
Heart Failure
Heart Failure
Last Section Update: 04/2025
Contributor(s): Shayna Sandhaus, PhD; Maureen Williams, ND
Table of Contents
- Overview
- Introduction
- Understanding the Heart and Heart Failure
- Heart Failure: Causes and Risk Factors
- What are the Signs and Symptoms of Heart Failure?
- How is Heart Failure Diagnosed?
- Heart Failure Treatment
- Novel and Emerging Therapies for Heart Failure
- Dietary and Lifestyle Consideration
- Nutrients
- Update History
- References
1 Overview
Summary and Quick Facts for Heart Failure
- Heart failure is a general term describing a syndrome in which the heart cannot pump enough blood to meet the body’s needs. About 5.7 million adults in the United States have heart failure and approximately 550,000 cases are diagnosed in the United States each year.
- This protocol will review the causes of and risk factors for heart failure, along with current standards of care and a number of emerging treatment strategies. Dietary and lifestyle strategies that can support overall cardiovascular health will also be reviewed, as will a variety of natural, integrative interventions shown in studies to support heart health.
- Standard treatment options for heart failure focus on limiting symptoms and attempting to reduce progression and mortality. A comprehensive strategy for minimizing risk and improving heart failure outcomes should address its underlying causes.
What is Heart Failure?
Heart failure is a condition in which the heart cannot pump enough blood to meet the body’s demands. Most cases of heart failure begin with the left ventricle being unable to pump blood efficiently to the body. As heart failure progresses, the body tries to keep up with tissue oxygen demands, but it cannot maintain this indefinitely.
Heart failure can occur when the heart becomes weakened or damaged. Many conditions can cause heart failure, the most common being coronary artery disease and ischemic heart disease.
The mortality rate from heart failure remains high despite medical advances. Natural integrative ingredients such as coenzyme Q10 and hawthorn may help improve outcomes for patients with heart failure.
What are the Risk Factors for Heart Failure?
- Family history of heart disease and other heart-related diseases
- Excessive alcohol consumption
- Smoking
- Lack of physical activity
- Poor dietary habits
- Obesity/overweight
- Concurrent health conditions, including:
- History of heart disease
- Diabetes
- High blood pressure
- Chronic obstructive pulmonary disease
- Kidney disease
- Depression
- Sleep apnea
- Certain medications, including some cancer drugs and antidepressants
What are the Signs and Symptoms of Heart Failure?
- Fatigue
- Difficulty breathing
- Decreased capacity for physical activity
- Fluid retention
- Frequent nighttime urination
- Irregular heartbeat
- Decreased appetite and/or nausea
- Difficulty concentrating, less alert
- In advanced heart failure, wheezing, cough with pink-tinged frothy sputum, abdominal discomfort or swelling, anorexia, and weight loss may occur
What are Conventional Medical Treatments for Heart Failure?
Note: Treatment considerations/recommendations vary depending on disease severity.
Stages A and B: For patients at high risk of heart failure, but without structural disease or symptoms of heart failure (Stage A); or patients with structural heart disease but without signs or symptoms of heart failure (Stage B):
- Diet and lifestyle improvement
- Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) for high blood pressure
- Beta blockers to lower heart rate and blood pressure
- Statins to lower cholesterol levels
Stage C: For patients who have structural heart disease with prior or current symptoms of heart failure, the same treatments are recommended, with the possible addition of:
- Angiotensin receptor-neprilysin inhibitors (ARNIs)
- Diuretics
- Aldosterone antagonists
- Cardiac glycosides
- Anticoagulants
- Sinoatrial current inhibitor
- SGLT2 inhibitors
- Medical devices, such as a pacemaker
- Continuous positive airway pressure (CPAP) (for patients with sleep apnea)
- Medically supervised exercise program
- Cardiac rehabilitation
Stage D: For patients with treatment-resistant heart failure:
- Heart transplant
- Biventricular pacemaker
- Left ventricle assist devices
What are Emerging Therapies for Heart Failure?
- Stem cell therapy
- Testosterone replacement therapy
- Vagus nerve stimulation
- Trimetazine, and others
What Dietary and Lifestyle Changes Can Be Beneficial for Heart Failure?
- Quit smoking and limit alcohol intake
- Follow a heart-healthy diet, such as DASH or the Mediterranean diet
- Ensure you are getting enough micronutrients, like potassium, magnesium, and B vitamins
- Physical activity (someone with heart failure should undergo exercise programs that are medically supervised)
- Manage stress and anxiety
- Maintain healthy blood sugar levels
What Natural Interventions May Be Beneficial for Heart Failure?
- Coenzyme Q10. Coenzyme Q10 (CoQ10) is essential for energy production in the mitochondria and is concentrated in heart muscle. CoQ10 deficiency is associated with heart failure. Several clinical studies have shown benefits from CoQ10 supplementation in heart failure, including lower risk of adverse cardiac events or death.
- Hawthorn. Extracts from hawthorn contain many beneficial phytochemicals, including oligomeric procyanidins. Hawthorn has been shown to improve left ventricle ejection fraction, cardiac efficiency, and blood pressure in patients with heart failure.
- Pyrroloquinoline quinone (PQQ). PQQ, like CoQ10, is involved in generating energy in the mitochondria. Preclinical studies suggest PQQ may benefit the heart muscle.
- Fish oil. Omega-3 fatty acids are well known for their positive impact on heart health. Clinical studies indicate supplementation with fish oil may improve certain parameters in patients with heart failure.
- Carnitine. Carnitine is important for cardiac energy metabolism. Clinical trials have shown that carnitine supplementation improved cardiac efficiency, left ventricle ejection fraction, and 3-year survival rates in heart failure patients.
- Many other ingredients may be beneficial for patients with heart failure, including creatine, taurine, D-ribose, and more.
2 Introduction
Heart failure is a general term describing a syndrome in which the heart cannot pump enough blood to meet the body’s needs. Heart failure can develop rapidly or gradually, and is most commonly caused by coronary artery disease, which occurs when fatty plaque deposits build up in the arteries supplying blood to the heart muscle.1 Other factors that can contribute to heart failure include structural heart muscle defects, cardiac valve disease, lung disease, coronary artery disease due to atherosclerosis, thyroid disease, and anemia.2-7
About 5.7 million adults in the United States have heart failure, and approximately 550,000 cases are diagnosed in the United States each year.8 Standard treatment options for heart failure focus on limiting symptoms and attempting to reduce progression and mortality. Despite medical advances, heart failure continues to reduce quality of life for those it affects, and the mortality rate due to heart failure remains high.9,10 However, several novel and integrative strategies appear promising.
For instance, an important insight in heart failure research came in 2014 with the publication of results of the Q-SYMBIO coenzyme Q10 (CoQ10) trial. This groundbreaking two-year study showed that CoQ10 supplementation significantly reduced the risk of a major cardiovascular event compared with placebo in subjects with moderate-to-severe heart failure.11 CoQ10 supplementation is especially important for individuals taking cholesterol-lowering statin drugs because statins block the biosynthesis of both cholesterol and CoQ10.12-16
A comprehensive strategy for minimizing risk and improving heart failure outcomes should also address its underlying causes. Therefore, readers are encouraged to review additional Life Extension protocols on atherosclerosis and cardiovascular disease, high blood pressure, cardiac arrhythmia, cholesterol management, weight loss, diabetes, kidney disease, and hyperthyroidism and hypothyroidism.
This protocol will review the causes of and risk factors for heart failure, along with current standards of care and a number of emerging treatment strategies. Dietary and lifestyle strategies that can support overall cardiovascular health will be reviewed as well, as will a variety of natural, integrative interventions shown in studies to support heart health.
3 Understanding the Heart and Heart Failure
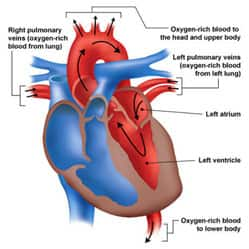
The human heart consists of left and right halves, which behave as two parallel “pumps” with distinct roles in circulation. Both the left and right side of the heart contain two chambers: a smaller atrium, at the top, which receives blood and transfers it to a larger, more muscular ventricle. The ventricles, situated at the bottom of the heart, pump blood from the heart into circulation.17
The right atrium receives low-oxygen blood from systemic circulation, and the right ventricle then pumps it to the lungs to become re-oxygenated. The left atrium of the heart receives high-oxygen blood from the lungs (pulmonary circulation), and the left ventricle then pumps it into systemic circulation. Thus, the two sides of the heart work in conjunction to collect oxygen-poor blood from peripheral tissues, send it to the lungs to pick up oxygen and deposit carbon dioxide, and redistribute the newly oxygenated blood to tissues and organs.17
Heart failure can occur when the heart becomes weakened or damaged (see “Heart Failure: Causes and Risk Factors”). The ventricles may become too stiff to fill properly or stretch too much to pump blood efficiently. Ejection fraction (EF) is a measure of the percentage of blood ejected from the left ventricle with each heartbeat, reflecting the efficiency of the heart’s pumping action. A normal left ventricular EF is 52 – 72% for men and 54 – 74% for women.401 In other words, a normally functioning heart will eject somewhere in the range of 50 – 75% of the total blood in the left ventricle with each heartbeat. Heart failure can occur with reduced EF or preserved EF. Whether EF is reduced or preserved may influence treatment decisions and the course of the condition.
As the heart begins to fail, the body tries to compensate to ensure that adequate oxygen is delivered to tissues. Signals from the nervous system, kidneys, and blood vessels result in fluid retention (to increase blood pressure in an attempt to better distribute oxygenated blood), increased heart rate and contractile force, and dilatation of the ventricle (to hold more blood) to increase ejection force.
Increases in blood volume and ventricular filling pressures cause blood to “back up” in systemic or pulmonary circulation and leak fluid into peripheral tissues, causing edema (swelling) in the lungs, abdomen, and extremities. This is termed “congestive” heart failure.18 Fluid can sometimes collect in the lungs, hindering breathing. This is known as pulmonary edema, which can cause respiratory distress if untreated.19
As heart failure progresses, the body tries to keep up with tissue oxygen demands. However, the heart is restricted in how much it can expand to hold more blood or increase its contractile force and rate, and the kidneys can only process so much water before fluid infiltrates other organs and tissues. Once compensation limits have been reached, the cardiovascular system can no longer satisfy tissue oxygen demands. This is called decompensated heart failure, which requires immediate medical intervention.18,20
Types of Heart Failure
Distinction is sometimes made between “left-sided” and “right-sided” heart failure. In left-sided heart failure, the left ventricle is primarily affected. Right-sided heart failure usually arises after left-sided heart failure progresses, and typically does not occur independently. In less common conditions, such as cor pulmonale (a lung problem), the right side of the heart may be primarily affected.
Left-sided heart failure: reduced and preserved ejection fraction. The left ventricle, the largest and most muscular of the four heart chambers, must generate a substantial amount of force to pump blood into the systemic circulation. Generally, heart failure begins with the left ventricle.1 In left-sided heart failure, the ability of the left ventricle to push oxygenated blood into circulation is compromised, meaning the heart must work harder to pump the same amount of blood.
There are two types of left-sided heart failure: heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).21 As previously noted, ejection fraction is a measure of the amount of blood that leaves the left ventricle and enters systemic circulation with each heartbeat. It represents how efficiently the left ventricle empties itself. In HFrEF, the ventricle cannot contract normally and lacks the force to adequately eject blood. In HFpEF, the ventricle is unable to relax and fill properly. Patients with HFrEF typically respond well to standard treatments and have a more favorable prognosis than those with HFpEF.22
Ejection fractions between 40‒55% may indicate cardiac damage, and an ejection fraction < 40%, termed reduced ejection fraction, indicates heart failure or significant heart muscle damage.23 An ejection fraction of > 75% may indicate hypertrophic cardiomyopathy, in which abnormally thick heart muscle makes it difficult for the heart to pump out enough blood.
The most common causes of heart failure are ischemic heart disease and coronary artery disease. Other causes include high blood pressure, valvular heart disease, congenital heart disease, and a variety of cardiomyopathies.24,25
Right-sided heart failure. The right side of the heart pumps oxygen-poor blood to the lungs so it can be oxygenated. Right-sided heart failure usually occurs as a result of left-sided failure. When the left ventricle fails, increased fluid pressure backs up through the pulmonary circulation and increases the resistance against which the right ventricle must pump. As the right side of the heart fails, blood backs up in the body's veins. This may cause swelling in the legs, ankles, and abdomen.26
4 Heart Failure: Causes and Risk Factors
Heart failure may be due to a variety of factors and causes, such as damage to the heart muscle of unknown origin (idiopathic cardiomyopathy), developmental abnormalities (eg, atrial septal defect), thyroid disease (eg, hyperthyroidism), and cardiac valve disease, among others. The most common cause of heart failure is ischemic heart disease due to coronary artery atherosclerosis. Recognition and mitigation of the various contributing factors may reduce heart failure risk and improve prognosis.27,28
Genetics and Family History
A family history of heart failure, cardiomyopathy (dysfunction of heart muscle), atherosclerotic disease, arrhythmia, skeletal myopathy (muscle disease involving skeletal muscle), or sudden cardiac death are well-known risk factors for heart failure.28-30
Diet and Lifestyle
Dietary and lifestyle factors associated with increased risk of heart failure include excessive alcohol consumption and nutritional deficiencies (B vitamins).30-32 Smoking is a major risk factor for developing heart failure, and quitting smoking was shown to have a significant effect on lowering morbidity and the risk of death in people with left ventricular dysfunction, an effect that was comparable to currently approved drugs.33,34
Physical inactivity, a known risk factor for many cardiovascular diseases, was shown to worsen the survival rate of patients with heart failure; a study reported that 2.5 years after being admitted to the hospital, only 25% of patients with a sedentary lifestyle were alive compared with 75% of physically active patients.35
Insufficient intake of fruits and vegetables is another risk factor associated with heart failure. A 22-year, prospective cohort study including 20,900 men assessed the association between heart failure and body weight, smoking, exercise, alcohol intake, and dietary habits, including fruit, vegetable, and breakfast cereal consumption. Healthy lifestyle habits were individually and jointly linked to a lower lifetime risk of heart failure, with the lowest risk (1 in 10) in the men who adhered to four or more factors, and the highest risk (1 in 5) in the men who adhered to none of the six factors.36 In addition, a diet with too much added sugar has been shown to increase risk of cardiovascular disease and mortality.37
Health Conditions Associated with Heart Failure
Heart disease. Atrial fibrillation, valve disease (including aortic stenosis, mitral regurgitation, tricuspid regurgitation), ischemic heart disease due to coronary artery atherosclerosis, and prior heart attack are associated with an increased risk of heart failure.30,38 Heart arrhythmias; cardiomyopathy caused by drug use, disease, infection, or alcohol abuse; and myocarditis from an infection may also cause heart disease. In addition, congenital heart defects may lead to heart failure.1
Hypertension. Hypertension (high blood pressure) increases heart failure risk two- to three-fold.39,40 Half of patients with acute heart failure have systolic blood pressure over 140 mmHg, and 70% have a history of high blood pressure.41 For more information, refer to the High Blood Pressure protocol.
Diabetes. Diabetics have a high rate of heart failure, and their heart failure prognosis is usually worse than in non-diabetics.42 Diabetics often have elevated lipid levels and hypertension, both risk factors for heart failure.
Chronic obstructive pulmonary disease (COPD). Long-standing obstructive pulmonary disease, often caused by tobacco abuse, is associated with heart failure, and when the two occur simultaneously, prognosis is worse than either alone.43 Advanced COPD typically contributes to right-sided heart failure (cor pulmonale).
Renal insufficiency/kidney disease. Heart failure can reduce blood flow to the kidneys, which may cause kidney failure if untreated. Evidence suggests heart failure is widespread in patients with chronic kidney disease and end-stage renal disease, and its prevalence increases with decreasing kidney function. Heart diseases is a strong predictor of mortality in dialysis patients.44
Overweight/obesity. The heart of an obese person must work harder than for a non-obese person. Having a high body mass index (BMI) is a risk factor for developing heart failure.45 Being overweight is linked to heart failure risk factors such as high blood pressure, diabetes, high blood lipid levels, metabolic syndrome, and an enlarged left ventricle. Obesity is also associated with sleep apnea and cardiomyopathy.19
Depression. Depression and heart failure often occur together, especially in older people. These conditions also seem to worsen one another in older individuals, a phenomenon termed “negative synergism.” Depression is often under-recognized in heart failure patients. Proper mental health screening and support is an important part of optimal care for those affected by heart failure.46 More information is available in Life Extension’s Depression protocol.
Other conditions. Other conditions less well-recognized to be linked with increased heart failure risk include iron overload, rheumatologic and connective tissue disorders, infection (HIV, infectious myocarditis), endocrine disorders (thyroid disease and growth hormone disorders), and amyloidosis and sarcoidosis.47
Medications That May Increase Heart Failure Risk
Certain medications may cause or worsen heart failure through toxicity, worsening hypertension levels, increasing the sodium load, or drug-drug interactions.58 Some drugs that cause or exacerbate heart failure include58:
- thiazolidinediones (a class of diabetes medications)
- antiarrhythmics (eg, dronedarone)
- anti-cancer drugs (eg, anthracyclines)
- targeted cancer therapies (eg, bevacizumab and lapatinib)
- hematologic medications (eg, anagrelide)
- certain antidepressants (eg, citalopram)
- pergolide (an anti-Parkinson medication)
- certain appetite suppressants
- pulmonary medications (eg, bosentan and epoprostenol)
- tumor necrosis factor-alpha (TNF-α) inhibitors
Prognostic Factors
Predictors of poor outcome and mortality in heart failure include having a reduced VO2 max capacity (the maximum intake of oxygen with increasing exercise intensity), older age, male gender, diabetes, a left ventricular ejection fraction of <45%, and a more advanced New York Heart Association (NYHA) heart failure classification.22 Anemia and depression have also been associated with poor outcomes in heart failure.59,60
Complications of Heart Failure
The prognosis of heart failure depends on the patient’s age and overall health, as well as the cause and severity of heart failure. Complications may include liver or kidney damage or failure, problems with the heart valves or heart rhythm, pulmonary congestion, anemia, muscle wasting, stroke, or pulmonary embolism. While there is no cure for heart failure, some people may experience improvements in heart function and symptoms with proper treatment, including medications, weight loss, exercise, a healthy diet, stress reduction, and dietary supplements, such as CoQ10, fish oil, and carnitine.1
5 What are the Signs and Symptoms of Heart Failure?
Some of the most prominent symptoms of heart failure are listed below.61 Some symptoms may not be apparent in mild heart failure, but will emerge as heart failure advances to moderate or severe stages.
- Fatigue and difficulty breathing (dyspnea), which can lead to decreased capacity for physical activity (exercise intolerance). In cases of mild heart failure, it may be difficult to breathe during physical activity, while in advanced heart failure it may be difficult for patients to breathe even at rest.
- Fluid retention, which may result in peripheral or pulmonary edema.28 However, not all people with heart failure will exhibit both exercise intolerance and edema.
- Frequent nighttime urination (nocturia).
