Wang C, et al. Determinants of hyperhomocysteinemia in healthy and hypertensive subjects: A population-based study and systematic review. Clin Nutr. Oct 2017;36(5):1215-1230. doi:10.1016/j.clnu.2016.11.011 https://www.ncbi.nlm.nih.gov/pubmed/27908565
- Tajima A, Kubo Y, Horiguchi S, Shoji K, Kawabata T. Relationship between Serum Homocysteine Concentration and Dietary Factors in Young Japanese Women. Nutrients. Nov 10 2023;15(22)doi:10.3390/nu15224740 https://www.ncbi.nlm.nih.gov/pubmed/38004134
- Challa F, Getahun T, Sileshi M, et al. Prevalence of Hyperhomocysteinaemia and Associated Factors among Ethiopian Adult Population in a 2015 National Survey. Biomed Res Int. 2020;2020:9210261. doi:10.1155/2020/9210261 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1155/2020/9210261?download=true
- Majda A, Zalewska-Puchala J, Bodys-Cupak I, Kaminska A, Kurowska A, Suder M. Comparison of Lifestyle of Catholics and Seventh-Day Adventists and the Relationship with Homocysteine as Risk Factor for Cardiovascular Diseases, a Cross-Sectional Study in Polish Males and Females. Int J Environ Res Public Health. Jan 4 2021;18(1)doi:10.3390/ijerph18010309 https://www.ncbi.nlm.nih.gov/pubmed/33406604
- Schenkelaars N, van Rossem L, Willemsen SP, Faas MM, Schoenmakers S, Steegers-Theunissen RPM. The intake of ultra-processed foods and homocysteine levels in women with(out) overweight and obesity: The Rotterdam Periconceptional Cohort. Eur J Nutr. Jun 2024;63(4):1257-1269. doi:10.1007/s00394-024-03334-w https://www.ncbi.nlm.nih.gov/pubmed/38383813
- Ubbink J, Levine AS. From Processed Foods to Ultraprocessed Foods: Evolution of an Industry Model and Impact on Dietary Quality, Health, and Society. Annual review of food science and technology . Apr 2025;16(1):1-24. doi:10.1146/annurev-food-111523-122028
- Gibson A, Woodside JV, Young IS, et al. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers--a randomized, crossover intervention study. QJM. Nov 2008;101(11):881-7. doi:10.1093/qjmed/hcn112 https://www.ncbi.nlm.nih.gov/pubmed/18790817
- Broz P, Rajdl D, Racek J, Trefil L, Stehlík P. Effect of Beer Consumption on Methylation and Redox Metabolism. Physiological research / Academia Scientiarum Bohemoslovaca . Aug 31 2022;71(4):573-582. doi:10.33549/physiolres.934863
- Kose S, Sozlu S, Bolukbasi H, Unsal N, Gezmen-Karadag M. Obesity is associated with folate metabolism. Int J Vitam Nutr Res. Jun 2020;90(3-4):353-364. doi:10.1024/0300-9831/a000602 https://www.ncbi.nlm.nih.gov/pubmed/31512572
- Wang J, You D, Wang H, et al. Association between homocysteine and obesity: A meta-analysis. J Evid Based Med. Sep 2021;14(3):208-217. doi:10.1111/jebm.12412 https://www.ncbi.nlm.nih.gov/pubmed/33145936
- Al Fatly M, Mulder MT, Roeters van Lennep J, Blom HJ, Berk KAC. The effect of diet-induced weight loss on circulating homocysteine levels in people with obesity and type 2 diabetes. Nutr J. Jan 3 2024;23(1):2. doi:10.1186/s12937-023-00908-y https://www.ncbi.nlm.nih.gov/pubmed/38167024
- Zarshenas N, Tapsell LC, Batterham M, Neale EP, Talbot ML. Changes in Anthropometric Measures, Nutritional Indices and Gastrointestinal Symptoms Following One Anastomosis Gastric Bypass (OAGB) Compared with Roux-en-y Gastric Bypass (RYGB). Obes Surg. Jun 2021;31(6):2619-2631. doi:10.1007/s11695-021-05284-2 https://pmc.ncbi.nlm.nih.gov/articles/PMC7901677/
- Buckner SL, Loenneke JP, Loprinzi PD. Single and combined associations of accelerometer-assessed physical activity and muscle-strengthening activities on plasma homocysteine in a national sample. Clin Physiol Funct Imaging. Nov 2017;37(6):669-674. doi:10.1111/cpf.12356 https://www.ncbi.nlm.nih.gov/pubmed/26916173
- Tu W, Yan F, Chao B, Ji X, Wang L. Status of hyperhomocysteinemia in China: results from the China Stroke High-risk Population Screening Program, 2018. Front Med. Dec 2021;15(6):903-912. doi:10.1007/s11684-021-0871-4 https://www.ncbi.nlm.nih.gov/pubmed/34893949
- Alomari MA, Khabour OF, Gharaibeh MY, Qhatan RA. Effect of physical activity on levels of homocysteine, folate, and vitamin B12 in the elderly. The Physician and sportsmedicine. 2016;44(1):68-73. doi:10.1080/00913847.2016.1135037 https://www.ncbi.nlm.nih.gov/pubmed/26831008
- Wang W, Li J, Ji P, Bian R, Xiong Y. Association between regular aerobic exercise and hyperhomocysteine in hypertensive patients. Postgrad Med. Jun 2020;132(5):458-464. doi:10.1080/00325481.2020.1743114 https://www.ncbi.nlm.nih.gov/pubmed/32167398
- Rajabi A, Akbar Nezhad Gharehlo A, Madadizadeh E, et al. The effect of 12 weeks of aerobic exercise training with or without saffron supplementation on diabetes-specific markers and inflammation in women with type 2 diabetes: A randomized double-blind placebo-controlled trial. Eur J Sport Sci . Jul 2024;24(7):899-906. doi:10.1002/ejsc.12125 https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/ejsc.12125?download=true
- Tan Q, Li Y, Guo Y. Exercise Training Improves Functions of Endothelial Progenitor Cells in Patients with Metabolic Syndrome. Arq Bras Cardiol . Jul 2021;117(1):108-117. Exercício Físico Melhora as Funções das Células Progenitoras Endoteliais em Pacientes com Síndrome Metabólica. doi:10.36660/abc.20200028 https://www.scielo.br/j/abc/a/ztz5DfztGZsg5gsny6yr6Qt/?lang=en&format=pdf
- Zheng G, Zheng X, Li J, et al. Effects of Tai Chi on Cerebral Hemodynamics and Health-Related Outcomes in Older Community Adults at Risk of Ischemic Stroke: A Randomized Controlled Trial. Journal of aging and physical activity . Sep 1 2019;27(5):678–687. doi:10.1123/japa.2018-0232
- Gu Y, Bai J, Li Y, Han L, Wang D. The effect of Tai Chi on plasma homocysteine in 1176 adults: a propensity score matching-based study. BMC cardiovascular disorders. Jan 29 2025;25(1):61. doi:10.1186/s12872-025-04519-9 https://www.ncbi.nlm.nih.gov/pubmed/39875831
- Kuebler U, Linnebank M, Semmler A, et al. Plasma homocysteine levels increase following stress in older but not younger men. Psychoneuroendocrinology. Aug 2013;38(8):1381-7. doi:10.1016/j.psyneuen.2012.12.003 https://www.ncbi.nlm.nih.gov/pubmed/23312061
- Chien LW, Chang HC, Liu CF. Effect of yoga on serum homocysteine and nitric oxide levels in adolescent women with and without dysmenorrhea. J Altern Complement Med. Jan 2013;19(1):20-3. doi:10.1089/acm.2011.0113 https://www.ncbi.nlm.nih.gov/pubmed/22963270
- Burns BC, Belani JD, Wittorf HN, Brailoiu E, Brailoiu GC. Choline-An Essential Nutrient with Health Benefits and a Signaling Molecule. Int J Mol Sci. Jul 24 2025;26(15)doi:10.3390/ijms26157159 https://mdpi-res.com/d_attachment/ijms/ijms-26-07159/article_deploy/ijms-26-07159.pdf?version=1753365306
- Wallace TC, Fulgoni VL, 3rd. Assessment of Total Choline Intakes in the United States. J Am Coll Nutr. 2016;35(2):108-12. doi:10.1080/07315724.2015.1080127 https://www.ncbi.nlm.nih.gov/pubmed/26886842
- Craig SA. Betaine in human nutrition. Am J Clin Nutr . Sep 2004;80(3):539-49. doi:10.1093/ajcn/80.3.539 https://www.ncbi.nlm.nih.gov/pubmed/15321791
- Lee JE, Jacques PF, Dougherty L, et al. Are dietary choline and betaine intakes determinants of total homocysteine concentration? Am J Clin Nutr. May 2010;91(5):1303-10. doi:10.3945/ajcn.2009.28456 https://www.ncbi.nlm.nih.gov/pubmed/20219967
- Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients . Sep 9 2013;5(9):3481-95. doi:10.3390/nu5093481 https://www.ncbi.nlm.nih.gov/pubmed/24022817