- Rapid or irregular heartbeat.
- Lack of appetite or nausea.
- Decreased mental alertness or difficulty concentrating.
- In advanced heart failure, wheezing, cough that produces pink-tinged frothy sputum, abdominal discomfort or swelling, anorexia, and weight loss may occur.
Some signs that may suggest heart failure include changes in heart size (cardiomegaly) and/or rhythm, impaired lung function, evidence of low blood oxygen, and abdominal swelling. These signs are typically progressive with the severity of heart failure.2
Classification and Staging of Heart Failure
Classifying heart failure on the basis of severity and clinical manifestations helps clarify what kinds of interventions may be necessary and what the prognosis may be. The New York Heart Association (NYHA) Functional Classification classifies patients with cardiac disease into one of four classes, based on symptoms and their degree of comfort at different levels of physical activity.
NYHA Functional Classification
- Class I patients have no physical limitations or any symptoms such as fatigue, palpitations, breathlessness, or chest pain.
- Class II patients are comfortable at rest and can typically perform everyday activities. Physical activity may result in fatigue, palpitations, breathlessness, or chest pain.
- Class III patients are comfortable at rest, but everyday activities cause fatigue, palpitations, breathlessness, or chest pain.
- Class IV patients cannot engage in any physical activity without discomfort. Symptoms of heart failure or chest pain may be present even at rest.62
Although the NYHA classification system helps cardiologists guide therapy for individual patients, it is subject to inter-observer variability. A second approach to heart failure classification was developed by the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Task Force on Practice Guidelines and is sometime used to supplement NYHA classification in the clinical setting.63,64
This system accounts for both the development and progression of heart failure.63 It identifies four stages, the first two (A and B) of which are not considered overt heart failure, but rather risk factors that predispose to heart failure. This scale attempts to help healthcare providers identify at-risk patients early.28
American College of Cardiology Foundation/American Heart Association Staging
At Risk for Heart Failure
- Stage A. These patients are at a high risk for heart failure, but do not have structural disease or symptoms of heart failure. This includes those with coronary artery disease or diabetes.
- Stage B. This stage includes those who have structural heart disease, such as left ventricular hypertrophy/dysfunction or chamber enlargement, but who do not have signs or symptoms of heart failure.
Heart Failure
- Stage C. These patients have structural heart disease with prior or current symptoms of clinical heart failure.
- Stage D. These patients have treatment-resistant heart failure requiring specialized intervention, such as transplantation, biventricular pacemakers, or left ventricle assist devices.28
Note that in the ACCF/AHA classification system, a patient cannot move backwards to a prior stage. That is, once a patient is classified as Stage C, they cannot be in Stage B again. In the NYHA system, which is based solely on symptoms, patients can move between classes.65
6 How is Heart Failure Diagnosed?
The initial approach to a patient with suspected heart failure relies heavily on a thorough medical history and careful clinical exam. However, signs and symptoms of heart failure are non-specific—they might be caused by a number of other conditions—so further testing is often necessary to make a conclusive diagnosis. A complete blood count, chemistry panel, and urinalysis, as well as a chest X-ray, are generally part of this workup. Blood testing for natriuretic peptides (BNP or N-terminal prohormone of brain natriuretic peptide [NT-proBNP]), an electrocardiogram, and an echocardiogram are also standard parts of an initial assessment.22,47,66
Electrocardiography and Imaging
An electrocardiogram (ECG) can be used to measure electric abnormalities, enlargement of the heart chambers, and arrhythmia. It is recommended as part of an initial evaluation of individuals with suspected heart failure.3,47,66
An echocardiogram is among the most useful diagnostic tests for heart failure.66 Echocardiography is an ultrasound technique that displays real-time images of the heart to visualize abnormalities in the heart muscle or valves, quantitate changes in the size of heart chambers, or detect abnormalities in blood flow. When combined with Doppler flow studies (which help visualize blood flow through the heart), it represents an important diagnostic approach for patients with heart failure.3 Echocardiography is also an important technique to estimate and monitor changes in left ventricular ejection fraction.
Other imaging techniques may also be used to evaluate the size and thickness of the heart chambers, detect myocardial damage, or detect pulmonary edema, including chest radiography (X-rays), computed tomography (CT or CAT scans), and magnetic resonance imaging (MRI).3,19,67,68 In particular, MRI is useful in helping determine the cause of heart failure and establishing prognosis. It can also help guide treatment.69
Biomarker Testing
B-type natriuretic peptide (BNP), a peptide hormone released mostly by cells of the ventricle (cardiomyocytes) in response to heart muscle stretch or injury, is a valuable biomarker both for diagnosing acute heart failure and predicting clinical outcomes.70-73 BNP normally functions to signal the kidneys to release sodium and water into the urine to lower blood volume, and thus, blood pressure. Serum levels of BNP, and its precursor fragment (NT-proBNP), rise proportionally with risk for cardiovascular disease.71 In one prospective cohort study of 380 people in Sweden, having a low BNP was one of the best predictors of survival to age 90 in men.74
Cardiac troponins (cTnI and cTnT) are regulatory proteins associated with muscle fibers in the heart that can be released into circulation upon cardiomyocyte damage or death. Quantitation of serum cardiac troponins is the gold standard for detecting acute damage to the heart muscle, such as from a heart attack.75 Cardiac troponins may also leak from cells during chronic diseases, such as heart failure.76 Measuring serum cTnT using a high-sensitivity assay (hs-cTnT) can be used in heart failure diagnosis and risk assessment.77-79 A recent meta-analysis of 16 studies with over 67,000 subjects found that there is a strong association between heart failure and cardiac troponins, and that its measurement was predictive of a heart failure event.80
In addition to these biomarkers, other markers of inflammation, oxidative stress, vascular dysfunction, and myocardial problems can mark heart failure.81,82 Measurements of soluble ST2, a member of the interleukin 1 receptor family and marker of cardiac distress,83 and galectin-3, a protein that plays a role in inflammation, cancer, and heart disease,84 can predict hospitalization and death. Monitoring multiple biomarkers may be useful to target heart failure therapies in the future, but further research is needed.
Additional tests that may help diagnose and monitor heart failure include thyroid function tests, especially thyroid-stimulating hormone (TSH), as hyperthyroidism and untreated hypothyroidism can cause heart failure. Standard blood tests to measure electrolyte levels and assess liver and kidney function (such as a chemistry panel and complete blood count [CBC]) may also be useful.47,66
Cardiovascular risk markers, such as homocysteine, insulin-like growth factor 1, C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), may also be assessed,30 although they are not specific for heart failure and may be more relevant for prognosis than diagnosis.85
7 Heart Failure Treatment
The suggested optimal treatment of heart failure is outlined in guidelines issued by the American College of Cardiology Foundation and American Heart Association (ACCF/AHA). These guidelines were updated in 2022.331 Recommendations in the guidelines are stratified by disease severity.
Treatment Recommendations for Patients at Risk for Heart Failure (ACCF/AHA stage A)
Patients with stage A heart failure have no symptoms of heart failure and no structural problems in their heart but are at high risk due to high blood pressure, atherosclerosis, type 2 diabetes, obesity, and/or metabolic syndrome. This category also includes those with a family history or genetic marker indicating high risk of cardiomyopathy, as well as those who have been exposed to cardiac toxins (eg, the anticancer drugs doxorubicin [Adriamycin] and daunomycin [Cerubidine]).331
The treatment approach to stage A heart failure begins with adopting a healthy diet and lifestyle, including exercising regularly, maintaining healthy body weight, and not smoking. Because of the close relationship between hypertension and heart failure, treatment of high blood pressure is paramount in patients with stage A heart failure.331
Medications
Antihypertensives. Use of antihypertensives (ie, medications that lower blood pressure) has been found to reduce the risk of developing heart failure.332 Intensive antihypertensive therapy targeting an optimal systolic blood pressure of <120 mm Hg may be indicated for those with high risk of heart failure who also have high blood pressure, but in general a treatment goal of <130/80 mm Hg is recommended.331
Sodium/glucose cotransporter-2 (SGLT2) inhibitors. SGLT2 inhibitors are a newer class of antidiabetic drugs that also protect the heart and kidneys. Canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) are drugs in this class. A growing body of evidence shows treatment with SGLT2 inhibitors reduces the risk of symptomatic heart failure and improves outcomes in symptomatic (stage C) heart failure patients with or without diabetes.333,334 Based on the strength of the evidence, SGLT2 inhibitor therapy is now recommended for stage A heart failure patients with type 2 diabetes, established cardiovascular disease, or high cardiovascular risk.331 Potential adverse side effects include urogenital yeast infections, especially in women, and potentially dangerous diabetic ketoacidosis.335
Cardiovascular medications. Any medications needed to treat existing cardiovascular risk factors like high cholesterol levels, atrial fibrillation, or peripheral or coronary artery disease are important for protecting patients with high risk of heart failure.331 Examples include cholesterol-lowering statins, aspirin, antiplatelet drugs, and anticoagulants.336,337
Treatment Recommendations for Patients with Pre-Heart Failure (ACCF/AHA stage B)
Stage B heart failure involves changes in structure and function of the heart, such as heart chamber enlargement, abnormal wall movement, valve disorders, or reduced ejection fraction. High levels of B-type natriuretic peptide (BNP) or cardiac troponin levels, which reflect heart muscle dysfunction, are also indicative of pre-heart failure. However, signs and symptoms of heart failure are not present in stage B.331
As in stage A, dietary and lifestyle strategies are paramount in managing stage B heart failure. In addition, therapies aimed at specific co-existing conditions affecting cardiovascular health continue to be important in stage B.331
Medications
The medical approach to treatment of stage A is also indicated for stage B heart failure patients, including SGLT2 inhibitor therapy if diabetes is present and cardiovascular medications as indicated for the individual.331 Additional medical recommendations are:
Antihypertensives. Guidelines for antihypertensive therapy are more specific for those with stage B heart failure than stage A.
- Angiotensin-converting enzyme (ACE) inhibitors. ACE inhibitors lower blood pressure by inhibiting activity of ACE and reducing synthesis of angiotensin II, a hormone that causes constriction of blood vessels and raises blood pressure. Enalapril (Vasotec) and lisinopril (Zestril) are examples of commonly prescribed ACE inhibitors. ACE inhibitors have been found to reduce progression, hospitalization, and death due to heart failure in patients with reduced left ventricular ejection fraction (≤40%).331,338
- Angiotensin II receptor blockers (ARBs). ARBs lower blood pressure by blocking interactions between angiotensin II and its receptors, limiting blood vessel constriction. Commonly prescribed ARBs include candesartan (Atacand) and valsartan (Diovan). Intriguingly, the ARB telmisartan (Micardis) has also been shown to reduce insulin resistance, a major contributor to heart disease.340ARBs have been found to reduce death and heart failure-related illness in patients with a low ejection fraction and a previous heart attack.331 However, because evidence of their benefits is not as strong as for ACE inhibitors, their use is usually restricted to cases of intolerance to ACE inhibitors due to cough or angioedema.341,342
- Beta blockers. Beta blockers lower blood pressure, heart rate, and heart contraction force by blocking a type of adrenaline receptor called beta-adrenoceptors. Beta blockers are added to treatment with ACE inhibitors or ARBs to improve their effectiveness in patients with pre-heart failure and reduced ejection fraction.343 Beta blockers such as carvedilol (Coreg), bisoprolol (Zebeta), and metoprolol (Lopressor) have been found to reduce damage to cardiac structure and function and improve outcomes in patients with reduced ejection fraction, with most of the evidence being in those who have had a heart attack.331,344,345 A meta-analysis of 11 randomized controlled trials including over 14,000 patients found beta blockers improved ejection fraction in heart failure patients with ejections fractions as high as 40–49%.346
Not all patients are able to tolerate ACE inhibitors with cough being a common side effect. ACE inhibitors can also cause a type of severe skin swelling called angioedema and must be used with caution in patients with low blood pressure, advanced kidney disease, and elevated blood potassium.339
Like ACE inhibitors, ARBs must be used with caution in patients with high blood potassium levels, advanced kidney disease, and low blood pressure.342
Common adverse side effects of beta blockers include fatigue, dizziness, nausea, and constipation; less commonly, difficulty breathing, increased blood glucose levels, insomnia, edema, and worsening of Raynaud syndrome may occur. Individuals with low blood pressure or a low heart rate may not be able to use beta blockers.347
Treatment Considerations for Patients with Heart Failure (ACCF/AHA stage C)
Patients with stage C heart failure have structural changes to the heart muscle and have or have experienced heart failure symptoms. The guideline recognizes the importance of self-care, patient education, social support, and an integrated team of healthcare providers to meet the complex needs of heart failure patients.331 A healthy diet and lifestyle are important for slowing progression; however, a sodium-restricted diet is no longer emphasized in recommendations due to lack of evidence of its benefit.331,348 Exercise training or cardiac rehabilitation may improve health-related quality of life.349 Annual influenza vaccination has been found to lower the rate of hospitalization and death, whether due to cardiovascular reasons or any cause, in heart failure patients, with cumulative benefits seen in those who were vaccinated regularly.350
Medications
Pharmacological treatment for stage C heart failure relies on the same key medications as stages A and B, with treatment beginning at very low doses that can be gradually increased as needed. Importantly, SGLT2 inhibitor therapy is a core recommendation for both diabetic and non-diabetic stage C heart failure patients. Additional medications recommended for stage C heart failure are:
Angiotensin receptor-neprilysin (ARN) inhibitors. The only ARN inhibitor with current FDA approval, Entresto, contains two agents: sacubitril and the ARB valsartan. By inhibiting the activity of the enzyme neprilysin, sacubitril slows the breakdown of natriuretic peptides. The combined effect of the two agents leads to blood vessel dilation and improved fluid balance. Sacubitril-valsartan appears to be more effective than traditionally used ACE inhibitors and ARBs for reducing hospitalizations and deaths in patients with mild-to-moderate symptomatic heart failure with reduced ejection fraction, and is recommended as an alternative to ACE inhibitors or ARBs in these patients.331,351,352 A meta-analysis determined the combination of ARN inhibitor, beta blocker, mineralocorticoid receptor antagonist (MRA), and a SGLT2 inhibitor reduced deaths more than any other treatment regimen in heart failure patients with reduced ejection fraction.353
Like ACE inhibitors, sacubitril-valsartan can cause angioedema, as well as low blood pressure, cough, high blood potassium levels, and kidney damage.354 Despite its relatively high cost, some evidence suggests including an ARN inhibitor early in heart failure treatment may ultimately save money.355
Mineralocorticoid receptor antagonists (MRAs). MRAs inhibit aldosterone, a hormone produced by the adrenal glands that increases the kidneys’ retention of sodium and water. Aldosterone inhibition decreases fluid retention, diminishing blood volume and blood pressure. Stage C heart failure patients with compromised ejection fraction have been found to benefit from MRAs, such as spironolactone (Aldactone) or eplerenone (Inspra).356 Emerging evidence suggests spironolactone may also decrease heart failure-related hospitalizations and improve cardiac function in patients with mildly reduced (40–49%) or preserved (≥50%) ejection fractions.357,358
When using MRAs, potassium, kidney function, and diuretic dosing should be carefully monitored to reduce risk of high blood potassium levels and kidney problems, which can be life-threatening.331 Spironolactone is also associated with gynecomastia, the swelling of breast tissue in males.356
Diuretics. Diuretics can be helpful in stage C heart failure patients with reduced ejection fraction who experience fluid retention. Diuretics work by altering the way the kidneys handle sodium or chloride, thus increasing urination. Loop diuretics like furosemide (Lasix), torsemide (Demadex), and bumetanide (Bumex) are the preferred choice for treating heart failure because of their more potent action compared with thiazide diuretics like hydrochlorothiazide (Microzide). Diuretics can improve symptoms and quality of life; however, there is uncertainty regarding their effects on outcomes in heart failure patients.359-361 The effectiveness of diuretics diminishes with prolonged use of high doses, high sodium intake, and the use of non-steroidal anti-inflammatory drugs (NSAIDs).362 Diuretics can cause electrolyte imbalance, and their use at the lowest dose needed to manage fluid retention is recommended.331
Other medications. In addition to core medical treatment with individualized combinations of SGLT2 inhibitors, renin-angiotensin system inhibitors (ARN inhibitors, ACE inhibitors, or ARBs), beta blockers, and MRAs, as well as diuretics for symptom relief, the following medications may be indicated for specific patients with symptomatic heart failure:
- Isosorbide dinitrate and hydralazine. Isosorbide dinitrate and hydralazine (BiDil) dilate blood vessels and lower blood pressure by relaxing smooth muscles in the vascular walls. This drug combination has been found to reduce complications and death rate specifically in African American heart failure patients with reduced ejection fraction and moderate-to-severe functional limitation.363,364 It may also help stage C heart failure patients who are unable to tolerate first-line therapy with ARN inhibitors, ACE inhibitors, or ARBs.331,364 Common adverse side effects of BiDil treatment are headache, digestive upset, and low blood pressure.364
- Sinoatrial current inhibitor. Increased resting heart rate is a risk factor for heart failure-related hospitalization and death.331,363 Ivabradine (Corlanor), classified as a sinoatrial current inhibitor, slows the heart rhythm and has been shown to reduce heart failure-related hospitalizations and deaths in stage C heart failure patients who have reduced ejection fraction, mild-to-moderate functional limitation, a normal heart rhythm, and a resting heart rate of 70 beats per minute or higher.364 However, it is recommended beta blocker therapy be optimized before considering the addition of ivabradine to treatment.331 Potential adverse side effects of ivabradine include arrhythmias and visual side effects.364
- Vericiguat. Vericiguat (Verquvo), as a guanylate cyclase stimulator, has a nitric oxide-like blood vessel-dilating effect. A large randomized controlled trial found vericiguat lowered the risks of heart failure-related hospitalization and cardiovascular death in patients with reduced ejection fraction and recently worsening heart failure.365 Its use may therefore be considered for this particular population of heart failure patients.331 Low blood pressure and anemia are the main adverse side effects of vericiguat.366
- Digoxin. Digoxin (Lanoxin) is a plant-derived compound known as a cardiac glycoside that has been used to treat cardiac problems for over 200 years, but its usefulness has declined as safer and more effective medical options have become available. Digoxin functions as a positive inotrope, meaning it increases the contractile force of the heart muscle, and also lowers heart rate.364 Digoxin may decrease symptoms and hospitalizations in stage C heart failure patients with reduced ejection fraction, and may be beneficial in patients who remain symptomatic despite optimized treatment with core medications.331,367 Digoxin’s benefits are countered by its potential to cause serious heart rhythm irregularities at doses not much higher than those needed to produce a therapeutic effect.367
- Omega-3 fatty acids. Omega-3 fatty acids, in conjunction with core medical therapies, have been shown to improve cardiac function and modestly decrease risks of cardiovascular events and death in heart failure patients with reduced ejection fraction.368,369 The majority of trials showing cardiovascular benefits have used highly purified ethyl esters of eicosapentaenoic acid (EPA), with or without docosahexaenoic acid (DHA).331,370 These omega-3 fatty acids are only available by prescription, and may increase the risk of bleeding more than over-the-counter supplements. A possible link between their use and increased risk of atrial fibrillation has been reported.370
- Potassium binders. Potassium-binding medications may be recommended to patients who are otherwise unable to optimize treatment with spironolactone or other potassium-raising medications (ACE inhibitors, ARBs, and ANR inhibitors).331 Sodium polystyrene sulfonate (Kayexalate) is an older potassium-binding drug that can only be used short-term due to potentially dangerous digestive toxicity; two newer drugs, patiromer (Veltassa) and sodium zirconium cyclosilicate (Lokelma), have shown promise in clinical trials.371 Adverse side effects of patiromer include low magnesium levels and digestive problems, and sodium zirconium cyclosilicate can cause problematic edema.331,372,373
- Anticoagulants and antiplatelets. Anticoagulants (eg, apixaban [Eliquis] and warfarin [Coumadin] and antiplatelets (eg, clopidogrel [Plavix] and aspirin) have not been found to have benefits in heart failure patients generally, and their use is only recommended for those with a co-occurring condition that increases blood clot risk, such as previous heart attack or stroke, venous thromboembolism, atrial fibrillation, and other cardiac conditions that trigger blood clotting.331 These drugs are associated with increased risk of serious bleeding, and dosing must be carefully considered.