- Zawieja E, Chmurzynska A. Betaine and aging: A narrative review of findings, possible mechanisms, research perspectives, and practical recommendations. Ageing Res Rev. Feb 2025;104:102634. doi:10.1016/j.arr.2024.102634
- Bortz J, Obeid R. The Shuttling of Methyl Groups Between Folate and Choline Pathways. Nutrients. Jul 30 2025;17(15)doi:10.3390/nu17152495 https://mdpi-res.com/d_attachment/nutrients/nutrients-17-02495/article_deploy/nutrients-17-02495.pdf?version=1753869611
- King JH, Kwan STC, Bae S, et al. Maternal choline supplementation alters vitamin B-12 status in human and murine pregnancy. J Nutr Biochem. Oct 2019;72:108210. doi:10.1016/j.jnutbio.2019.07.001
- Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. May 2003;133(5):1291-5. doi:10.1093/jn/133.5.1291 https://www.ncbi.nlm.nih.gov/pubmed/12730412
- Olthof MR, Verhoef P. Effects of betaine intake on plasma homocysteine concentrations and consequences for health. Curr Drug Metab . Feb 2005;6(1):15-22. doi:10.2174/1389200052997366 https://www.ncbi.nlm.nih.gov/pubmed/15720203
- Atkinson W, Slow S, Elmslie J, Lever M, Chambers ST, George PM. Dietary and supplementary betaine: effects on betaine and homocysteine concentrations in males. Nutr Metab Cardiovasc Dis. Dec 2009;19(11):767-73. doi:10.1016/j.numecd.2009.01.004 https://www.ncbi.nlm.nih.gov/pubmed/19346114
- Zawieja E, Drabińska N, Jeleń H, Szwengiel A, Durkalec-Michalski K, Chmurzynska A. Betaine supplementation modulates betaine concentration by methylenetetrahydrofolate reductase genotype, but has no effect on amino acid profile in healthy active males: A randomized placebo-controlled cross-over study. Nutr Res. Jul 2024;127:63-74. doi:10.1016/j.nutres.2024.05.003 https://www.sciencedirect.com/science/article/abs/pii/S0271531724000691?via%3Dihub
- Cholewa JM, Wyszczelska-Rokiel M, Glowacki R, et al. Effects of betaine on body composition, performance, and homocysteine thiolactone. J Int Soc Sports Nutr. Aug 22 2013;10(1):39. doi:10.1186/1550-2783-10-39 https://www.ncbi.nlm.nih.gov/pubmed/23967897
- Jakubowski H, Glowacki R. Chemical biology of homocysteine thiolactone and related metabolites. Advances in clinical chemistry . 2011;55:81-103. doi:10.1016/b978-0-12-387042-1.00005-8 https://www.ncbi.nlm.nih.gov/pubmed/22126025
- Jieru P, Zhang S, Cai L, et al. Dietary choline intake and health outcomes in U.S. adults: exploring the impact on cardiovascular disease, cancer prevalence, and all-cause mortality. Journal of health, population, and nutrition . May 6 2024;43(1):59. doi:10.1186/s41043-024-00528-0 https://www.ncbi.nlm.nih.gov/pubmed/38711145
- Li W, Liu S, Meng X, Liu H. A nutrient wide association study of cardiovascular disease prevalence in older adults from NHANES 2007 to 2018. Sci Rep. Apr 13 2025;15(1):12710. doi:10.1038/s41598-025-97143-8 https://www.nature.com/articles/s41598-025-97143-8.pdf
- Dawson SL, Bowe SJ, Crowe TC. A combination of omega-3 fatty acids, folic acid and B-group vitamins is superior at lowering homocysteine than omega-3 alone: A meta-analysis. Nutr Res. Jun 2016;36(6):499-508. doi:10.1016/j.nutres.2016.03.010 https://www.ncbi.nlm.nih.gov/pubmed/27188895
- Dzupina A, Pella D, Zenuch P, et al. The Role of Omega-3 Polyunsaturated Fatty Acids in Patients with Metabolic Syndrome and Endothelial Dysfunction. Medicina (Kaunas). Dec 30 2024;61(1)doi:10.3390/medicina61010043 https://mdpi-res.com/d_attachment/medicina/medicina-61-00043/article_deploy/medicina-61-00043.pdf?version=1735569281
- Sohouli MH, Roshan MM, Olusola OF, et al. Impact of Omega-3 supplementation on homocysteine levels in humans: A systematic review and meta-regression analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis . Sep 2022;32(9):2013-2025. doi:10.1016/j.numecd.2022.05.008 https://www.ncbi.nlm.nih.gov/pubmed/35843792