Medical Devices
Medical devices can have an important role in improving outcomes and survival in select patients with heart failure, as a complement to optimal medical therapy.331,374 Implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy (CRT) are examples of such devices. ICDs are similar to pacemakers, and they monitor the heart’s rhythm to ensure it beats at an optimal rate. CRT is a pacemaker that sends timed electrical impulses to the heart’s left and right ventricles so they pump more efficiently. For those not suited for either ICD or CRT, options available include an implantable device that stimulates pressure receptors in the carotid artery, vagus nerve stimulation, and cardiac contractility modulators that stimulate stronger ventricular function, although it is unknown whether these interventions reduce hospitalization or death rates.331
Surgeries
In patients whose symptomatic heart failure is complicated by atrial fibrillation, a procedure called ablation may relieve symptoms and improve quality of life, and has been found to reduce heart failure-related hospitalizations and deaths.331,375 The procedure involves using a catheter to direct heating or freezing to a small region of the heart, resulting in scarring that interrupts the origination of abnormal heart beats.376 Coronary artery bypass graft surgery can improve survival in select heart failure patients with ejection fraction ≤35% and advanced coronary artery disease.331
Interventions for Valvular Heart Disease
Valvular heart disease is a known risk factor for heart failure. This is true for multiple types of cardiac valve disease, including aortic stenosis and mitral and tricuspid valve regurgitation.401 Aortic stenosis is the most prevalent cardiac valve pathology worldwide, and heart failure is a leading cause of death in people with aortic stenosis.402 Aortic valve replacement is the main treatment for aortic stenosis, and can be performed both through major invasive surgery as well as a much less invasive method called transcatheter aortic valve replacement (TAVR). Another term, transcatheter aortic valve implantation (TAVI), refers to the same procedure as TAVR; usage of this term is more common in Europe whereas “TAVR” is more common in the United States.403,404 The advent of TAVR has made valve replacement for aortic stenosis available to many more patients who would be at undue risk from major surgery. As adoption of TAVR has grown, it is now becoming increasingly available to people who have low-risk aortic stenosis as well—and is now the preferred therapy for aortic stenosis for most patients.405
TAVR’s relative low risk has made it the leading procedure for aortic stenosis, with some saying it has revolutionized the treatment of aortic stenois.405,406 Deciding on TAVR or traditional surgical aortic valve replacement should be based on risk-benefit profile, shared decision-making between patient and physician, and patient-specific clinical indications.331
A retrospective observational study in patients with heart failure found symptomatic improvement after TAVR, regardless of class of disease. However, patients with reduced ejection fraction experienced the least improvement.407 In a randomized controlled trial that included 178 patients (average age 77 years) who had heart failure with reduced ejection fraction and moderate aortic stenosis, TAVR was not superior to surveillance for a composite outcome of all-cause death, disabling stroke, and hospitalizations. TAVR did, however, result in an improvement in symptomatic quality of life.408 An earlier meta-analysis compared surgical aortic valve replacement to TAVR and found a relative survival benefit at two years for TAVR overall, regardless of the specific valve device implanted or baseline surgical risk profile. The clearest benefit was in patients with femoral access TAVR and in women. In men, and when thoracic access was used, TAVR was not superior to surgery.409 A follow-up analysis confirmed the survival benefit of TAVR relative to surgery and found that stroke risk was lower with the TAVR procedure, while major vascular complications and permanent pacemaker implantations were lower with surgery.410
Until recently, studies had not specifically addressed whether TAVR would provide benefits over surveillance among people with asymptomatic severe aortic stenosis. A randomized controlled trial published in late 2024 addressed this question. Among 901 patients with asymptomatic severe aortic stenosis enrolled in this study, TAVR reduced the occurrence of stroke, unplanned cardiovascular hospitalization, or death from any cause, compared with active surveillance. Moreover, TAVR resulted in markedly reduced risk of hospitalization for heart failure compared with surveillance, although this was an exploratory endpoint so needs to be replicated in other studies.411
Emerging evidence suggests SGLT2 inhibitors may be associated with improved TAVR outcomes. In a randomized controlled trial, 1,222 individuals with a history of heart failure and severe aortic stenosis who were undergoing TAVR were given either 10 mg of the SGLT2 inhibitor dapagliflozin daily plus standard care or standard care alone, beginning at hospital discharge, and followed for one year. All subjects also had at least one of these conditions: diabetes, left ventricular systolic dysfunction, or renal insufficiency. The primary outcome of interest was a composite of death from any cause or worsening of heart failure. Compared with placebo, the treated group was 28% less likely to experience the composite outcome, although the rate of death from any cause did not differ significantly between the groups. Subjects in the dapagliflozin group were 37% less likely to experience a worsening of heart failure and more likely to develop genital infections or low blood pressure—known side effects of SGLT-2 inhibitors.412
These trial results provide some confirmation for the findings of an earlier observational study in 311 patients with diabetes and severe aortic stenosis undergoing TAVR, 75 of whom were being treated with SGLT2 inhibitors. One year after the procedure, SGLT2 inhibitor users had a higher likelihood of left ventricular recovery. Remarkably, those with especially low baseline left ventricular ejection fraction (≤ 30%) were particularly likely to benefit. Those not treated with SGLT2 inhibitors were more likely to develop worse extra-valvular cardiac damage, while SGLT2 inhibitor users experienced more beneficial cardiac remodeling. After two years, SGLT2 inhibitor use was associated with lower rates of major adverse cardiovascular events, all-cause death, and heart failure. These findings remained significant after results were adjusted for confounding factors.413,414
Advanced Heart Failure (ACCF/AHA stage D)
Stage D heart failure is an advanced form of the condition that is not responsive to core heart failure medications. This stage is characterized by severe cardiac dysfunction; unplanned hospital visits within the previous year due to progressive heart failure; and severe symptoms, including unintentional weight loss, fatigue, and shortness of breath while performing daily tasks (eg, getting dressed or bathing) or at rest.380
Diuretics for symptom relief are a mainstay of treatment for advanced heart failure. Inotropic drugs, kidney dialysis, and the use of mechanical circulatory support may also be part of life-extending care for some patients with advanced heart failure, and heart transplant may be an option for select patients. In addition, a re-evaluation of any co-existing health problems that may be contributing to heart failure may lead to treatments that improve the clinical status of some patients with advanced heart failure. It is important to consider the individual circumstances of each stage D heart failure patient and decide whether and when end-of-life hospice care and palliative treatments are appropriate.331,380
8 Novel and Emerging Therapies for Heart Failure
Stem Cell Therapy
Stem cell therapy for cardiac regeneration is an emerging and continuously evolving field. Stem cells are renewable, unspecialized precursor cells that can transform into specialized cell types.144-146 Acute myocardial infarction, chronic ischemic heart failure, cardiomyopathy, and left ventricle dysfunction are all associated with a loss of cardiomyocytes (cardiac muscle cells) that was previously considered irreversible. Adult cardiomyocytes have a limited capacity for self-repair; however, research suggests stem cells could offer a novel approach to replacing or repairing damaged cells and tissues.147,148 Multiple preclinical studies suggest stem cells may help reduce inflammatory response and cardiac fibrosis, and also help with the recovery of damaged cardiac tissue, yet much remains to be discovered and clarified.149
Several clinical studies have been conducted with stem cells as a potential treatment for heart failure and other cardiac-related conditions. Several reviews and meta-analyses indicate that stem cell treatments appear to benefit heart failure patients, with those treated experiencing lower mortality rates and fewer cardiac events.147,150,151 However, the authors of these articles recommend interpreting the results with caution, as many of the studies were of lower quality and had minimal benefits. Other clinical trials have shown no benefit from stem cell treatments.152,153
Some scientists pointed out that different methods of processing stem cells may have led to the contradictory clinical outcomes.154 The initial hype surrounding stem cell therapy also likely led to clinical testing before the science was fully appreciated and understood, leading to inconsistent results.148,155 Some studies that led to the early excitement have since been discovered to be faulty, and several were retracted.155 As such, the science behind whether stem cells may benefit heart failure patients is still not clear and will require more reproducible, well-designed studies.
The use of stem cells as a therapy for heart failure is an interesting and potentially important area; however, more high-quality studies (clinical and preclinical) are needed before its usefulness can be determined and implemented as a standard treatment.
Testosterone
Testosterone is a male hormone that helps regulate bone density, fat distribution, muscle strength, red blood cell production, sperm production, and sex drive. Inadequate levels of testosterone can contribute to cardiovascular diseases, including heart failure. However, this connection is overlooked by many mainstream physicians.156,157 A decline in circulating testosterone may exacerbate exercise intolerance and muscle mass loss (cachexia) seen in heart failure patients.158,159
An estimated 25% of men with heart failure have evidence of testosterone deficiency.160 A recent study involving 167 Chinese men with chronic heart failure measured their testosterone levels and followed them for at least three years. Patients in the low testosterone group had worse cardiac function and higher mortality and hospital readmission rates.161 A review of eight published studies indicated testosterone replacement therapy can enhance exercise capacity and muscle strength, but not ejection fraction, blood pressure, and other markers of cardiac health. More quality research is needed to understand the association between testosterone levels and clinical outcomes in heart failure patients.162 Another study randomized 39 men with heart failure and testosterone deficiency to an exercise training, intramuscular testosterone injection, or training and testosterone group. The combination group showed the most improved muscle nerve activity, muscle wasting, and functional capacity.163
Some evidence suggests testosterone replacement may also benefit women with heart failure. Testosterone therapy was shown to improve exercise capacity (six-minute walk test and muscle performance) and insulin resistance in a study of 36 women with stable heart failure.164 Testosterone therapy may be useful in cardiovascular events such as heart failure, angina, and ischemia.165
Individuals with heart failure should consider testing their testosterone levels using an inexpensive blood test. If levels are suboptimal, testosterone replacement therapy may relieve some heart failure symptoms. More randomized, controlled clinical trials are required to clarify testosterone’s role in cardiac health. More information about testosterone replacement therapy is available in the Male Hormone Restoration and Female Hormone Restoration protocols.
Vagus Nerve Stimulation
Each of two vagus nerves carries signals from the brain to the heart to control heart rate as part of the parasympathetic nervous system. In chronic heart failure, vagal activity is reduced, increasing heart rate and mortality.166-168 Vagus nerve stimulation is an approved treatment for depression and epilepsy that does not respond to drug therapy, and may also be useful in the treatment of chronic heart failure. In a multi-center open-label trial, implantation of an electro-stimulator device around the right vagus nerve and chronic nerve stimulation for one year significantly improved quality of life, ejection fraction, and a six-minute walk test in 23 NYHA class II/III patients.167
The INOVATE-HF (Increase of Vagal Tone in Heart Failure) trial was a multinational, randomized, controlled trial that examined 707 people with heart failure and reduced ejection fraction. The study found that vagus nerve stimulation improved 6-minute walk distance, NYHA functional classification, and quality of life scores, but did not improve risk of death or a cardiac event.169 Further research is warranted to better understand optimal delivery of this therapy, and if specific patient subgroups are more likely to benefit from this therapy.
Trimetazidine
As heart failure develops, perturbations in energy metabolism in the heart compromise its function. The failing heart muscle cells are unable to derive energy from fatty acids, the primary energy source for the healthy heart. Therefore, declining cardiac function in heart failure is compounded by inefficient fatty acid utilization.170,171
The drug trimetazidine (TMZ) has garnered interest because it has been shown to help overcome impaired cardiac fatty acid metabolism. TMZ boosts glucose utilization in the heart, lessening the reliance on fatty acids for energy.309,310 Animal research suggests TMZ may also help mitigate cardiac fibrosis, which contributes to heart failure progression.311 Some clinical evidence suggests TMZ, along with conventional therapies, improves symptoms, cardiac function, and prognosis in some patients with heart failure.172,312 However, much of this evidence comes from small studies that lack rigorous design and execution, so should be viewed as preliminary until larger, well-designed trials evaluate the effects of TMZ in people with heart failure.
A meta-analysis of three randomized clinical trials involving 326 heart failure patients found that TMZ, when provided as an add-on therapy, offered a protective effect, reduced all-cause mortality, and increased survival rates.173 Another meta-analysis of 19 randomized controlled trials involving nearly 1,000 chronic heart failure patients found TMZ treatment improved clinical symptoms and cardiac function and reduced cardiac hospitalizations and serum levels of BNP and C-reactive protein.174
In a comprehensive analysis of studies including 884 subjects with chronic heart failure, TMZ reduced hospitalization for cardiac causes by 57%. Moreover, TMZ was associated with improved left ventricular ejection fraction, exercise capacity, left ventricular end-diastolic diameter, and NYHA functional classification.175
In another review of published studies including data on 955 heart failure patients, TMZ was associated with improved left ventricular ejection fraction, left ventricular end-systolic volume, NYHA classification, and exercise capacity. Most impressively, TMZ use was associated with a 71% reduction in all-cause mortality and 58% reduction in cardiovascular events.176
However, a randomized double-blind study in 60 patients with stable, nonischemic heart failure found 35 mg of TMZ twice daily did not result in significant changes to left ventricular ejection fraction, exercise capacity, oxygen uptake, or quality of life.177 Another randomized controlled trial published in February 2019 found TMZ failed to improve exercise capacity among patients with hypertrophic cardiomyopathy whose mean age was 50 years.313 A randomized trial conducted in Bangladesh during 2015–2016 found that glyceryl trinitrate outperformed TMZ in improving NYHA classification at six and 12 weeks in patients with ischemic cardiomyopathy.314 Some evidence suggests diabetics with heart failure may benefit from TMZ, but not all studies have confirmed the benefits for this group.315
Despite over 40 years of published studies,178 TMZ has not received FDA approval. Marketed as Vastarel MR in Europe, scientific research shows TMZ has the capability to protect vulnerable, oxygen-deprived heart muscle. However, concerns about lack of long-term data on heart attack and cardiac mortality make regulatory approval difficult.
The authors of a literature review of TMZ and heart failure concluded the following315:
“… we cannot recommend using trimetazidine in [CHF patients] as a result of the significant limitations connected with these studies—meta-analyses based on unpowered studies and the retrospective character of [a key study]. A well designed, randomized clinical study, placebo-controlled, with well selected endpoints, appropriate patient group, and follow-up duration is still needed to possibly recommend the use of trimetazidine in HF patients… There are still no answers to key questions as to the role of this drug in selected cardiovascular conditions as well as whether this drug can reduce mortality in any group of patients with cardiovascular disease.”
TMZ can cause side effects such as Parkinsonism (ie, slowed or stiff movements, speech disturbances, hand tremors, and disequilibrium), which could contribute to a fall in older populations.179,180 A 2019 population-based study found TMZ use was a significant predictor of new-onset Parkinsonism symptoms. The researchers called for close monitoring of patients prescribed TMZ for emergence of Parkinsonism symptoms.316 Another study found that while TMZ often produces Parkinsonism, drug withdrawal generally results in resolution of symptoms in patients with mild, symmetrical Parkinsonism.317
Some trials are underway,318 and recent review articles319,320 suggest there is interest in continuing to explore the potential benefits of TMZ. Future trials will help clarify what role TMZ has to play in the management of heart failure and related conditions.
Other Potential Therapies
Gene therapy holds some promise for treating heart failure. Efforts are underway to enhance heart muscle sensitivity to calcium via the SERCA2a gene, a protein that pumps calcium into cardiac muscle cells. Other calcium-handling proteins may be candidates for future gene therapy work. Antagomirs are a class of drug that block microRNA, which play a role in gene expression and protein synthesis. Preliminary research suggests antagomirs improve cardiac function.10
Another potential therapy involves CD31 agonist peptides. CD31, a transmembrane glycoprotein present on endothelial cells and white blood cells, may play a protective role in heart failure. In a recent randomized study, mice with both preserved and reduced ejection fractions received either 2.5 mg/kg of CD31 or placebo by subcutaneous infusion. Daily treatment with CD31 improved ejection fraction and left ventricle filling pressure. In mice with a preserved ejection fraction, it prevented diastolic left ventricle dysfunction. Researchers concluded CD31 improved heart function and may be useful in treating heart failure.181 Ultrafiltration therapy can be used for fluid reduction in patients with refractory heart failure (ie, those with advanced heart failure who experience symptoms while at rest) that are not responsive to other medical therapies, such as diuretics. Ultrafiltration removes sodium and water from the blood across a semipermeable membrane and a pressure gradient to make plasma water. It improves congestion, cardiac output, and lowers right atrial and pulmonary pressures.182
In early 2019 the FDA approved a device to treat patients with chronic, moderate-to-severe heart failure who remain symptomatic despite receiving optimal medical therapy, and who lack other treatment options.303 The Optimizer Smart System has been shown in clinical trials to improve walking distance, decrease symptoms, improve quality of life, and improve cardiovascular outcomes.304-306 This device may also decrease mortality in these patients.304,307
The Optimizer device is implanted in a minimally-invasive procedure under local anaesthesia,308 and has been associated with a low rate of complications.304 A trial published in 2019 found that, in patients with left ventricular ejection fraction between 25% and 45%, hospitalizations decreased by 75% compared to before implantation of the device. In this group of patients, three-year survival did not differ from that predicted by an established model; however, among those with a left ventricular ejection fraction of 35‒45%, three-year survival was significantly better than that predicted by the same model.305
9 Dietary and Lifestyle Consideration
Cessation of Tobacco and Excessive Alcohol Use
Intake of more than 7–8 alcoholic drinks per day for more than five years may increase the risk of cardiovascular dysfunction that can lead to heart failure. Patients with a history of alcohol overconsumption are encouraged to abstain from drinking.3 However, light-to-moderate drinking (up to one drink daily for women and two drinks daily for men) may be associated with a reduced risk of heart failure compared with those who abstain from drinking.32,183,184
Smoking is a major risk factor for many medical conditions, including cardiovascular diseases. Stopping smoking was shown to provide benefits for patients with congestive heart failure similar to benefits offered by primary drugs.33,185 Several other studies found people who quit smoking have a lower risk of cardiovascular disease.186
The DASH Diet and Mediterranean Diet
The DASH (Dietary Approaches to Stop Hypertension) eating plan, which is rich in fruits, vegetables, whole grains, and low-fat dairy products, has been shown to lower systolic blood pressure by 8‒14 mmHg187,188 and is often recommended for people with heart failure.189,190 The Mediterranean diet, which is similar to the DASH diet in emphasizing fruits, vegetables, and whole grains, is also a healthy dietary pattern for those with heart failure.189
Specifically restricting dietary sodium intake remains controversial in the context of heart failure.191-194 However, both the DASH and Mediterranean dietary patterns generally do not contain large amounts of sodium relative to the typical Western diet. Until large, randomized, controlled trials can address the question of whether specifically restricting dietary sodium is optimal for heart failure patients, adhering to a diet rich in unprocessed plant-based foods is a good option.195
Monitor Micronutrient Sufficiency
Deficiencies in micronutrients, such as potassium, calcium, magnesium, and zinc play an important role in the progression of heart failure. These nutrients help maintain the proper relaxation and contraction of heart muscle cells. A comprehensive literature review suggests micronutrients improve health outcomes in heart failure patients, including symptoms, work capacity of the heart, and left ventricular ejection fraction.196
In a recent multi-center, longitudinal study, 246 heart failure patients were asked to fill out four-day food diaries. Analysis of these diaries revealed micronutrient deficiency to be a strong, independent predictor of one-year hospitalization or death rates, particularly in patients with comorbid depressive symptoms. The most common dietary deficiencies were calcium, folate, magnesium, zinc, and vitamins C, D, E, and K. These results suggest promoting a varied diet may prevent micronutrient deficiencies, and diet quality plays a role in heart failure outcomes.197
The frequency of malnutrition increases with degree of heart failure severity, ranging from an estimated 22% in NYHA class II patients to 63% in class III patients.31 Micronutrient insufficiency is of particular concern among patients on certain heart failure medications. Further research is needed to document the effects of micronutrients on quality of life and heart failure patient survival.