- Jerneren F, Cederholm T, Refsum H, et al. Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer's Disease: The OmegAD Study. J Alzheimers Dis. 2019;69(1):189-197. doi:10.3233/JAD-181148 https://www.ncbi.nlm.nih.gov/pubmed/30958356
- Maltais M, de Souto Barreto P, Bowman GL, et al. Omega-3 Supplementation for the Prevention of Cognitive Decline in Older Adults: Does It Depend on Homocysteine Levels? J Nutr Health Aging. 2022;26(6):615-620. doi:10.1007/s12603-022-1809-5 https://www.ncbi.nlm.nih.gov/pubmed/35718871
- Hooper C, De Souto Barreto P, Coley N, et al. Cross-Sectional Associations of Total Plasma Homocysteine with Cortical beta-Amyloid Independently and as a Function of Omega 3 Polyunsaturated Fatty Acid Status in Older Adults at Risk of Dementia. J Nutr Health Aging . 2017;21(10):1075-1080. doi:10.1007/s12603-017-0989-x https://www.ncbi.nlm.nih.gov/pubmed/29188863
- van Soest APM, van de Rest O, Witkamp RF, Cederholm T, de Groot L. DHA status influences effects of B-vitamin supplementation on cognitive ageing: a post-hoc analysis of the B-proof trial. Eur J Nutr . Oct 2022;61(7):3731-3739. doi:10.1007/s00394-022-02924-w https://link.springer.com/content/pdf/10.1007/s00394-022-02924-w.pdf
- Oulhaj A, Jerneren F, Refsum H, Smith AD, de Jager CA. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J Alzheimers Dis. 2016;50(2):547-57. doi:10.3233/JAD-150777 https://www.ncbi.nlm.nih.gov/pubmed/26757190
- Jerneren F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD. Brain atrophy in cognitively impaired elderly: the importance of long-chain omega-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. Jul 2015;102(1):215-21. doi:10.3945/ajcn.114.103283 https://www.ncbi.nlm.nih.gov/pubmed/25877495
- Kondakci G, Aydin AF, Dogru-Abbasoglu S, Uysal M. The effect of N-acetylcysteine supplementation on serum homocysteine levels and hepatic and renal oxidative stress in homocysteine thiolactone-treated rats. Arch Physiol Biochem. May 2017;123(2):128-133. doi:10.1080/13813455.2016.1273365 https://www.ncbi.nlm.nih.gov/pubmed/28100069
- Aydin AF, Kondakci G, Hatipoglu S, Dogru-Abbasoglu S, Uysal M. N-Acetylcysteine supplementation decreased brain lipid and protein oxidations produced by experimental homocysteine thiolactone exposure: Relevance to neurodegeneration. Pathophysiology : the official journal of the International Society for Pathophysiology . Jun 2018;25(2):125-129. doi:10.1016/j.pathophys.2018.02.004 https://www.ncbi.nlm.nih.gov/pubmed/29500146
- Faghfouri AH, Zarezadeh M, Tavakoli-Rouzbehani OM, et al. The effects of N-acetylcysteine on inflammatory and oxidative stress biomarkers: A systematic review and meta-analysis of controlled clinical trials. Eur J Pharmacol. Oct 5 2020;884:173368. doi:10.1016/j.ejphar.2020.173368 https://www.sciencedirect.com/science/article/abs/pii/S001429992030460X?via%3Dihub
- Yilmaz H, Sahin S, Sayar N, et al. Effects of folic acid and N-acetylcysteine on plasma homocysteine levels and endothelial function in patients with coronary artery disease. Acta cardiologica. Dec 2007;62(6):579-85. doi:10.2143/AC.62.6.2024017 https://www.ncbi.nlm.nih.gov/pubmed/18214123
- Shankle WR, Hara J, Barrentine LW, Curole MV. CerefolinNAC Therapy of Hyperhomocysteinemia Delays Cortical and White Matter Atrophy in Alzheimer's Disease and Cerebrovascular Disease. J Alzheimers Dis . Oct 4 2016;54(3):1073-1084. doi:10.3233/JAD-160241 https://www.ncbi.nlm.nih.gov/pubmed/27567825
- Hildebrandt W, Sauer R, Bonaterra G, Dugi KA, Edler L, Kinscherf R. Oral N-acetylcysteine reduces plasma homocysteine concentrations regardless of lipid or smoking status. Am J Clin Nutr . Nov 2015;102(5):1014-24. doi:10.3945/ajcn.114.101964 https://www.ncbi.nlm.nih.gov/pubmed/26447155