Potassium and zinc. Diuretic use is associated with electrolyte depletion. Potassium is essential for normal heart rhythm and function. Conversely, ACE inhibitors and ARBs decrease the excretion of potassium and may lead to elevated potassium levels. ACE inhibitors, ARBs, and thiazide increase urinary excretion of zinc.31
Magnesium. Loop diuretics increase renal excretion of magnesium and other essential minerals.31 In a study of 68 patients admitted to the hospital for heart failure, 38% presented with low magnesium levels at admission and 72% had excessive urinary magnesium loss.198 Several clinical trials have investigated the use of magnesium in heart failure patients. A recent analysis of 40 trials including over one million participants concluded that increasing dietary magnesium intake lowered risk of stroke, diabetes, heart failure, and mortality.199
B-vitamins. Chronic therapy with diuretics, which are administered to many patients with heart failure, may prevent the reabsorption of thiamine and increase its urinary excretion, contributing to thiamine deficiency. A study in 25 patients with heart failure found that furosemide use at 80 mg or more per day was associated with a 98% prevalence of thiamine deficiency.31 Deficiencies of several vitamins, including riboflavin, pyridoxine, folic acid, and B12 have also been documented in heart failure patients. Riboflavin, B12, and folic acid play a role in homocysteine metabolism. Homocysteine is an amino acid that can cause damage to the inner lining of blood vessels (the endothelium), and elevated homocysteine levels have been associated with a poor prognosis in heart failure patients.200,201
Exercise
Exercise training is a valuable addition to other heart failure interventions. Regular exercise that provokes mild-to-moderate shortness of breath is beneficial, and best undertaken in a structured, medically-supervised program.202,203 In clinically stable patients able to participate, cardiac rehabilitation improves heart-related quality of life, functional capacity, endothelial function, and reduces hospitalizations and mortality.47 Exercise training is considered suitable for most heart failure patients in NYHA class I‒III. 204
Published studies evaluating the efficacy of exercise training in heart failure patients report improvements in skeletal muscle oxygen utilization, diastolic function, symptoms and quality of life measures; increased exercise capacity, muscle strength and endurance; and reductions in inflammatory cytokines (eg, TNF-α and IL-6), NYHA functional class, hospital stays and mortality.205
In addition to formal structured exercise programs that may include aerobic and resistance exercise components, lifestyle approaches that emphasize activities such as brisk walking, taking stairs, gardening, and house work are also considered valuable.204
Regular physical activity can also help maintain a healthy weight, which in turn promotes optimal cardiovascular health.
Maintain Healthy Blood Sugar Levels
Diabetes and insulin resistance are major risk factors for heart failure. Diabetes not only increases risk of heart failure, but also worsens the outcome of patients with existing heart failure.3 The diabetic heart is more susceptible to ischemic (low oxygen) injury, myocardial infarction, and oxidative damage.206 Strategies for maintaining blood sugar control are reviewed in Life Extension’s Diabetes and Glucose Control protocol.
For those with diabetes and heart failure, the choice of diabetes medication may be complex. Metformin is often the first-line drug of choice for managing blood sugar in diabetics without overt heart disease. It was historically contraindicated in patients with heart failure due to concerns over increased risk of lactic acidosis. However, accumulating evidence suggests the risk of lactic acidosis may not be as pronounced as once thought. In fact, studies suggest metformin may reduce heart failure risk in diabetic patients and improve two-year survival rates in those with heart failure.207,208 Metformin is now commonly prescribed to diabetics with heart failure.209
Emerging evidence increasingly favors another class of diabetes medication, SGLT-2 inhibitors, in the management of diabetes in people with heart failure.209,210 (Refer to the Novel and Emerging Therapies section for more information).
Reduce Stress
Reducing stress levels may also promote optimal heart health. Anxiety is a serious mood disorder that affects many heart failure patients. Feeling anxious can make the heart beat faster, which in turn increases breathing rates and blood pressure levels. This can exacerbate heart failure, as the heart is already struggling to meet the body’s demand for oxygen-rich blood. In addition, stress may affect lifestyle behaviors that influence heart disease, such as alcohol consumption, overeating, smoking, and physical inactivity.211
In one review of six prospective cohort studies, there was a significant association between hospitalization and anxiety.212 Further research is needed to determine if anxiety can help predict a cardiac event or hospitalization in those with chronic heart failure. Research on meditation (focused mental practices that improve concentration and mindfulness) suggests a benefit on cardiovascular risk, especially in addition to tradition treatments.213 However, randomized trials with large cohorts are needed to clarify the role this method of stress reduction plays in cardiac health.
10 Nutrients
Coenzyme Q10
Coenzyme Q10 (CoQ10) has a central role in maintaining proper cardiac function and producing cellular energy in the mitochondria. It is also a potent antioxidant that helps maintain healthy blood sugar levels, preserve cognitive function, and support optimal heart health. CoQ10 is concentrated in healthy heart muscle, and CoQ10 deficiency is associated with heart failure.214,215 In one randomized controlled study, patients with moderate-to-severe heart failure who received 100 mg CoQ10 three times daily in addition to standard treatment showed improved symptoms and reduced risk of major cardiovascular events.11
In another trial that assessed circulating levels of CoQ10 in 257 cardiac patients, those with in-hospital mortalities had significantly lower levels of CoQ10.216 In a recent analysis of 14 randomized controlled trials, which included over 2,000 patients with heart failure, supplementation with CoQ10 resulted in a 31% lower mortality rate and an increased exercise capacity as compared with placebo.217
An examination of seven systematic reviews suggests CoQ10 supplementation is beneficial for heart failure patients,218 while another systematic review of 28 trials found CoQ10 enhanced exercise capacity, improved symptoms, and lowered blood pressure levels in heart failure patients.219 Other research indicates heart failure patients with lower CoQ10 levels have up to a two-fold risk of dying as compared to those with higher CoQ10 levels.220
As shown in several studies conducted by Life Extension Scientific Advisory Board Member Peter H. Langsjoen, MD, FACC, CoQ10 supplementation is especially important for individuals on cholesterol-lowering statin therapy (HMG CoA reductase inhibitors). Statin medications block the biosynthesis of both cholesterol and CoQ10, and they worsen heart muscle dysfunction in heart failure patients.12-15 In one study, diastolic dysfunction (heart muscle weakness) occurred in 70% of previously normal patients treated with 20 mg per day of Lipitor for six months. This heart muscle dysfunction was reversible with 100 mg of CoQ10 three times daily.15
Three comprehensive reviews have investigated 19 different clinical trials on the use of CoQ10 in heart failure.221-223 The results of 13 randomized controlled trials, encompassing 395 participants, revealed that CoQ10 supplementation led to a statistically significant average net increase of 3.7% in ejection fraction. For individuals with heart failure, Life Extension suggests an optimal CoQ10 blood level of 4 μg/mL.
Pycnogenol in combination with CoQ10 (PycnoQ10) may improve exercise capacity, ejection fraction, and edema in heart failure patients. In a single-blind, 12-week observational study of patients with NYHA class II or III heart failure, PycnoQ10 was well tolerated and improved systolic and diastolic function as well as heart and respiratory rate. Further research is warranted.224
Hawthorn
Hawthorn (Crataegus spp.) is a traditional cardiovascular tonic of plant origin that has been in use since the Middle Ages. Hawthorn extracts contain dozens of biologically active molecules, including flavonoids and polyphenols. The hawthorn-derived phytochemicals most thoroughly studied in humans are oligomeric procyanidins (OPCs). A typical hawthorn dose provides between 30 and 340 mg a day of procyanidins.226-228
Hawthorn extracts are believed to lower blood pressure by dilating coronary and peripheral blood vessels, inhibiting ACE, anti-oxidative and anti-inflammatory effects, and mild diuretic activity.229,230 Hawthorn’s efficacy in the treatment of heart failure has been demonstrated in over 4,000 patients, with significant reductions in subjective discomfort ratings, improved left ventricular ejection fraction, and increased cardiac efficiency.231 A recent meta-analysis of randomized clinical trials including over 600 subjects concluded that Hawthorn extract improves parameters such as maximal workload, left ventricle ejection fraction, and rate pressure product (a measure of the stress put on the cardiac muscle).232
The SPICE trial was a large, randomized controlled study of 2,681 NYHA class II or III patients with a left ventricular ejection fraction ≤ 35%. A 900 mg/day dose of a standardized extract from hawthorn leaves and flowers (providing 169 mg OPCs) significantly reduced cardiac mortality, and sudden cardiac death was significantly reduced for the subgroup of patients with a left ventricular ejection fraction ≥ 25%.233,234 In the HERB chronic heart failure trial, which was a placebo-controlled trial of 120 patients with NYHA class II or III heart failure, 900 mg/day standardized hawthorn extract improved left ventricular ejection fraction compared with the control group.235
Pyrroloquinoline quinone
Pyrroloquinoline quinone (PQQ), a cofactor for several energy-generating reactions in the mitochondria of the cell, may stimulate the production of new mitochondria (mitochondrial biogenesis) through interactions with mitochondrial regulatory genes.236 Impaired mitochondrial function has been implicated in heart failure development.237 In a controlled trial involving cell lines and animal models, formulations of nanocurcumin and PQQ helped modulate hypoxia-induced hypertrophy (enlargement) of human heart cells.238
In animal models of ischemic injury (depriving the heart muscle of oxygen), treatment or pretreatment with PQQ reduced the extent of ischemic damage and degree of lipid peroxidation. In addition, PQQ improved ventricular function and reduced arrhythmias (irregular heartbeats).239,240
Omega-3 Fats
Omega-3 fatty acids from fish oil, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been shown to reduce inflammation, oxidative stress, triglyceride levels, and thrombosis risk.381,382 A meta-analysis of eight studies involving nearly 317,000 individuals found that those who consumed the highest level of dietary omega-3 fats had a 16% lower risk of heart failure than those whose omega-3 consumption was lowest.383 An analysis of six studies, comprising over 17,000 participants with a median follow-up of 9.7 years, found that the highest blood levels of EPA and DHA, compared to the lowest, were associated with a 41% lower risk of heart failure.384
Omega-3 fatty acids may also improve the course of heart failure.383 A meta-analysis of 12 randomized placebo-controlled trials that included a total of 81,364 participants confirmed that omega-3 fish oil supplementation reduced recurrent heart failure-related hospitalizations.385 VITAL-HF (Vitamin D and Omega-3 Trial‒Heart Failure), an analysis of study data examining fish oil supplementation and heart failure outcomes, found a significant reduction in recurrent hospitalizations for heart failure in those who received fish oil omega-3 supplementation compared with placebo.386 Another later analysis of the VITAL study data found that first hospitalizations for heart failure were reduced by more than 30% in people with type 2 diabetes. This protective effect was not observed in non-diabetics, and the protective benefit was greater in Black participants than in people of other racial backgrounds.387
A meta-analysis of findings from 12 randomized controlled trials with 2,162 participants found omega-3 fatty acid supplementation improved left ventricular ejection fraction in heart failure patients, with greater improvement in those with preserved ejection fraction (≥50%). Daily dosages of 1,000‒3,000 mg, and greater than 3,000 mg, yielded improvement in left ventricular ejection fraction, whereas doses of less than 1,000 mg did not. Longer duration of omega-3 supplementation was associated with greater improvement.388 A meta-analysis of 13 randomized controlled trials found that as dosage of omega-3 fish oil increased, so did cardiovascular protection.389
Carnitine
L-carnitine is an amino acid that aids in transporting fatty acid into the mitochondria. In decompensated heart failure, L-carnitine metabolism is altered, and cardiac energy metabolism is compromised.248 Research indicates carnitine deficiency is associated with cardiomyopathy, and 1.5–6 grams of L-carnitine daily increased exercise capacity in addition to improving left ventricle ejection fraction and clinical outcomes.214 A meta-analysis of 17 randomized controlled trials, including 1,624 patients, found supplementation with L-carnitine improved clinical systems in congestive heart failure patients, including overall cardiac efficiency, left ventricle ejection fraction, and cardiac output.249 A recent literature review concluded that L-carnitine helps transport fatty acids into the mitochondria, resulting in reduced oxidative stress and inflammation. Carnitine supplementation protects against ventricular dysfunction and cardiac arrhythmias, and may help reduce hypertension, diabetes mellitus, insulin resistance, and hyperlipidemia.250
Several studies evaluating the role of L-carnitine or its analog, propionyl-L-carnitine, in heart failure have shown statistically significant increases in exercise capacity, maximum exercise time, peak heart rate, and peak oxygen consumption.251 A study that administered 30 mg/kg propionyl-L-carnitine supplementation to 30 heart failure patients demonstrated a reduced pulmonary artery pressure, improved exercise capacity, increased oxygen utilization, and reduced ventricular size.252
Improvements in ejection fraction (13.6% after 180 days) were observed in a 60-patient study of NYHA class II and III heart failure patients who received 1.5 grams propionyl-L-carnitine per day in addition to their conventional treatments (digitalis and diuretics).253 Another trial, which enrolled 80 patients with NYHA class III or IV heart failure caused by dilated cardiomyopathy (heart disease in which the ventricles become enlarged and unable to adequately pump blood), demonstrated a significantly improved 3-year survival after supplementation with L-carnitine.254
Creatine
Creatine helps ensure the chemical energy supply to muscle tissue. Most research focusing on creatine has targeted its potential use in skeletal muscle metabolism, but a few studies have investigated its potential to improve heart muscle energetics in cardiovascular disease.255
A systematic review of creatine supplementation in patients with heart failure, ischemic heart disease, or acute myocardial infarction analyzed six randomized trials that collectively enrolled 1,226 patients with heart failure. Four of the trials demonstrated a significant reduction in dyspnea (breathing difficulty) in patients with heart failure receiving creatine, creatine phosphate, or phosphocreatinine.255,256
In a randomized double-blind study, 100 subjects engaged in an 8-week exercise program, followed a prepared diet regimen, and received 5 grams/day of creatine monohydrate. The control group received only the diet and exercise intervention. At the end of the study, the treatment group demonstrated reduced inflammatory markers, including IL-6, and improved endothelial functioning.257
Taurine
Taurine, an amino acid found in the heart, may serve as a cardioprotective agent. In a double-blind, placebo-controlled, randomized study, 16 patients with heart failure received 500 mg of taurine three times daily for two weeks. Taurine supplementation enhanced physical function and improved cardiovascular functional capacity.258 In another randomized placebo-controlled trial, heart failure patients with an ejection fraction less than 50% were given 500 mg taurine three times daily for two weeks. The subjects exercised before and after supplementation. Inflammatory and atherogenic (arterial plaque) markers were decreased in the treatment group, suggesting taurine is a cardioprotective agent.259
In a randomized placebo-controlled clinical trial that enrolled 29 NYHA class II or III heart failure patients with a left ventricular ejection fraction < 50% (average 29.27%), subjects received 500 mg taurine three times daily or placebo. After two weeks, exercise capacity increased significantly in the taurine group compared with placebo.260 Another study that compared taurine (3 grams/day) to low-dose CoQ10 (30 mg/day) supplementation in 17 patients with congestive heart failure (ejection fraction < 50%) revealed a significant improvement in ejection fraction in the taurine group after six weeks, as shown by echocardiography.261
D-ribose
D-ribose, a naturally-occurring pentose sugar that is a key component in adenosine triphosphate (ATP), may aid in energy generation and functional recovery in patients with heart failure and ischemic heart disease. Multiple preclinical studies have demonstrated that supplementation with D-ribose following myocardial ischemia (when blood flow to the heart is blocked or reduced, and the heart muscle is deprived of oxygen) enhanced the regeneration of ATP.262
Research supports the use of D-ribose for optimal cardiovascular health. In a pilot study of 11 NYHA class II‒IV patients, supplementation with 5 grams/dose D-ribose for six weeks led to some improvements in tissue Doppler velocity (a measure of the heart’s velocity while beating) and the velocity ratio of diastolic filling to heart valve relaxation. Researchers conclude that D-ribose may be beneficial for patients in heart failure, but further research with larger cohort sizes is needed to substantiate these benefits.263
In another trial of 15 patients with NYHA class II or III heart failure and chronic coronary artery disease, the administration of D-ribose (5 grams, three times daily) improved cardiac functional parameters as assessed by echocardiography and quality of life scores.264 D-ribose supplementation improved respiratory parameters during exercise in 44% of patients in one study.265 Significant benefits of daily oral D-ribose in NYHA class II and III patients were reported in a double-blind, randomized, crossover trial. D-ribose supplementation significantly improved left atrial functional parameters, quality of life, and physical function activity scores in the treatment group.266
Arjuna (Terminalia arjuna)
The arjuna tree is native to India, where its bark has been used in Ayurvedic medicine for centuries due to the belief that it may have cardiotonic properties—that is, it may improve the efficiency and function of the heart muscle.396 Arjuna contains a wide variety of bioactive compounds such as triterpenoids, tannins, and flavonoids. These compounds and others in arjuna appear to offer beneficial effects that may be relevant to heart health, as demonstrated in preclinical studies, including decreasing lipid peroxidation and generation of free radicals, modulating inflammation, and protecting against fibrosis.397
The cardiotonic properties of arjuna have been demonstrated in healthy individuals. For instance, two randomized controlled trials in healthy people found that 400 mg daily of an arjuna extract called Oxyjun for eight weeks resulted in a 3.6–3.7% increase in left ventricular ejection fraction, representing a statistically significant improvement compared with placebo.394,395
Some preliminary trials have observed that arjuna may provide small benefits for people with heart failure.398,399 However, as of early 2025, the totality of the evidence is mixed and inconclusive. For example, an arjuna extract was unable to increase left ventricular ejection fraction in a study involving 100 people who had heart failure with left ventricular ejection fraction ≤ 40%. Participants were randomized to receive 750 mg arjuna extract or a placebo twice daily for 12 weeks. Results showed that the group receiving arjuna had no significant improvements in the primary outcome of left ventricular ejection fraction compared with placebo. Nevertheless, the arjuna group had a significant improvement in secondary outcomes compared with placebo: distance covered in the 6-minute walk test, symptom severity and stability, and oxidative stress biomarkers.400 More research is needed to determine whether arjuna can provide meaningful benefits for people with heart failure.
Vitamin D
A number of observational studies have suggested an association between low vitamin D levels and chronic heart failure,201,281 particularly among the elderly.282 For example, in a study of 548 patients re-hospitalized with heart failure, 75% were vitamin D deficient (defined as < 20 ng/mL for this study), and for each 10 ng/mL decrease in vitamin D levels, the risk of all-cause mortality increased by 10%.283
The contribution of vitamin D deficiency to the pathology of heart failure as well as its protective effects for cardiovascular health are most likely exerted by several mechanisms, including effects on the hypertensive hormone angiotensin II, influence on vascular endothelial function, effects on systemic inflammation, and impact on the risk of cardiovascular mortality.201,281,284 Vitamin D may affect BNP and parathyroid hormone levels, as well as enhance heart contractility.285 A synthetic vitamin D analog (paricalcitol) decreased inflammation and cell death in mice following experimental heart attack, while transgenic mice that lacked the vitamin D receptor showed decreased survival following a heart attack.286
Intervention trials of vitamin D for heart failure have had mixed results. In a prospective study, 100 patients with heart failure (NYHA class I‒III) received 50,000 IU vitamin D every week for eight weeks, followed by 50,000 IU every month for two months. At the end of the study, patients on supplemental vitamin D saw improvements in exercise capacity and reductions in NYHA heart failure scores.287 Another intervention showed that the administration of 2,000 IU vitamin D daily for nine months in 93 patients with heart failure had an anti-inflammatory effect.288,289
In a recent randomized double-blind trial, 40 NYHA class II‒III patients with vitamin D deficiency or insufficient vitamin D levels received either 10,000 IU vitamin D3 daily or a placebo for six months. Researchers found that in high doses, vitamin D3 replenished diminished vitamin stores, improved quality of life scores, and normalized BNP, parathyroid hormone, and C-reactive protein levels.285 More research is warranted to determine if these results occur at lower dosages.
Intervention trials using vitamin D have demonstrated modest results for lowering blood pressure. A review of 11 randomized controlled trials, including 716 subjects, found a small reduction in systolic (3.6 mmHg) and diastolic (3.1 mmHg) blood pressure at doses of 800‒2,900 IU vitamin D daily in individuals with high blood pressure.290 In another analysis of randomized controlled studies, the effect of vitamin D replacement on heart failure was mixed. It reduced the risk of mortality in one study, but did not affect heart function, exercise capacity, or quality of life in two others.201
Resveratrol
Resveratrol, a polyphenol found in grapes, nuts, and red wine, has potent antioxidant activity. A randomized controlled study in which rats with induced post-infarction heart failure were given 15 mg/kg body weight per day of resveratrol showed that resveratrol supplementation decreased the severity of heart failure, improved left ventricular function, decreased collagen deposits in the myocardium, lowered oxidative stress, and attenuated inflammatory signaling pathways.291 A recent literature review of clinical data found that resveratrol reduces inflammation, promotes endothelial function, supports healthy blood pressure levels, and helps reduce biomarkers of cardiovascular disease.292
Resveratrol may play a role in myocardial ischemia, myocarditis, cardiac hypertrophy, and heart failure. Possible mechanism of action include reducing oxidative stress and inflammation, improving calcium handling, decreasing apoptosis, and modifying inflammatory pathways.291 Further research is warranted to fully understand resveratrol’s role in heart health.
Selenium
Selenium is a cofactor necessary for a number of cellular metabolic processes. In an animal model of hypertension leading to heart failure, a selenium-free diet was associated with high mortality (70%); however, supplementation with 50 or 100 mcg/kg resulted in survival rates of 78% and 100%, respectively.293 In humans, severe selenium deficiency has been firmly linked to a reversible form of heart failure. Known as Keshan disease, the condition is potentially fatal if untreated.294,295 Several studies also suggest less severe selenium deficiency may be associated with heart failure.294
In a recent randomized, double-blind, placebo-controlled trial involving 53 patients with congestive heart failure, 200 µg selenium per day for 12 weeks reduced serum insulin levels, decreased LDL cholesterol levels, increased HDL cholesterol levels, reduced C-reactive protein levels, and elevated the plasma antioxidant capacity.296
Magnesium
Magnesium is a mineral found in the body that is a cofactor in over 600 enzymatic reactions,415 including protein synthesis and blood pressure and blood glucose regulation. It is also required for energy production and bone development, and plays a role in muscle contraction, nerve impulses, and maintaining a normal heart rhythm. Magnesium deficiency is common in people with congestive heart failure, and is believed to worsen clinical outcomes in this population.297 Recent research suggested risk factors for cardiovascular disease, including cholesterol and blood pressure levels, are associated with low serum levels and dietary intake of magnesium.298
A recent literature review focusing on epidemiological research suggests high magnesium intake is associated with lower risk of cardiovascular risk factors and cardiovascular disease, including coronary heart disease and stroke. A recent meta-analysis demonstrated a strong association between heart failure and increment of 100 mg/day of magnesium intake.299 Further randomized controlled clinical trials will help elucidate magnesium’s role in heart health.
In another trial, 300 mg magnesium citrate was found to improve heart rate variability (ie, the time variation between heartbeats) after five weeks of supplementation.300 Magnesium oxide, at a dose of 800 mg daily for three months, improved arterial elasticity compared with placebo in individuals with chronic heart failure.301 In another study, magnesium orotate (6,000 mg daily for one month, 3,000 mg daily for 11 months) or placebo was given to patients with severe congestive heart failure. The survival rate after one year of supplementation was 76% for the magnesium group vs. 52% for the placebo group. The authors concluded “Magnesium orotate may be used as adjuvant therapy in patients on optimal treatment for severe congestive heart failure, increasing survival rate and improving clinical symptoms and patient’s quality of life.”302
L-carnosine
L-carnosine is a dipeptide (two amino acids linked together) made in the body from the amino acids L-histidine and beta-alanine. It is abundant in skeletal muscles and the brain. Meat and fish are the main dietary sources of L-carnosine as well as beta-alanine. Preclinical evidence shows that L-carnosine may provide benefit to the heart muscles through several mechanisms, including support for calcium homeostasis, defense against oxidative stress, and detoxification of reactive molecules such as advanced glycation end products and oxidized lipids.390 L-carnosine precursor supplementation (ie, L-histidine and beta-alanine) combined with exercise training has shown benefits in animal models of heart failure.391,392
A randomized controlled clinical trial included 50 people who had stable congestive heart failure with severe left-ventricular systolic dysfunction and were receiving standard medical treatment. The participants were randomized to either continue with the standard treatment or receive 500 mg L-carnosine per day in addition to standard treatment. The results showed that, compared to baseline, those who received L-carnosine had significant improvements in six-minute walk time and subjective rated quality of life at six months. The L-carnosine group also experienced significant improvements in exercise capacity and aerobic fitness compared to the group that continued with only standard treatment.393
2025
- Apr: Updated section on interventions for valvular heart disease in Heart Failure Treatment
- Feb: Added section on interventions for valvular heart disease to Heart Failure Treatment
- Feb: Updated section on arjuna (Terminalia arjuna) in Nutrients
2024
- May: Added section on L-carnosine to Nutrients
2022
- May: Updated Heart Failure Treatment
- May: Updated section on omega-3 fats in Nutrients
2019
- Mar: Comprehensive update & review
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Clinic M. Heart Failure. 2017.
- Foley PM. Clinical Key. First Consult; Heart Failure. Available at: www.clinicalkey.com. Accessed 7/19/2013. 2012.
- Hunt, S. A., Abraham, W. T., Chin, M. H., 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):e1–e90.
- Heidenreich, P. A., Albert, N. M., Allen, L. A., et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement From the American Heart Association. Circ Heart Fail. 2013; 6(3):606-19.
- Brum PC, Bacurau AV, Medeiros A, Ferreira JC, Vanzelli AS, Negrao CE. Aerobic exercise training in heart failure: impact on sympathetic hyperactivity and cardiac and skeletal muscle function. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.]. Sep 2011;44(9):827-835.
- Iwanaga Y, Miyazaki S. Heart failure, chronic kidney disease, and biomarkers--an integrated viewpoint. Circulation journal: official journal of the Japanese Circulation Society. Jul 2010;74(7):1274-1282.
- Ferri FF. Ferri's Clinical Advisor. Heart Failure. Available at: www.clinicalkey.com. Accessed 8/8/2013. 2013.
- CDC. Heart Failure Fact Sheet. 2019.
- Savarese G. Global Public Health Burden of Heart Failure. Cardiac failure review. 2017;3(1):7-11.
- Braunwald E. The war against heart failure: the Lancet lecture. The Lancet. 2015;385(9970):812-824.
- Mortensen SA. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2(6):641-649.
- Langsjoen PH, Langsjoen JO, Langsjoen AM, Lucas LA. Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. BioFactors. 2005;25(1-4):147-152.
- Langsjoen P, Littarru G, Silver M. Potential role of concomitant coenzyme Q~ 1~ 0 with statins for patients with hyperlipidemia. Current Topics in Nutraceutical Research. 2005;3(3):149.
- Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H. Lovastatin decreases coenzyme Q levels in humans. Proceedings of the National Academy of Sciences of the United States of America. Nov 1990;87(22):8931-8934.
- Silver MA, Langsjoen PH, Szabo S, Patil H, Zelinger A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. The American journal of cardiology. Nov 15 2004;94(10):1306-1310.
- Rubinstein J, Aloka F, Abela GS. Statin therapy decreases myocardial function as evaluated via strain imaging. Clin Cardiol. 2009;32(12):684-9.
- Marieb, E., and Hoehn, K. Human anatomy & physiology. 8 ed. San Francisco: Pearson Benjamin Cummings. 2010.
- Goldman, L. Schafer, A.L. Goldman's Cecil Medicine, Twenty-Fourth Edition. Heart Failure. Pathophysiology and Diagnosis. Available at: https://www.clinicalkey.com. Accessed 7/10/2013.
- Association AH. Causes of Heart Failure. 2017.
- Mangini S, Pires PV, Braga FGM, Bacal F. Decompensated heart failure. Einstein (Sao Paulo, Brazil). 2013;11(3):383-391.
- Gazewood JD, Turner PL. Heart Failure with Preserved Ejection Fraction: Diagnosis and Management. American family physician. 2017;96(9):582-588.
- Inamdar AA, Inamdar AC. Heart Failure: Diagnosis, Management and Utilization. J Clin Med. 2016;5(7):62.
- American Heart Association. Ejection Fraction Heart Failure Measurement. https://www.heart.org/en/health-topics/heart-failure/diagnosing-heart-failure/ejection-fraction-heart-failure-measurement. Last reviewed 5/31/2017. Accessed 3/4/2019.
- Pazos-López P, Peteiro-Vázquez J, Carcía-Campos A, García-Bueno L, de Torres JPA, Castro-Beiras A. The causes, consequences, and treatment of left or right heart failure. Vascular health and risk management. 2011;7:237-254.
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nature reviews. Cardiology. 2016;13(6):368-378.
- American Heart Association. Types of Heart Failure. https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/types-of-heart-failure. Accessed 02/19/2019.
- NHLBI. What Is Heart Failure? - NHLBI, NIH. National Heart, Lung, and Blood Institute. 2012; Available at: http://www.nhlbi.nih.gov/health/health-topics/topics/hf/ [Accessed May 15, 2013].
- Hunt, S. A., Abraham, W. T., Chin, M. H., ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235.
- Abdel-Qadir, H. M., Lee, D.S. The contribution of familial and heritable risks in heart failure. Curr Opin Cardiol. 2007;22(3):214-9.
- Kenchaiah, S., Narula, J., and Vasan, R. S. Risk factors for heart failure. Med. Clin. North Am. 2004;88(5):1145–72.
- Dunn, S. P., Bleske, B., Dorsch, M., Macaulay, T., Van Tassell, B., and Vardeny, O. Nutrition and heart failure: impact of drug therapies and management strategies. Nutrition in Clinical Practice. 2009;24(1):60–75.
- Bryson, C. L., Mukamal, K. J., Mittleman, M. A., The Association of Alcohol Consumption and Incident Heart Failure. J Am Coll Cardiol. 2006;48(2):305–11.
- Suskin, N., Sheth, T., Negassa, A., Yusuf, S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;37(6):1677-82.
- Conard, M.W., Haddock, C.K., Poston, W.S., Spertus, J.A., and the Cardiovascular Outcomes Research Consortium. The impact of smoking status on the health status of heart failure patients. Congest Heart Fail. 2009;15(2):82-6.
- Oerkild, B., Frederiksen, M., Hansen, J.F., Prescott, E. Self-reported physical inactivity predicts survival after hospitalization for heart disease. Eur J Cardiovasc Prev Rehabil. 2011;18(3):475-80.
- Djoussé L, Driver JA, Gaziano JM. Relation Between Modifiable Lifestyle Factors and Lifetime Risk of Heart Failure. JAMA. 2009;302(4):394-400.
- Yang Q. Added sugar intake and cardiovascular diseases mortality among us adults. JAMA Internal Medicine. 2014;174(4):516-524.
- Heist, E. K., and Ruskin, J. N. Atrial fibrillation and congestive heart failure: risk factors, mechanisms, and treatment. Prog Cardiovasc Dis. 2006;48(4):256–69.
- Britton, K.A., Gaziano, J.M., Djoussé, L. Normal systolic blood pressure and risk of heart failure in US male physicians. Eur J Heart Fail. 2009;11(12):1129-34.
- Kannel, W.B. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5(2):167-73.
- Harinstein, M. E., Filippatos, G. S., and Gheorghiade, M. Acute heart failure syndromes: epidemiology, risk stratification and prognostic factors. Acute Card Care. 2009;11(2):77–82.
- Lehrke M, Marx N. Diabetes Mellitus and Heart Failure. The American journal of cardiology. 2017;120(1s):S37-s47.
- de Miguel Diez J, Morgan JC, Garcia RJ. The association between COPD and heart failure risk: a review. International journal of chronic obstructive pulmonary disease. 2013;8:305-312.
- Segall L, Nistor I, Covic A. Heart Failure in Patients with Chronic Kidney Disease: A Systematic Integrative Review. J BioMed Research International. 2014;2014:21.
- Vestberg D, Rosengren A, Olsson M, Gudbjornsdottir S, Svensson AM, Lind M. Relationship Between Overweight and Obesity With Hospitalization for Heart Failure in 20,985 Patients With Type 1 Diabetes: A population-based study from the Swedish National Diabetes Registry. Diabetes care. Jun 11 2013.
- Liguori I, Russo G, Curcio F, et al. Depression and chronic heart failure in the elderly: an intriguing relationship. J Geriatr Cardiol. Jun 2018;15(6):451-459.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62(16):e147-239.
- NHLBI. Sleep Apnea. Health topics. https://www.nhlbi.nih.gov/health-topics/sleep-apnea. Accessed 02/07/19.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5(2):136-143.
- Costanzo MR. Mechanisms and Clinical Consequences of Untreated Central Sleep Apnea in Heart Failure. Journal of the American College of Cardiology. 2015;65(1):72.
- Khattak HK. Obstructive Sleep Apnea in Heart Failure: Review of Prevalence, Treatment with Continuous Positive Airway Pressure, and Prognosis. Texas Heart Institute journal. 2018;45(3):151-161.
- Gottlieb, D.J., Yenokyan, G., Newman, A.B., O'Connor, G.T., Punjabi, N.M., Quan, S.F., et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352-60.
- Drager LF, Polotsky VY, et al. Obstructive Sleep Apnea: An Emerging Risk Factor for Atherosclerosis. Chest. 2011;140:534-42.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive Sleep Apnea: The Most Common Secondary Cause of Hypertension Associated with Resistant Hypertension. Hypertension. 2011;58:811-17.
- Aronsohn RS, Whitmore H et al. Impact of Untreated Obstructive Sleep Apnea on Glucose control in Type 2 Diabetes. American Journal of Respiratory Critical Care, 2010; 181; 507-513.
- Nieto FJ, Peppard PE, Young T, et al. Sleep disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012 May 20. [Epub ahead of print].
- Yaggi HK, Concato J. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. NEJM. 2005;353(19):2034-41.
- Page RL, O’Bryant CL, Cheng D, et al. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2016;134(6):e32-e69.
- Díez-López C, Lupón J, de Antonio M, et al. Hemoglobin Kinetics and Long-term Prognosis in Heart Failure. Revista Española de Cardiología (English Edition). 2016;69(09):820-826.
- Mbakwem A, Aina F, Amadi C. Depression in Patients with Heart Failure: Is Enough Being Done? Cardiac Failure Review. 2016;2(2).
- Mayo Clinic. High blood pressure (hypertension). Symptoms & causes. https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/symptoms-causes/syc-20373410. Last updated 5/12/2018. Accessed 3/5/2019.
- N.Y. Criteria Committee, N. Y. H. A. Diseases of the Heart and Blood Vessels. Nomenclature and Criteria for Diagnosis ... Sixth Edition. [With Illustrations.]. 1964.
- Jessup, M., Abraham, W. T., Casey, D. E., 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016.
- Brozena, S. C., and Jessup, M. The new staging system for heart failure. What every primary care physician should know. Geriatrics. 2003;58(6):31–6–quiz38.
- Cardiology Review. ACC/AHA Heart Failure Classification. https://www.healio.com/cardiology/learn-the-heart/cardiology-review/topic-reviews/accaha-heart-failure-classification. Accessed 3/5/2019.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2016;18(8):891-975.
- Association AH. Cardiomyopathy. 2016.
- Mangalat, D., Kalogeropoulos, A., Georgiopoulou, V., Stillman, A., Butler, J. Value of Cardiac CT in Patients With Heart Failure. Curr Cardiovasc Imaging Rep. 2009;2(6):410-417.
- Peterzan MA, Rider OJ, Anderson LJ. The Role of Cardiovascular Magnetic Resonance Imaging in Heart Failure. Cardiac failure review. 2016;2(2):115-122.