- Santulli G, Kansakar U, Varzideh F, Mone P, Jankauskas SS, Lombardi A. Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview. Nutrients. Sep 30 2023;15(19)doi:10.3390/nu15194236 https://mdpi-res.com/d_attachment/nutrients/nutrients-15-04236/article_deploy/nutrients-15-04236-v2.pdf?version=1741688739
- Tzang CC, Lin WC, Lin LH, et al. Insights into the cardiovascular benefits of taurine: a systematic review and meta-analysis. Nutr J. Aug 15 2024;23(1):93. doi:10.1186/s12937-024-00995-5 https://www.ncbi.nlm.nih.gov/pubmed/39148075
- Tzang CC, Chi LY, Lin LH, et al. Taurine reduces the risk for metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutr Diabetes. May 16 2024;14(1):29. doi:10.1038/s41387-024-00289-z
- Sun Q, Wang J, Wang H, et al. Effect of Long-Term Taurine Supplementation on the Lipid and Glycaemic Profile in Adults with Overweight or Obesity: A Systematic Review and Meta-Analysis. Nutrients. Dec 27 2024;17(1)doi:10.3390/nu17010055 https://mdpi-res.com/d_attachment/nutrients/nutrients-17-00055/article_deploy/nutrients-17-00055-v2.pdf?version=1735552480
- Zhang Z, Zhao L, Zhou Y, et al. Taurine ameliorated homocysteine-induced H9C2 cardiomyocyte apoptosis by modulating endoplasmic reticulum stress. Apoptosis. May 2017;22(5):647-661. doi:10.1007/s10495-017-1351-9 https://www.ncbi.nlm.nih.gov/pubmed/28229251
- Nonaka H, Tsujino T, Watari Y, Emoto N, Yokoyama M. Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation . Sep 4 2001;104(10):1165-70. doi:10.1161/hc3601.093976 https://www.ncbi.nlm.nih.gov/pubmed/11535574
- Zulli A, Lau E, Wijaya BP, et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension. Jun 2009;53(6):1017-22. doi:10.1161/HYPERTENSIONAHA.109.129924 https://www.ncbi.nlm.nih.gov/pubmed/19398656
- Ahn CS. Effect of taurine supplementation on plasma homocysteine levels of the middle-aged Korean women. Adv Exp Med Biol . 2009;643:415-22. doi:10.1007/978-0-387-75681-3_43 https://www.ncbi.nlm.nih.gov/pubmed/19239173
- Van Hove JLK, Freehauf CL, Ficicioglu C, et al. Biomarkers of oxidative stress, inflammation, and vascular dysfunction in inherited cystathionine beta-synthase deficient homocystinuria and the impact of taurine treatment in a phase 1/2 human clinical trial. Journal of inherited metabolic disease . May 2019;42(3):424-437. doi:10.1002/jimd.12085 https://www.ncbi.nlm.nih.gov/pubmed/30873612
- Papakostas GI, Cassiello CF, Iovieno N. Folates and S-adenosylmethionine for major depressive disorder. Can J Psychiatry . Jul 2012;57(7):406-13. doi:10.1177/070674371205700703 https://www.ncbi.nlm.nih.gov/pubmed/22762295
- Bhatia P, Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundamental & clinical pharmacology . Dec 2015;29(6):522-8. doi:10.1111/fcp.12145 https://www.ncbi.nlm.nih.gov/pubmed/26376956
- Karas Kuzelicki N. S-Adenosyl Methionine in the Therapy of Depression and Other Psychiatric Disorders. Drug Dev Res. Nov 2016;77(7):346-356. doi:10.1002/ddr.21345 https://www.ncbi.nlm.nih.gov/pubmed/27594595
- Anderson S, Panka J, Rakobitsch R, Tyre K, Pulliam K. Anxiety and Methylenetetrahydrofolate Reductase Mutation Treated With S-Adenosyl Methionine and Methylated B Vitamins. Integr Med (Encinitas) . Apr 2016;15(2):48-52. https://www.ncbi.nlm.nih.gov/pubmed/27330489
- Mischoulon D, Alpert JE, Arning E, Bottiglieri T, Fava M, Papakostas GI. Bioavailability of S-adenosyl methionine and impact on response in a randomized, double-blind, placebo-controlled trial in major depressive disorder. J Clin Psychiatry. Jun 2012;73(6):843-8. doi:10.4088/JCP.11m07139 https://www.ncbi.nlm.nih.gov/pubmed/22687580