- Weber, M., Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92(6):843-9.
- Di Angelantonio, E., Chowdhury, R., Sarwar, N., B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120(22):2177–87.
- Kim H-N, Januzzi JL. Natriuretic Peptide Testing in Heart Failure. Circulation. 2011;123(18):2015-2019.
- Gaggin HK, Januzzi JL. Cardiac Biomarkers and Heart Failure. Latest in Cardiology: Expert Analysis https://www.acc.org/latest-in-Cardiology/%20articles/%202015/%2002/09/13/00/cardiac-biomarkers-and-heart-failure. Accessed 02/11/2019.
- Göran Nilsson PH, John Ohrvik. How to live until 90 – Factors predicting survival in 75-year-olds from the general population. Healthy Aging Research. 2014;3.
- Nagarajan, V., Hernandez, A. V., and Tang, W. H. W. Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart. 2012;98(24):1778–86.
- Nagarajan, V., and Tang, W. H. W. Biomarkers in advanced heart failure: diagnostic and therapeutic insights. Congest Heart Fail. 2011;17(4):169–74.
- Wang, M., Liao, Y. Value of quantitative analysis of serum cTnT in diagnosis of cardiac disease and myocardial injury. J Tongji Med Univ. 2000;20(1):53-4.
- Nishio, Y., Sato, Y., Taniguchi, R., Shizuta, S., Doi, T., Morimoto, T., Kimura, T., Kita, T. Cardiac troponin T vs other biochemical markers in patients with congestive heart failure. Circ J. 2007;71(5):631-5.
- McQueen, M. J., Kavsak, P. A., Xu, L., Shestakovska, O., and Yusuf, S. Predicting myocardial infarction and other serious cardiac outcomes using high-sensitivity cardiac troponin T in a high-risk stable population. Clin. Biochem. 2013;46(1-2):5–9.
- Evans JDW, Dobbin SJH, Pettit SJ, Di Angelantonio E, Willeit P. High-Sensitivity Cardiac Troponin and New-Onset Heart Failure: A Systematic Review and Meta-Analysis of 67,063 Patients With 4,165 Incident Heart Failure Events. JACC: Heart Failure. 2018;6(3):187-197.
- Magnussen C, Blankenberg S. Biomarkers for heart failure: small molecules with high clinical relevance. Journal of internal medicine. 2018;283(6):530-543.
- Nooralam Ansari* AH, Mohammad Owais. A study of inflammatory markers and their correlation with severity, in patients with chronic heart failure. Biomedical Research. 2012;23(3).
- McCarthy CP, Januzzi JL, Jr. Soluble ST2 in Heart Failure. Heart failure clinics. 2018;14(1):41-48.
- Gehlken C, Suthahar N, Meijers WC, de Boer RA. Galectin-3 in Heart Failure: An Update of the Last 3 Years. Heart failure clinics. 2018;14(1):75-92.
- van Kimmenade, R. R. J., and Januzzi, J. L. Emerging biomarkers in heart failure. Clin Chem. 2012;58(1):127–38.
- Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. Nov 15 2011;124(20):2253-2263.
- Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. Journal of the American College of Cardiology. Sep 21 2010;56(13):1001-1012.
- Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. Journal of cardiac failure. Nov 2010;16(11):888-900.
- Ambrosy AP, Lewis GD, Malhotra R, et al. Identifying responders to oral iron supplementation in heart failure with a reduced ejection fraction: a post-hoc analysis of the IRONOUT-HF trial. Journal of cardiovascular medicine (Hagerstown, Md). 2018.
- Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, . . . Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. American heart journal. Apr 2013;165(4):575-582.e573.
- Comin-Colet J, Enjuanes C, Gonzalez G, Torrens A, Cladellas M, Merono O, . . . Bruguera J. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. European journal of heart failure. Jul 15 2013.
- Filippatos G, Farmakis D, Colet JC, Dickstein K, Luscher TF, Willenheimer R, . . . Anker SD. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. European journal of heart failure. Jul 18 2013.
- Kapoor M, Schleinitz MD, Gemignani A, Wu WC. Outcomes of patients with chronic heart failure and iron deficiency treated with intravenous iron: a meta-analysis. Cardiovascular & hematological disorders drug targets. Mar 1 2013;13(1):35-44.
- Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. European journal of heart failure. Apr 2012;14(4):423-429.
- Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, . . . Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. European heart journal. Aug 2010;31(15):1872-1880.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Colvin MM, . . . Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6).
- Westenbrink BD, Visser FW, Voors AA, Smilde TD, Lipsic E, Navis G, . . . van Veldhuisen DJ. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. European heart journal. Jan 2007;28(2):166-171.
- Shah R, Agarwal AK. Anemia associated with chronic heart failure: current concepts. Clinical interventions in aging. 2013;8:111-122.
- Pereira CA, Roscani MG, Zanati SG, Matsubara BB. Anemia, heart failure and evidence-based clinical management. Arquivos brasileiros de cardiologia. Jul 2013;101(1):87-92.
- Kilicgedik A, Dundar C, Tigen MK. Anemia in heart failure. Anadolu kardiyoloji dergisi: AKD = the Anatolian journal of cardiology. Feb 2012;12(1):65-70.
- Vrtovec B, Poglajen G, Haddad F. Stem cell therapy in patients with heart failure. Methodist Debakey Cardiovasc J. Jan-Mar 2013;9(1):6-10.
- Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015). Stem Cell Res Ther. Jun 4 2016;7(1):82.
- NLM. U.S. National Library of Medicine. Stem Cells. https://medlineplus.gov/stemcells.html. Last updated 9/16/2016. Accessed 9/29/2016.
- Katarzyna R. Adult Stem Cell Therapy for Cardiac Repair in Patients After Acute Myocardial Infarction Leading to Ischemic Heart Failure: An Overview of Evidence from the Recent Clinical Trials. Curr Cardiol Rev. 2017;13(3):223-231.
- Wernly B, Mirna M, Rezar R, et al. Regenerative Cardiovascular Therapies: Stem Cells and Beyond. Int J Mol Sci. Mar 21 2019;20(6).
- Yun CW, Lee SH. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int J Mol Sci. Feb 24 2019;20(4).
- Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. Dec 24 2016;12:Cd007888.
- Nguyen PK, Rhee JW, Wu JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol. Oct 1 2016;1(7):831-841.
- Yau TM, Pagani FD, Mancini DM, et al. Intramyocardial Injection of Mesenchymal Precursor Cells and Successful Temporary Weaning From Left Ventricular Assist Device Support in Patients With Advanced Heart Failure: A Randomized Clinical Trial. Jama. Mar 26 2019;321(12):1176-1186.
- Bartunek J, Terzic A, Davison BA, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. Mar 1 2017;38(9):648-660.
- Wernly B, Goncalves I, Kiss A, et al. Differences in Stem Cell Processing Lead to Distinct Secretomes Secretion-Implications for Differential Results of Previous Clinical Trials of Stem Cell Therapy for Myocardial Infarction. Biotechnol J. Sep 2017;12(9).
- Luscher TF. Back to square one. Eur Heart J. Apr 1 2019;40(13):1031-1033.
- Kalicinska E, Wojtas K, Majda J, Doehner W, Haehling SV, Banasiak W, . . . Jankowska EA. Anabolic deficiencies in men with systolic heart failure: do co-morbidities and therapies really contribute significantly? The aging male: the official journal of the International Society for the Study of the Aging Male. Jun 26 2013.
- Tirabassi G, Gioia A, Giovannini L, Boscaro M, Corona G, Carpi A, . . . Balercia G. Testosterone and cardiovascular risk. Internal and emergency medicine. Apr 2013;8 Suppl 1:S65-69.
- Volterrani, M., Rosano, G., and Iellamo, F. Testosterone and heart failure. Endocrine. 2012;42(2):272–7.
- Aukrust, P., Ueland, T., Gullestad, L., and Yndestad, A. Testosterone: a novel therapeutic approach in chronic heart failure? J Am Coll Cardiol. 2009;54(10):928–9.
- Giagulli VA, Guastamacchia E, De Pergola G, Iacoviello M, Triggiani V. Testosterone deficiency in male: a risk factor for heart failure. Endocrine, metabolic & immune disorders drug targets. 2013;13(1):92-99.
- Han Y, Sun W, Sun G, et al. A 3-year observation of testosterone deficiency in Chinese patients with chronic heart failure. Oncotarget. 2017;8(45):79835-79842.
- Wang W, Jiang T, Li C, et al. Will testosterone replacement therapy become a new treatment of chronic heart failure? A review based on 8 clinical trials. Journal of thoracic disease. 2016;8(5):E269-277.
- Dos Santos MR, Sayegh AL, Bacurau AV, et al. Effect of Exercise Training and Testosterone Replacement on Skeletal Muscle Wasting in Patients With Heart Failure With Testosterone Deficiency. Mayo Clinic proceedings. 2016;91(5):575-586.
- Iellamo, F., Volterrani, M., Caminiti, G., et al. Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol. 2010;56(16):1310–6.
- Kloner RA. Testosterone and Cardiovascular Disease. J Am Coll Cardiol. 2016;67(5):545-557.
- Bibevski, S., Dunlap, M.E. Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev. 2011;16(2):129-35.
- De Ferrari, G. M., Crijns, H. J. G. M., Borggrefe, M. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. European Heart Journal. 2011;32(7):847–55.
- Sabbah, H.N. Electrical vagus nerve stimulation for the treatment of chronic heart failure. Cleve Clin J Med. 2011;78 Suppl 1:S24-9.
- Gold MR, Van Veldhuisen DJ, Hauptman PJ, et al. Vagus Nerve Stimulation for the Treatment of Heart Failure. Journal of the American College of Cardiology. 2016;68(2):149.
- Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovascular research. Feb 15 2009;81(3):412-419.
- Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiological reviews. Jan 2010;90(1):207-258.
- Lopatin YM. Rationale and benefits of trimetazidine by acting on cardiac metabolism in heart failure. International journal of cardiology. 2016;203:909-915.
- Grajek S, Michalak M. The effect of trimetazidine added to pharmacological treatment on all-cause mortality in patients with systolic heart failure. Cardiology. 2015;131(1):22-29.
- Zhou X, Chen J. Is treatment with trimetazidine beneficial in patients with chronic heart failure? PLoS One. 2014;9(5):e94660.
- Zhang L, Lu Y, Jiang H, Zhang L, Sun A, Zou Y, Ge J. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. Journal of the American College of Cardiology. Mar 6 2012;59(10):913-922.
- Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart (British Cardiac Society). Feb 2011;97(4):278-286.
- Winter JL, Castro PF, Quintana JC, et al. Effects of trimetazidine in nonischemic heart failure: a randomized study. Journal of cardiac failure. 2014;20(3):149-154.
- Brodbin P, O'Connor CA. Trimetazidine in the treatment of angina pectoris. The British journal of clinical practice. Sep 1968;22(9):395-396.
- Masmoudi K, Masson H, Gras V, Andrejak M. Extrapyramidal adverse drug reactions associated with trimetazidine: a series of 21 cases. Fundamental & clinical pharmacology. Apr 2012;26(2):198-203.
- Montastruc JL, Sommet A, Olivier P, Bagheri H, Gony M, Lapeyre-Mestre M, . . . Rascol O. [Drugs, Parkinson's disease and parkinsonian syndroms: recent advances in pharmacovigilance]. Therapie. Jan-Feb 2006;61(1):29-38.
- Syvannarath V, Delbosc S, Escoubet B, et al. Treatment with a CD31 agonist peptide improves the outcome of experimental heart failure with either reduced or preserved ejection fraction. Archives of Cardiovascular Diseases Supplements. 2018;10(2):197-198.
- Costanzo MR. Ultrafiltration In The Management Of Heart Failure. US Cardiology Review. 2008;5(1):66-69.
- Djousse L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Current atherosclerosis reports. Apr 2008;10(2):117-120.
- Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. May 2002;121(5):1638-1650.
- CDC (Centers for Disease Control and Prevention) (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2010. 6, Cardiovascular Diseases. http://www.ncbi.nlm.nih.gov/books/NBK53012/.
- Clair, C., Rigotti, N.A., Porneala, B., Fox, C.S., D'Agostino, R.B., Pencina, M.J., Meigs, J.B. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309(10):1014-21.
- Sacks, F., Svetkey, L., Vollmer, W., and Appel, L. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001; 344(1):3-10.
- Tejada, T., Fornoni, A., Lenz, O., Materson, B.J. Nonpharmacologic therapy for hypertension: does it really work?. Curr Cardiol Rep. 2006;8(6):418-24.
- Kerley CP. Dietary patterns and components to prevent and treat heart failure: a comprehensive review of human studies. Nutr Res Rev. Aug 16 2018:1-27.
- Rifai L, Silver MA. A Review of the DASH Diet as an Optimal Dietary Plan for Symptomatic Heart Failure. Prog Cardiovasc Dis. Mar-Apr 2016;58(5):548-554.
- Brooks M. Benefit of Salt Restriction in Heart Failure Uncertain. Medscape Medical News https://www.medscape.com/viewarticle/904603. Accessed 02/12/2019.
- Doukky R, Avery E, Mangla A, et al. Impact of Dietary Sodium Restriction on Heart Failure Outcomes. JACC. Heart failure. 2016;4(1):24-35.
- Hummel SL, Konerman MC. Dietary Sodium Restriction in Heart Failure: A Recommendation Worth its Salt?∗. JACC: Heart Failure. 2016/01/01/ 2016;4(1):36-38.
- Yancy CW. The Uncertainty of Sodium Restriction in Heart Failure: We Can Do Better Than This∗. JACC: Heart Failure. 2016/01/01/ 2016;4(1):39-41.
- Kerley CP. A Review of Plant-based Diets to Prevent and Treat Heart Failure. Card Fail Rev. May 2018;4(1):54-61.
- Dragan S, Buleu F, Christodorescu R, et al. Benefits of multiple micronutrient supplementation in heart failure: A comprehensive review. Critical reviews in food science and nutrition. 2018:1-17.
- Lennie Terry A, Andreae C, Rayens Mary K, et al. Micronutrient Deficiency Independently Predicts Time to Event in Patients With Heart Failure. Journal of the American Heart Association. 2018;7(17):e007251.
- Ceremuzyński, L., Gebalska, J., Wolk, R., and Makowska, E. Hypomagnesemia in heart failure with ventricular arrhythmias. Beneficial effects of magnesium supplementation. Acta Med Scand. 2000;247(1):78–86.
- Fang. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose–response meta-analysis of prospective cohort studies. BMC Med. 2016;14(1):210.
- Azizi-Namini, P., Ahmed, M., Yan, A. T., and Keith, M. The role of B vitamins in the management of heart failure. Nutr Clin Pract. 2012;27(3):363–74.
- Krim, S. R., Campbell, P., Lavie, C. J., and Ventura, H. Micronutrients in chronic heart failure. Curr Heart Fail Rep. 2013;10(1):46–53.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. Aug 2016;18(8):891-975.
- American Heart Association. Cardiac Rehab for Heart Failure. Health Topics https://www.heart.org/en/health-topics/heart-failure/treatment-options-for-heart-failure/cardiac-rehab-for-heart-failure. Accessed 02/15/2019.
- Piepoli MF, Conraads V, Corrà U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13(4):347-357.
- Downing, J., and Balady, G. J. The role of exercise training in heart failure. J Am Coll Cardiol. 2011;58(6):561–9.
- Ansley, D. M., and Wang, B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013;229(2):232–41.
- Papanas, N., Maltezos, E., and Mikhailidis, D. P. Metformin and heart failure: never say never again. Expert Opin Pharmacother. 2012;13(1):1–8.
- Facila L, Fabregat-Andres O, Bertomeu V, et al. Metformin and risk of long-term mortality following an admission for acute heart failure. J Cardiovasc Med (Hagerstown). Feb 2017;18(2):69-73.
- Martens P, Janssens J, Ramaekers J, Dupont M, Mullens W. Contemporary choice of glucose lowering agents in heart failure patients with type 2 diabetes. Acta Cardiol. Feb 8 2019:1-7.
- Saad M, Gomceli U, Ravi P, Lacoste AG, Shah N, Vittorio TJ. The metabolic model of heart failure: the role of sodium glucose co-transporter-2 (SGLT-2) inhibition. Drugs Context. 2018;7:212549.
- Association AH. Stress and Heart Health. 2014.
- Vongmany J, Hickman LD, Lewis J, Newton PJ, Phillips JL. Anxiety in chronic heart failure and the risk of increased hospitalisations and mortality: A systematic review. European journal of cardiovascular nursing: journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology. 2016;15(7):478-485.
- Levine GN, Lange RA, Bairey‐Merz CN, et al. Meditation and Cardiovascular Risk Reduction: A Scientific Statement From the American Heart Association. J Am Heart Assoc. 2017;6(10):e002218.
- Aggarwal M, Bozkurt B, Panjrath G, et al. Lifestyle Modifications for Preventing and Treating Heart Failure. Journal of the American College of Cardiology. 2018;72(19):2391.
- Rosenfeldt, F. L., Haas, S. J., Krum, H. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. 2007;21(4):297–306.
- Shimizu M. Low circulating coenzyme Q10 during acute phase is associated with inflammation, malnutrition, and in-hospital mortality in patients admitted to the coronary care unit. Heart and vessels. 2017;32(6):668-673.
- Lei L. Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials. BMC cardiovascular disorders. 2017;17(1):196-196.
- Jafari M, Mousavi SM, Asgharzadeh A, Yazdani N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart Journal. 2018;70:S111-S117.
- Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. BioFactors (Oxford, England). 2003;18(1-4):91-100.
- Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, Richards AM. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. Journal of the American College of Cardiology. Oct 28 2008;52(18):1435-1441.
- Soja, A. M., and Mortensen, S. A. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Mol. Aspects Med. 1997;18 Suppl:S159–68.
- Sander, S., Coleman, C. I., Patel, A. A., Kluger, J., and White, C. M. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J. Card. Fail. 2006;12(6):464–72.
- Fotino, A. D., Thompson-Paul, A. M., and Bazzano, L. A. Effect of coenzyme Q₁₀ supplementation on heart failure: a meta-analysis. American Journal of Clinical Nutrition. 2013;97(2):268–75.
- Belcaro G, Cesarone MR, Dugall M, et al. Investigation of Pycnogenol(R) in combination with coenzymeQ10 in heart failure patients (NYHA II/III). Panminerva medica. 2010;52(2 Suppl 1):21-25.
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, . . . McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. The New England journal of medicine. Jul 18 2002;347(3):161-167.
- Rigelsky JM, Sweet BV. Hawthorn: pharmacology and therapeutic uses. American journal of health-system pharmacy: AJHP: official journal of the American Society of Health-System Pharmacists. Mar 1 2002;59(5):417-422.
- Urbonaviciute A, Jakstas V, Kornysova O, Janulis V, Maruska A. Capillary electrophoretic analysis of flavonoids in single-styled hawthorn (Crataegus monogyna Jacq.) ethanolic extracts. Journal of chromatography. A. Apr 21 2006;1112(1-2):339-344.
- Yang B, Liu P. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. Journal of the science of food and agriculture. Jun 2012;92(8):1578-1590.
- Schröder, D., Weiser, M., Klein, P. Efficacy of a homeopathic Crataegus preparation compared with usual therapy for mild (NYHA II) cardiac insufficiency: results of an observational cohort study. Eur J Heart Fail. 2003;5(3):319-26.
- Furey, A., and Tassell, M. Towards a systematic scientific approach in the assessment of efficacy of an herbal preparation: Hawthorn (Crataegus spp.). European Journal of Heart Failure. 2008;10(12):1153–7.
- Koch, E., and Malek, F. A. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders--preclinical and clinical studies. Planta Med. 2011;77(11):1123–8.
- Cicero AFG, Colletti A. Nutraceuticals and Dietary Supplements to Improve Quality of Life and Outcomes in Heart Failure Patients. Current pharmaceutical design. 2017;23(8):1265-1272.
- Holubarsch CJF, Colucci WS, Meinertz T, et al. Survival and Pronosis: Investigation of Crataegus Extract WS 1442 in congestive heart failure (SPICE) – rationale, study design and study protocol. European Journal of Heart Failure. 2000;431-37.
- Holubarsch, C. J. F., Colucci, W. S., Meinertz, T., Gaus, W., Tendera, M., Survival and Prognosis: Investigation of Crataegus Extract WS 1442 in CHF (SPICE) trial study group. The efficacy and safety of Crataegus extract WS 1442 in patients with heart failure: the SPICE trial. Eur. J. Heart Fail. 2008;10(12):1255–63.
- Zick, S. M., Vautaw, B. M., Gillespie, B., and Aaronson, K. D. Hawthorn Extract Randomized Blinded Chronic Heart Failure (HERB CHF) trial. Eur. J. Heart Fail. 2009;11(10):990–9.
- Rucker, R., Chowanadisai, W., and Nakano, M. Potential physiological importance of pyrroloquinoline quinone. Altern Med Rev. 2009;14(3):268–77.
- Hamilton DJ. Mechanisms of disease: is mitochondrial function altered in heart failure? Methodist DeBakey cardiovascular journal. Jan-Mar 2013;9(1):44-48.
- Nehra. Nanocurcumin–pyrroloquinoline formulation prevents hypertrophy–induced pathological damage by relieving mitochondrial stress in cardiomyocytes under hypoxic conditions. Experimental & Molecular Medicine. 2017;49.
- Zhu, B.-Q., Simonis, U., Cecchini, G., et al. Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 2006;11(2):119–28.
- Zhu, B.-Q., Zhou, H.-Z., Teerlink, J. R., and Karliner, J. S. Pyrroloquinoline quinone (PQQ) decreases myocardial infarct size and improves cardiac function in rat models of ischemia and ischemia/reperfusion. Cardiovasc Drugs Ther. 2004;18(6):421–31.
- Siscovick. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation. 2017;135.
- Marik, P. E., and Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32(7):365–72.
- Geleijnse, J. M., Giltay, E. J., Grobbee, D. E., Donders, A. R. T., and Kok, F. J. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J. Hypertens. 2002;20(8):1493–9.
- Wang C, Xiong B, Huang J. The Role of Omega-3 Polyunsaturated Fatty Acids in Heart Failure: A Meta-Analysis of Randomised Controlled Trials. Nutrients. 2017;9(1):18.
- Xin, W., Wei, W., and Li, X. Effects of fish oil supplementation on cardiac function in chronic heart failure: a meta-analysis of randomised controlled trials. Heart. 2012;98(22):1620–5.
- Mehra, M. R., Lavie, C. J., Ventura, H. O., and Milani, R. V. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J. Heart Lung Transplant. 2006;25(7):834–8.
- Jiang W, Whellan DJ, Adams KF, et al. Long-Chain Omega-3 Fatty Acid Supplements in Depressed Heart Failure Patients: Results of the OCEAN Trial. JACC Heart Fail. Oct 2018;6(10):833-843.
- Moreira da Silva Guimaraes S, de Souza Cruz WM, de Souza Weigert G, et al. Decompensated Chronic Heart Failure Reduces Plasma L-carnitine. Archives of medical research. 2018;49(4):278-281.
- Song X, Qu H, Yang Z, Rong J, Cai W, Zhou H. Efficacy and Safety of L-Carnitine Treatment for Chronic Heart Failure: A Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2017;2017:6274854.
- Wang ZY, Liu YY, Liu GH, Lu HB, Mao CY. l-Carnitine and heart disease. Life sciences. 2018;194:88-97.
- Soukoulis, V., Dihu, J. B., Sole, M. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol. 2009;54(18):1660–73.
- Anand, I., Chandrashekhan, Y., De Giuli, F. Acute and chronic effects of propionyl-L-carnitine on the hemodynamics, exercise capacity, and hormones in patients with congestive heart failure. Cardiovasc Drugs Ther. 1998;12(3):291–9.
- Mancini, M., Rengo, F., Lingetti, M., Sorrentino, G. P., and Nolfe, G. Controlled study on the therapeutic efficacy of propionyl-L-carnitine in patients with congestive heart failure. Arzneimittelforschung. 1992;42(9):1101–4.
- Rizos, I. Three-year survival of patients with heart failure caused by dilated cardiomyopathy and L-carnitine administration. Am. Heart J. 2000; 139(2 Pt 3):S120-3.
- Glickman-Simon, R., and Ehrlich, A. Leeches, creatine, xylitol, spinal manipulation, acupuncture. Explore (NY). 2012;8(3):206–9.
- Horjus, D. L., Oudman, I., van Montfrans, G. A., and Brewster, L. M. Creatine and creatine analogues in hypertension and cardiovascular disease. Cochrane Database Syst Rev. 2011;(11):CD005184.
- Hemati F, Rahmani A, Asadollahi K, Soleimannejad K, Khalighi Z. Effects of Complementary Creatine Monohydrate and Physical Training on Inflammatory and Endothelial Dysfunction Markers Among Heart Failure Patients. Asian journal of sports medicine. 2016;7(1):e28578-e28578.
- Ahmadian M, Dabidi Roshan V, Ashourpore E. Taurine Supplementation Improves Functional Capacity, Myocardial Oxygen Consumption, and Electrical Activity in Heart Failure. Journal of dietary supplements. 2017;14(4):422-432.
- Ahmadian M, Roshan VD, Aslani E, Stannard SR. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther Adv Cardiovasc Dis. 2017;11(7):185-194.
- Beyranvand, M. R., Khalafi, M. K., Roshan, V. D., Choobineh, S., Parsa, S. A., and Piranfar, M. A. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol. 2011;57(3):333–7.
- Azuma, J., Sawamura, A., and Awata, N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn. Circ. J. 1992;56(1):95–9.
- Shecterle, L. M., Wagner, S., and St Cyr, J. A. A sugar for congestive heart failure patients. Ther Adv Cardiovasc Dis. 2011;5(2):95–7.
- Bayram M, St. Cyr JA, Abraham WT. d-Ribose aids heart failure patients with preserved ejection fraction and diastolic dysfunction: a pilot study. Ther Adv Cardiovasc Dis. 2015;9(3):56-65.
- Omran H, Illien S, MacCarter D, et al. D-Ribose improves diastolic function and quality of life in congestive heart failure patients: a prospective feasibility study. The European Journal of Heart Failure. 2003; 5: 615-19.
- Vijay, N., MacCarter, D., Shecterle, L. M., and St Cyr, J. A. D-ribose benefits heart failure patients. J Med Food. 2008;11(1):199–200.
- Omran, H., McCarter, D., St Cyr, J., and Lüderitz, B. D-ribose aids congestive heart failure patients. Exp Clin Cardiol. 2004;9(2):117–8.
- Beveridge, L. A., and Witham, M. D. Vitamin D and the cardiovascular system. Osteoporos Int. 2013; 24(8):167-80.
- Catarina Magalhães Porto VDLS, João Soares Brito da Luz, Brivaldo Markman Filho,and Vera Magalhães da Silveira. Association between vitamin D deficiency and heart failure risk in the elderly. ESC Heart Failure. 2018;5:63-74.
- Liu, L. C. Y., Voors, A. A., van Veldhuisen, D. J., et al. Vitamin D status and outcomes in heart failure patients. Eur. J. Heart Fail. 2011;13(6):619–25.
- Pourdjabbar, A., Dwivedi, G., and Haddad, H. The role of vitamin D in chronic heart failure. Curr. Opin. Cardiol. 2013;28(2):216–22.
- Moretti HD, Colucci VJ, Berry BDJBCD. Vitamin D3 repletion versus placebo as adjunctive treatment of heart failure patient quality of life and hormonal indices: a randomized, double-blind, placebo-controlled trial. BMC Cardiovasc Disord. 2017;17(1):274.
- Bae, S., Singh, S. S., Yu, H., Lee, J. Y., Cho, B. R., and Kang, P. M. Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J Appl Physiol. 2013;114(8):979–87.
- Amin, A., Minaee, S., Chitsazan, M., Naderi, N., Taghavi, S., and Ardeshiri, M. Can Vitamin D Supplementation Improve the Severity of Congestive Heart Failure? Congest Heart Fail. 2013; Mar 21. doi: 10.1111/chf.12026. [Epub ahead of print].
- Witham, M. D., Crighton, L. J., Gillespie, N. D., Struthers, A. D., and McMurdo, M. E. T. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3(2):195–201.
- Schleithoff, S. S., Zittermann, A., Tenderich, G., Berthold, H. K., Stehle, P., and Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9.
- Witham, M. D., Nadir, M. A., and Struthers, A. D. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J. Hypertens. 2009;27(10):1948–54.
- Riba. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxidative Medicine and Cellular Longevity. 2017.
- Berman. The therapeutic potential of resveratrol: a review of clinical trials. npj Precision Oncology. 2017.
- Lymbury, R.S., Marino, M.J., Perkins, A.V. Effect of dietary selenium on the progression of heart failure in the ageing spontaneously hypertensive rat. Mol Nutr Food Res. 2010;54(10):1436-44.
- McKeag, N.A., McKinley, M.C., Woodside, J.V., Harbinson, M.T., McKeown, P.P. The role of micronutrients in heart failure. J Acad Nutr Diet. 2012;112(6):870-86.
- Saliba, W., El Fakih, R., Shaheen, W. Heart failure secondary to selenium deficiency, reversible after supplementation. Int J Cardiol. 2010;141(2):e26-7.
- Raygan F, Behnejad M, Ostadmohammadi V, et al. Selenium supplementation lowers insulin resistance and markers of cardio-metabolic risk in patients with congestive heart failure: a randomised, double-blind, placebo-controlled trial. British Journal of Nutrition. 2018;120(1):33-40.
- DiNicolantonio JJ, Liu J, O’Keefe JH. Magnesium for the prevention and treatment of cardiovascular disease. Open Heart. 2018;5(2):e000775.
- Supplements NIoHOoD. Magnesium: Fact Sheet for Professionals. 2018.
- Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, Salas-Salvadó J. Dietary Magnesium and Cardiovascular Disease: A Review with Emphasis in Epidemiological Studies. Nutrients. 2018;10(2):168.
- Almoznino-Sarafian D, Sarafian G, Berman S, Shteinshnaider M, Tzur I, Cohen N, Gorelik O. Magnesium administration may improve heart rate variability in patients with heart failure. Nutrition, metabolism, and cardiovascular diseases: NMCD. Nov 2009;19(9):641-645.
- Fuentes JC, Salmon AA, Silver MA. Acute and chronic oral magnesium supplementation: effects on endothelial function, exercise capacity, and quality of life in patients with symptomatic heart failure. Congestive heart failure (Greenwich, Conn.). Jan-Feb 2006;12(1):9-13.
- Stepura OB, Martynow AI. Magnesium orotate in severe congestive heart failure (MACH). International journal of cardiology. Jan 9 2009;131(2):293-295.
- FDA. U.S. Food and Drug Administration. FDA approves new device for treating moderate to severe chronic heart failure in patients. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634103.htm. In: United States Dept of Health and Human Services. 3/21/2019. Accessed 3/27/2019.
- Abraham WT, Kuck KH, Goldsmith RL, et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart failure. 2018;6(10):874-883.
- Anker SD, Borggrefe M, Neuser H, et al. Cardiac contractility modulation improves long-term survival and hospitalizations in heart failure with reduced ejection fraction. European journal of heart failure. 2019.
- Kuschyk J, Roeger S, Schneider R, et al. Efficacy and survival in patients with cardiac contractility modulation: long-term single center experience in 81 patients. Int J Cardiol. 2015;183:76-81.
- Kloppe A, Lawo T, Mijic D, Schiedat F, Muegge A, Lemke B. Long-term survival with Cardiac Contractility Modulation in patients with NYHA II or III symptoms and normal QRS duration. Int J Cardiol. 2016;209:291-295.
- Impulse Dynamics. Implantation. https://www.impulse-dynamics.com/us/physicians/implantation/. Copyright 2019. Accessed 3/27/2019.
- Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. Mar 17 2000;86(5):580-588.
- Zheng S, Du Y, Peng Q, Fan X, Li J, Chen M. Trimetazidine Protects Against Atherosclerosis by Changing Energy Charge and Oxidative Stress. Medical science monitor : international medical journal of experimental and clinical research. 2018;24:8459-8468.
- Liu X, Gai Y, Liu F, et al. Trimetazidine inhibits pressure overload-induced cardiac fibrosis through NADPH oxidase-ROS-CTGF pathway. Cardiovasc Res. Oct 1 2010;88(1):150-158.
- Wen J, Ma X, Zhang L, et al. Therapeutic efficacy and safety of Shexiang Baoxin Pill combined with trimetazidine in elderly patients with heart failure secondary to ischaemic cardiomyopathy: A systematic review and meta-analysis. Medicine (Baltimore). Dec 2018;97(51):e13580.
- Coats CJ, Pavlou M, Watkinson OT, et al. Effect of Trimetazidine Dihydrochloride Therapy on Exercise Capacity in Patients With Nonobstructive Hypertrophic Cardiomyopathy: A Randomized Clinical Trial. JAMA Cardiol. Feb 6 2019.
- Hoque MH, Kabir FI, Arzu J, et al. Comparison between Glyceryl Trinitrate and Trimetazidine in Ischaemic Cardiomyopathy Patients. Mymensingh Med J. Jan 2019;28(1):114-119.
- Chrusciel P, Rysz J, Banach M. Defining the role of trimetazidine in the treatment of cardiovascular disorders: some insights on its role in heart failure and peripheral artery disease. Drugs. Jun 2014;74(9):971-980.
- Kwon J, Yu YM, Kim S, Jeong KH, Lee E. Association between Trimetazidine and Parkinsonism: A Population-Based Study. Neuroepidemiology. Mar 4 2019;52(3-4):220-226.
- Pinter D, Kovacs M, Harmat M, Juhasz A, Janszky J, Kovacs N. Trimetazidine and parkinsonism: A prospective study. Parkinsonism Relat Disord. Jan 4 2019.
- ClinicalTrials.gov. Trimetazidine clinical trials, recruiting or not yet recruiting. 2019. https://clinicaltrials.gov/ct2/results?recrs=ab&cond=&term=trimetazidine&cntry=&state=&city=&dist=. Accessed Apr. 16, 2019.
- Guarini G, Huqi A, Morrone D, Capozza PFG, Marzilli M. Trimetazidine and Other Metabolic Modifiers. Eur Cardiol. Dec 2018;13(2):104-111.
- Zou H, Zhu XX, Ding YH, et al. Trimetazidine in conditions other than coronary disease, old drug, new tricks? Int J Cardiol. May 1 2017;234:1-6.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. Journal of the American College of Cardiology. 2022;79(17):e263-e421. doi:doi:10.1016/j.jacc.2021.12.012. https://pubmed.ncbi.nlm.nih.gov/35363499/
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure--meta-analyses of randomized trials. J Hypertens. Mar 2016;34(3):373-84; discussion 384. doi:10.1097/hjh.0000000000000848.
- Yeong T, Mai AS, Lim OZH, et al. Can glucose-lowering medications improve outcomes in non-diabetic heart failure patients? A Bayesian network meta-analysis. ESC Heart Fail. Apr 2022;9(2):1338-1350. doi:10.1002/ehf2.13822. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8934935/pdf/EHF2-9-1338.pdf
- Pandey AK, Dhingra NK, Hibino M, Gupta V, Verma S. Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a meta-analysis. ESC Heart Fail. Apr 2022;9(2):942-946. doi:10.1002/ehf2.13805. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8934917/pdf/EHF2-9-942.pdf
- Xu D, Chandler O, Wee C, et al. Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i) as a Primary Preventative Agent in the Healthy Individual: A Need of a Future Randomised Clinical Trial? Review. Frontiers in Medicine. 2021-August-23 2021;8doi:10.3389/fmed.2021.712671. https://www.frontiersin.org/article/10.3389/fmed.2021.712671
- Hink U, Voigtländer T. Necessity of Antiaggregation and Anticoagulation and Its Prognostic Impact: A Cardiologist's View. Visc Med. Aug 2020;36(4):264-273. doi:10.1159/000509896. https://www.karger.com/Article/Pdf/509896
- Lee MMY, Sattar N, McMurray JJV, Packard CJ. Statins in the Prevention and Treatment of Heart Failure: a Review of the Evidence. Curr Atheroscler Rep. Jul 27 2019;21(10):41. doi:10.1007/s11883-019-0800-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6660504/pdf/11883_2019_Article_800.pdf
- Bratsos S. Efficacy of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor-Neprilysin Inhibitors in the Treatment of Chronic Heart Failure: A Review of Landmark Trials. Cureus. Jan 19 2019;11(1):e3913. doi:10.7759/cureus.3913. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6426571/pdf/cureus-0011-00000003913.pdf
- Goyal A, Cusick AS, Thielemier B. ACE Inhibitors. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Wang Y, Qiao S, Han DW, et al. Telmisartan Improves Insulin Resistance: A Meta-Analysis. Am J Ther. Nov/Dec 2018;25(6):e642-e651. doi:10.1097/mjt.0000000000000733.
- Tai C, Gan T, Zou L, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on cardiovascular events in patients with heart failure: a meta-analysis of randomized controlled trials. BMC cardiovascular disorders. Oct 5 2017;17(1):257. doi:10.1186/s12872-017-0686-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5629775/pdf/12872_2017_Article_686.pdf
- Hill RD, Vaidya PN. Angiotensin II Receptor Blockers (ARB). StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Bao J, Kan R, Chen J, et al. Combination pharmacotherapies for cardiac reverse remodeling in heart failure patients with reduced ejection fraction: A systematic review and network meta-analysis of randomized clinical trials. Pharmacol Res. Jul 2021;169:105573. doi:10.1016/j.phrs.2021.105573. https://www.sciencedirect.com/science/article/abs/pii/S1043661821001572?via%3Dihub
- Kotecha D, Flather MD, Altman DG, et al. Heart Rate and Rhythm and the Benefit of Beta-Blockers in Patients With Heart Failure. J Am Coll Cardiol. Jun 20 2017;69(24):2885-2896. doi:10.1016/j.jacc.2017.04.001. https://www.sciencedirect.com/science/article/pii/S0735109717368936?via%3Dihub
- Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. Bmj. Jan 16 2013;346:f55. doi:10.1136/bmj.f55. https://www.bmj.com/content/bmj/346/bmj.f55.full.pdf
- Cleland JGF, Bunting KV, Flather MD, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. Jan 1 2018;39(1):26-35. doi:10.1093/eurheartj/ehx564. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5837435/pdf/ehx564.pdf
- Farzam K, Jan A. Beta Blockers. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Patel Y, Joseph J. Sodium Intake and Heart Failure. Int J Mol Sci. Dec 13 2020;21(24)doi:10.3390/ijms21249474. https://mdpi-res.com/d_attachment/ijms/ijms-21-09474/article_deploy/ijms-21-09474.pdf
- Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. Jan 29 2019;1(1):Cd003331. doi:10.1002/14651858.CD003331.pub5.
- Modin D, Jørgensen ME, Gislason G, et al. Influenza Vaccine in Heart Failure. Circulation. Jan 29 2019;139(5):575-586. doi:10.1161/circulationaha.118.036788.
- Burnett H, Earley A, Voors AA, et al. Thirty Years of Evidence on the Efficacy of Drug Treatments for Chronic Heart Failure With Reduced Ejection Fraction: A Network Meta-Analysis. Circulation Heart failure. Jan 2017;10(1):e003529. doi:10.1161/circheartfailure.116.003529. https://www.ahajournals.org/doi/pdf/10.1161/CIRCHEARTFAILURE.116.003529?download=true
- She J, Lou B, Liu H, et al. ARNI versus ACEI/ARB in Reducing Cardiovascular Outcomes after Myocardial Infarction. ESC Heart Fail. Dec 2021;8(6):4607-4616. doi:10.1002/ehf2.13644.
- Tromp J, Ouwerkerk W, van Veldhuisen Dirk J, et al. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure With Reduced Ejection Fraction. JACC: Heart Failure. 2022/02/01 2022;10(2):73-84. doi:10.1016/j.jchf.2021.09.004. https://doi.org/10.1016/j.jchf.2021.09.004 https://www.sciencedirect.com/science/article/abs/pii/S221317792100442X?via%3Dihub
- Nicolas D, Kerndt CC, Reed M. Sacubitril/Valsartan. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Grant ADM, Chew DS, Howlett JG, Miller RJH. Cost-Effectiveness of Earlier Transition to Angiotensin Receptor Neprilysin Inhibitor in Patients With Heart Failure and Reduced Ejection Fraction. CJC Open. 2020/11/01/ 2020;2(6):447-453. doi:https://doi.org/10.1016/j.cjco.2020.05.009. https://www.sciencedirect.com/science/article/pii/S2589790X20300688
- Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC cardiovascular disorders. Dec 1 2016;16(1):246. doi:10.1186/s12872-016-0425-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5134129/pdf/12872_2016_Article_425.pdf
- Xiang Y, Shi W, Li Z, et al. Efficacy and safety of spironolactone in the heart failure with mid-range ejection fraction and heart failure with preserved ejection fraction: A meta-analysis of randomized clinical trials. Medicine (Baltimore). Mar 2019;98(13):e14967. doi:10.1097/md.0000000000014967. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6456096/pdf/medi-98-e14967.pdf
- Kapelios CJ, Murrow JR, Nührenberg TG, Montoro Lopez MN. Effect of mineralocorticoid receptor antagonists on cardiac function in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Heart failure reviews. May 2019;24(3):367-377. doi:10.1007/s10741-018-9758-0.
- Kapelios CJ, Bonou Μ, Malliaras K, et al. Association of loop diuretics use and dose with outcomes in outpatients with heart failure: a systematic review and meta-analysis of observational studies involving 96,959 patients. Heart failure reviews. Jan 2022;27(1):147-161. doi:10.1007/s10741-020-09995-z.
- Antonietta CM, Calvi E, Faggiano A, et al. Impact of Loop Diuretic on Outcomes in Patients with Heart Failure and Reduced Ejection Fraction. Curr Heart Fail Rep. Feb 2022;19(1):15-25. doi:10.1007/s11897-021-00538-7. https://link.springer.com/article/10.1007/s11897-021-00538-7
- Tomasoni D, Vishram-Nielsen JKK, Pagnesi M, et al. Advanced heart failure: guideline-directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. Mar 30 2022;doi:10.1002/ehf2.13859. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/ehf2.13859?download=true
- Shams E, Bonnice S, Mayrovitz HN. Diuretic Resistance Associated With Heart Failure. Cureus. Jan 2022;14(1):e21369. doi:10.7759/cureus.21369. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8852330/pdf/cureus-0014-00000021369.pdf
- Taylor AL, Ziesche S, Yancy CW, et al. Early and sustained benefit on event-free survival and heart failure hospitalization from fixed-dose combination of isosorbide dinitrate/hydralazine: consistency across subgroups in the African-American Heart Failure Trial. Circulation . Apr 3 2007;115(13):1747-53. doi:10.1161/circulationaha.106.644013.
- Greenberg B. Medical Management of Patients With Heart Failure and Reduced Ejection Fraction. Korean circulation journal. Mar 2022;52(3):173-197. doi:10.4070/kcj.2021.0401. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8907986/pdf/kcj-52-173.pdf
- Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. New England Journal of Medicine. 2020;382(20):1883-1893. doi:10.1056/NEJMoa1915928. https://www.nejm.org/doi/full/10.1056/NEJMoa1915928
- Vyas A, Onteddu N. Vericiguat. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Parikh RR, Patel KR, Pergolizzi JV, Jr., Breve F, Magnusson P. Effects of Digoxin in Heart Failure (HF) With Reduced Ejection Fraction (EF). Cureus. Mar 2022;14(3):e22778. doi:10.7759/cureus.22778. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8971068/pdf/cureus-0014-00000022778.pdf
- Liu J, Meng Q, Zheng L, et al. Effect of omega-3 polyunsaturated fatty acids on left ventricular remodeling in chronic heart failure: a systematic review and meta-analysis. Br J Nutr. Mar 4 2022:1-35. doi:10.1017/S0007114521004979. https://www.ncbi.nlm.nih.gov/pubmed/35241186
- Oppedisano F, Mollace R, Tavernese A, et al. PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling. Nutrients. Aug 26 2021;13(9)doi:10.3390/nu13092965. https://mdpi-res.com/d_attachment/nutrients/nutrients-13-02965/article_deploy/nutrients-13-02965.pdf
- Patel D, Busch R. Omega-3 Fatty Acids and Cardiovascular Disease: A Narrative Review for Pharmacists. J Cardiovasc Pharmacol Ther. Nov 2021;26(6):524-532. doi:10.1177/10742484211023715. https://www.ncbi.nlm.nih.gov/pubmed/34191622 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8547235/pdf/10.1177_10742484211023715.pdf
- Rakisheva A, Marketou M, Klimenko A, Troyanova-Shchutskaia T, Vardas P. Hyperkalemia in heart failure: Foe or friend? Clin Cardiol. Jul 2020;43(7):666-675. doi:10.1002/clc.23392. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7368299/pdf/CLC-43-666.pdf
- NIH. National Library of Medicine/MedlinePlus. Patiromer. Available at https://medlineplus.gov/druginfo/meds/a616012.html Last updated 05/15/2017. Accessed 04/08/2022. 2017;
- NIH. National Library of Medicine/MedlinePlus. Sodium Zirconium Cyclosilicate. Available at https://medlineplus.gov/druginfo/meds/a618035.html Last updated 07/15/2018. Accessed 04/08/2022. 2018;
- Breitenstein A, Steffel J. Devices in Heart Failure Patients-Who Benefits From ICD and CRT? Front Cardiovasc Med. 2019;6:111. doi:10.3389/fcvm.2019.00111. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6700378/pdf/fcvm-06-00111.pdf
- Marrouche NF, Brachmann J, Andresen D, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. New England Journal of Medicine. 2018;378(5):417-427. doi:10.1056/NEJMoa1707855. https://www.nejm.org/doi/full/10.1056/NEJMoa1707855
- Ghzally Y, Ahmed I, Gerasimon G. Catheter Ablation. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Butler J, Filippatos G, Jamal Siddiqi T, et al. Empagliflozin, Health Status, and Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation. Jan 18 2022;145(3):184-193. doi:10.1161/circulationaha.121.057812.
- Packer M, Butler J, Zannad F, et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. Oct 19 2021;144(16):1284-1294. doi:10.1161/circulationaha.121.056824.
- Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nature Reviews Cardiology. 2022/02/01 2022;19(2):100-116. doi:10.1038/s41569-021-00605-5. https://doi.org/10.1038/s41569-021-00605-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8420965/pdf/41569_2021_Article_605.pdf
- Metra M, Dinatolo E, Dasseni N. The New Heart Failure Association Definition of Advanced Heart Failure. Cardiac failure review. Feb 2019;5(1):5-8. doi:10.15420/cfr.2018.43.1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6396060/pdf/cfr-05-5.pdf
- Djuricic I, Calder PC. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients. 2021;13(7):2421. https://www.mdpi.com/2072-6643/13/7/2421 https://mdpi-res.com/d_attachment/nutrients/nutrients-13-02421/article_deploy/nutrients-13-02421.pdf?version=1626337299
- Skulas-Ray AC, Wilson PWF, Harris WS, et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation. Sep 17 2019;140(12):e673-e691. doi:10.1161/cir.0000000000000709.
- Liao J, Xiong Q, Yin Y, Ling Z, Chen S. The Effects of Fish Oil on Cardiovascular Diseases: Systematical Evaluation and Recent Advance. Front Cardiovasc Med. 2021;8:802306. doi:10.3389/fcvm.2021.802306. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8767101/pdf/fcvm-08-802306.pdf
- Zheng S, Qiu M, Wu JHY, et al. Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure. Therapeutic advances in chronic disease. 2022;13:20406223221081616. doi:10.1177/20406223221081616. https://journals.sagepub.com/doi/pdf/10.1177/20406223221081616
- Barbarawi M, Lakshman H, Barbarawi O, et al. Omega-3 supplementation and heart failure: A meta-analysis of 12 trials including 81,364 participants. Contemporary clinical trials. Aug 2021;107:106458. doi:10.1016/j.cct.2021.106458.
- Djoussé L, Cook NR, Kim E, et al. Supplementation With Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure. Circulation. Mar 3 2020;141(9):784-786. doi:10.1161/circulationaha.119.044645. https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.119.044645?download=true
- Djoussé L, Cook NR, Kim E, et al. Diabetes Mellitus, Race, and Effects of Omega-3 Fatty Acids on Incidence of Heart Failure Hospitalization. JACC: Heart Failure. 2022/04/01/ 2022;10(4):227-234. doi:https://doi.org/10.1016/j.jchf.2021.12.006. https://www.sciencedirect.com/science/article/pii/S2213177922000543 https://www.sciencedirect.com/science/article/abs/pii/S2213177922000543?via%3Dihub
- Liu J, Meng Q, Zheng L, et al. Effect of omega-3 polyunsaturated fatty acids on left ventricular remodeling in chronic heart failure: a systematic review and meta-analysis. Br J Nutr. Mar 4 2022:1-35. doi:10.1017/S0007114521004979. https://www.ncbi.nlm.nih.gov/pubmed/35241186
- Hu Y, Hu FB, Manson JE. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J Am Heart Assoc . Oct 2019;8(19):e013543. doi:10.1161/jaha.119.013543.
- Creighton JV, de Souza Goncalves L, Artioli GG, et al. Physiological Roles of Carnosine in Myocardial Function and Health. Adv Nutr. Oct 2 2022;13(5):1914-1929. doi:10.1093/advances/nmac059. https://pubmed.ncbi.nlm.nih.gov/35689661/
- Stefani GP, Capalonga L, da Silva LR, Dal Lago P. β-Alanine and l-histidine supplementation associated with combined training increased functional capacity and maximum strength in heart failure rats. Exp Physiol. May 2020;105(5):831-841. doi:10.1113/ep088327. https://pubmed.ncbi.nlm.nih.gov/32125738/
- Stefani GP, Capalonga L, da Silva LR, et al. Effects of aerobic and resistance exercise training associated with carnosine precursor supplementation on maximal strength and V̇O(2max) in rats with heart failure. Life Sci. Oct 1 2021;282:119816. doi:10.1016/j.lfs.2021.119816. https://pubmed.ncbi.nlm.nih.gov/34273376/
- Lombardi C, Carubelli V, Lazzarini V, et al. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition. Jan 2015;31(1):72-8. doi:10.1016/j.nut.2014.04.021. https://pubmed.ncbi.nlm.nih.gov/25287762/
- Girandola RN, Srivastava S. Effect of E-OJ-01 on Cardiac Conditioning in Young Exercising Adults: A Randomized Controlled Trial. Am J Ther. May 2017;24(3):e298-e307. doi:10.1097/MJT.0000000000000542. https://pubmed.ncbi.nlm.nih.gov/27930383/
- Srivastava S, Girandola RN, Abedon B. Effect of E-OJ-01 on Left Ventricular Ejection Fraction and Myocardial Oxygen Consumption: A Randomized, Double-Blind, Placebo-Controlled Study. J Multidiscip Healthc. 2022;15:2511-2525. doi:10.2147/JMDH.S381028. https://pubmed.ncbi.nlm.nih.gov/36349244/
- Kaur N, Shafiq N, Negi H, et al. Terminalia arjuna in Chronic Stable Angina: Systematic Review and Meta-Analysis. Cardiol Res Pract. 2014;2014:281483. doi:10.1155/2014/281483. https://pubmed.ncbi.nlm.nih.gov/24600529/
- Kumar V, Sharma N, Saini R, et al. Therapeutic potential and industrial applications of Terminalia arjuna bark. J Ethnopharmacol. Jun 28 2023;310:116352. doi:10.1016/j.jep.2023.116352. https://pubmed.ncbi.nlm.nih.gov/36933876/
- Dwivedi S, Jauhari R. Beneficial effects of Terminalia arjuna in coronary artery disease. Indian heart journal. Sep-Oct 1997;49(5):507-10. https://pubmed.ncbi.nlm.nih.gov/9505018/
- Bharani A, Ganguly A, Bhargava KD. Salutary effect of Terminalia Arjuna in patients with severe refractory heart failure. Int J Cardiol. May 1995;49(3):191-9. doi:10.1016/0167-5273(95)02320-v. https://www.ncbi.nlm.nih.gov/pubmed/7649665
- Maulik SK, Wilson V, Seth S, et al. Clinical efficacy of water extract of stem bark of Terminalia arjuna (Roxb. ex DC.) Wight & Arn. in patients of chronic heart failure: a double-blind, randomized controlled trial. Phytomedicine. Oct 15 2016;23(11):1211-9. doi:10.1016/j.phymed.2016.02.007. https://www.ncbi.nlm.nih.gov/pubmed/26988798
- Chioncel O, Adamo M, Nikolaou M, et al. Acute heart failure and valvular heart disease: A scientific statement of the Heart Failure Association, the Association for Acute CardioVascular Care and the European Association of Percutaneous Cardiovascular Interventions of the European Society of Cardiology. European journal of heart failure. 2023;25(7):1025-1048. doi:https://doi.org/10.1002/ejhf.2918. https://pubmed.ncbi.nlm.nih.gov/37312239/
- Minamino-Muta E, Kato T, Morimoto T, et al. Causes of Death in Patients with Severe Aortic Stenosis: An Observational study. Scientific Reports. 2017/11/07 2017;7(1):14723. doi:10.1038/s41598-017-15316-6. https://pubmed.ncbi.nlm.nih.gov/29116212/
- Cutlip D. Transcatheter aortic valve implantation: Periprocedural and postprocedural management. UpToDate. Updated 9/9/2024. Accessed 1/29/2025, https://www.uptodate.com/contents/transcatheter-aortic-valve-implantation-periprocedural-and-postprocedural-management
- Leon MB, Mack MJ. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Chapter 74: Transcatheter Aortic Valve Replacement. 12 ed. Elsevier Inc.; 2022.
- Parikh PB, Mack M, Stone GW, et al. Transcatheter aortic valve replacement in heart failure. European journal of heart failure. Feb 2024;26(2):460-470. doi:10.1002/ejhf.3151. https://pubmed.ncbi.nlm.nih.gov/38297972/
- Cahill TJ, Terre JA, George I. Over 15 years: the advancement of transcatheter aortic valve replacement. Annals of Cardiothoracic Surgery. 2020;9(6):442-451. https://pubmed.ncbi.nlm.nih.gov/33312902/
- Fischer-Rasokat U, Renker M, Liebetrau C, et al. Outcome of patients with heart failure after transcatheter aortic valve implantation. PLoS One. 2019;14(11):e0225473. doi:10.1371/journal.pone.0225473. https://pubmed.ncbi.nlm.nih.gov/31770401/
- Van Mieghem NM, Elmariah S, Spitzer E, et al. Transcatheter Aortic Valve Replacement in Patients With Systolic Heart Failure and Moderate Aortic Stenosis: TAVR UNLOAD. J Am Coll Cardiol. Oct 22 2024;doi:10.1016/j.jacc.2024.10.070. https://pubmed.ncbi.nlm.nih.gov/39480381/
- Siontis GCM, Praz F, Pilgrim T, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. European Heart Journal. 2016;37(47):3503-3512. doi:10.1093/eurheartj/ehw225. https://pubmed.ncbi.nlm.nih.gov/27389906/
- Siontis GCM, Overtchouk P, Cahill TJ, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. European Heart Journal. 2019;40(38):3143-3153. doi:10.1093/eurheartj/ehz275. https://pubmed.ncbi.nlm.nih.gov/31329852/
- Généreux P, Schwartz A, Oldemeyer JB, et al. Transcatheter Aortic-Valve Replacement for Asymptomatic Severe Aortic Stenosis. New England Journal of Medicine. 2025;392(3):217-227. doi:doi:10.1056/NEJMoa2405880. https://pubmed.ncbi.nlm.nih.gov/39466903/
- Raposeiras-Roubin S, Amat-Santos IJ, Rossello X, et al. Dapagliflozin in Patients Undergoing Transcatheter Aortic-Valve Implantation. New England Journal of Medicine. 2025;392(14):1396-1405. doi:doi:10.1056/NEJMoa2500366. https://pubmed.ncbi.nlm.nih.gov/40162639/
- Paolisso P, Belmonte M, Gallinoro E, et al. SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc Diabetol. Nov 21 2024;23(1):420. doi:10.1186/s12933-024-02504-8. https://pubmed.ncbi.nlm.nih.gov/39574095/
- Paolisso P, Belmonte M, Gallinoro E, et al. Correction: SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc Diabetol. Jan 13 2025;24:14. doi:10.1186/s12933-024-02560-0. https://pubmed.ncbi.nlm.nih.gov/39806470/
- de Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1-46. doi: 10.1152/physrev.00012.2014. https://pubmed.ncbi.nlm.nih.gov/25540137/
